Abstract
Necrotizing enterocolitis (NEC), a major cause of morbidity and mortality in premature infants, occurs after the introduction of oral feedings in conjunction with initial bacterial colonization of the gut and is hypothesized to be due to an immature (inappropriate) enterocyte response to bacterial stimuli. To test this hypothesis, we compared the enterocyte IL-8 response to inflammatory stimuli [lipopolysaccharide (LPS) and IL-1β] in immature vs. mature human small intestine. Initial in vitro studies comparing confluent Caco-2 cells, a model for mature human enterocytes, with a primary human fetal intestinal cell line (H4 cells) demonstrated that after inflammatory stimulation fetal cells secreted more IL-8 (LPS, 8-fold; IL-1β, 20-fold) than Caco-2 cells. IL-8 mRNA activity in fetal compared to Caco-2 cells was proportionately increased by the same magnitude with both stimuli. To validate the in vitro observations, small intestinal organ cultures from fetuses vs. older children were exposed to LPS and IL-1β. Again in human organ cultures from fetuses compared to older children, IL-8 secretion was greater (LPS, 2.5-fold; IL-1β, 200-fold) and mRNA activity after stimulation was comparably higher, suggesting that increased transcription of the IL-8 gene may account for the excessive response. Using immunohistochemical staining to identify the cellular source of IL-8, activity was noted predominantly in villous and crypt epithelium but also in a few immunoresponsive lymphoid cells. The observation that immature human enterocytes react with excessive pro-inflammatory cytokine production after inflammatory stimulation may help in part explain why prematures exposed to initial colonizing bacteria develop necrotizing enterocolitis.
As the incidence of premature births increases (1) and infants are born at earlier gestational ages (22–27 wk), a major challenge to neonatologists becomes coping with an immature gastrointestinal tract unprepared for bacterial colonization within the extrauterine environment. With the use of surfactant to reduce the incidence of respiratory distress syndrome in prematures (2), the major medical challenge in newborn intensive care nurseries becomes necrotizing enterocolitis (NEC), an inflammatory disease of the distal small intestine and proximal colon occurring principally in premature infants after the introduction of oral feedings (3). There is strong evidence that the initial bacterial colonization of the newborn intestine plays a pivotal role in the development of NEC (4–6). Extensive recent research studies have suggested that pathologic microorganisms, particularly Gram-negative bacteria, communicate (“crosstalk”) with the intestinal epithelium by attachment to microvillus membrane glycoproteins or glycolipids receptors. Through this communication, these pathologic microorganisms co-opt physiologic signal transduction pathways to modify effector responses in enterocyte structure and/or function for their own purposes. For example, bacterial cell surface lipopolysaccharide [endotoxin, lipopolysaccharide (LPS)] interacts with the enterocyte to stimulate transcription and translation of pro-inflammatory cytokines [tumor necrosis factor (TNF), IL-6, and IL-8] presumably via the activation of the transcription factor, NF-κB (7–9). IL-8 is a chemokine that stimulates migration of neutrophils from intravascular to interstitial and luminal sites (7). NEC is characterized by an extensive hemorrhagic inflammatory necrosis of the distal small bowel and proximal colon with extensive infiltration of neutrophils (10). We have previously hypothesized that the pathogenesis of NEC is in part due to an immature (inappropriate) intestinal epithelial immunologic response to luminal bacterial stimuli (11). Because the disease develops principally in prematures fed artificially at a time when initial Gram-negative bacterial colonization occurs (12), we choose to study the response of immature human enterocytes to inflammatory stimuli using the pro-inflammatory cytokine, IL-8, as the effector response.
Therefore, in this study, we have examined the interaction of endotoxin and IL-1β with human small intestine from fetuses, neonates, and older children. To study the enterocyte response to endotoxin developmentally, we have used a primary crypt-like human fetal enterocyte cell line (H4 cells) developed in this laboratory (13) and a human organ culture technique (14), which allows fetal intestine and biopsy samples from human neonatal and infant intestine to remain viable for several days to determine their response to inflammatory stimuli. We herein provide strong evidence to suggest that the pathophysiologic inflammatory response in premature infants at a time when NEC occurs is in part related to an excessive IL-8 stimulation after initial colonization of the intestine with Gram-negative organisms.
Materials and Methods
Chemicals.
Ultra-pure D-glucose, sucrose, hydrocortisone hemisuccinate, and BSA were obtained from Sigma. Cell culture media, DMEM, cell culture media-RL (CMRL 1066), FBS, nonessential amino acids, methionine, b-retinyl acetate, glutamine, penicillin, and gentamicin were obtained from GIBCO/BRL. Tissue culture plastics were obtained from Fisher Scientific. All other chemicals were either reagent or molecular grade. IL-1 β and IL-8 ELISA kits (Quantikine) were obtained from R & D Systems. Protein concentrations were measured using a BCL kit (Pierce) against BSA standards in a colorimetric assay according to the manufacturer's protocol. Glucose was measured using the Trinder 100 kit from Sigma. It was necessary to dissolve the glucose oxidase reagent in 1 M Tris buffer, pH 7.6, to inhibit contaminating invertase.
Human Intestinal Tissue.
Human small intestinal samples were obtained from prostaglandin/saline-induced aborted fetus at the age of 18–21 wk with informed consent from all concerned parties according to the regulations of the Committee for the Protection of Human Subjects from Research Risks at the Brigham and Women's Hospital and the Human Investigation Committee at the Massachusetts General Hospital. To maintain sterility, tissues were collected only from fetuses in which the abdomen had not been previously opened. These tissues were then transported to the laboratory in ice-cold fresh CMRL 1066 media containing 40 μg/ml of penicillin and gentamicin. Tissues were processed as described below and rewashed in fresh ice-cold CMRL media. The gestational age averaged between 18 and 21 wk as determined by standard tables for developmental age.
Small intestinal (duodenal) mucosal biopsies from infants and older children were obtained by endoscopy with informed consent in the Pediatric Endoscopy Suite at MGH when these children were endoscoped for diagnostic purposes. Only biopsies from patients without any histologic abnormalities were used in these studies. Tissues were transported to the laboratory in ice-cold CMRL 1066 media containing 40 μg/ml penicillin and gentamicin and processed (15). Samples of intestine from all specimens tested were assayed for sucrase enzymatic activity before and after treatment with endotoxin and IL-1β. Samples of tissues before and after treatment were also obtained for histologic examination.
Intestinal Cell Lines.
The H4 cell line was developed from a 20-wk-old normal fetal small intestine by our laboratory and characterized in detail (13). The Caco-2/15 cell line at confluency is an established cell model for mature differentiated enterocytes (16). The cells were placed into 6-well Falcon plates (Fisher Scientific) at an initial density of 1 × 105 cells/cm2. Both cell lines were grown in DMEM (GIBCO/BRL) supplemented with 10% FCS, 2 mM glutamine, 100,000 units/liter penicillin, 100 gm/liter streptomycin, 0.1 mM MEM, nonessential amino acid, and 10 mM Hepes buffer in a humid atmosphere of 95% O2/5% CO2, as described previously (43). On day 7 after both cultures were grown in duplicate as described previously (43) and allowed to reach confluence, cultures were changed into a serum-free media with or without 1 mM sodium butyrate. After 24 h, the cells were treated with media containing either 50 μg/ml of LPS, 1 ng/ml IL-1β, or media alone as a control. After 24 h, the media were collected and stored at −20°C for subsequent analysis of IL-8 secretion. Cells were extracted with lysis buffer and centrifuged to clear particulate matter. Supernatants were collected and the protein concentration was determined using a BCL kit (Pierce) with BSA as a standard. The capacity of LPS and IL-1β to stimulate IL-8 secretion were quantitated by an ELISA assay and expressed as ng/mg of total cellular protein. Using trypan blue exclusion, approximately 95–98% of the cells were determined to be viable before and after each experiment.
Intestinal Organ Culture.
To confirm any differences in the endotoxin/IL-1β response between fetal human primary immature cells and mature confluent human Caco-2 colon cancer cells, we compared the endotoxin/IL-1β response in human small intestinal tissues from fetuses, neonates, and children. Proximal small bowel was stripped of its mesentery, split longitudinally, washed in fresh culture media, and cut into explants (5 × 5 mm2), for deposit onto a Falcon organ culture dish maintained in culture media at 37°C in an environment of 95% O2/5%CO2 and saturated water vapor as described previously (17). The culture medium was CMRL 1066 supplemented with glucose (5 g/liter), methionine (1 μg/liter), tricine buffer (20 mmol, pH 7.4), hydrocortisone hemisuccinate (0.5 μg/liter), b-retinyl acetate (1 mg/liter), glutamine (3 mmol/liter), 5% FBS (HyClone), penicillin G (100 units/liter), and gentamicin (50 mg/liter). Initially organ cultures were mounted on triangular wire-mesh grids as described by MacDonald and Spencer (14), but subsequent experiments demonstrated that the wire mesh was not necessary for the viability of the organ culture if cultures were grown on organ culture dishes for 24 h. The tissues were allowed to equilibrate for up to 12 h before beginning the experiments.
At least 12 explants were cultured as described above from each fetal intestinal sample. The cultures were allowed to equilibrate for 12 h before one-half of them were treated with media containing either 50 μg/ml of LPS or IL-1β (1 ng/ml) or with media alone as control. Six separate fetuses were used for 12 organ culture experiments. The intestinal biopsies were grouped into three categories: biopsies up to 2 yr were grouped as infants, from 2 to 8 yr were grouped as young children, and from 8 to 14 yr were grouped as older children. For organ cultures, at least 6–8 biopsies were obtained from the duodenum of each patient. One was stored for sucrase activity, another frozen for histology, and the remaining biopsies were treated in duplicate with media containing either LPS, IL-1β, or media alone. The media and tissue were collected and stored as described above 24 h after treatment with LPS, IL-1β, or media alone. The capacity of LPS and IL-1β to induce IL-8 was quantitated by an ELISA assay and expressed and ng/mg of total tissue protein. Tissue was homogenized in 9 vol of 0.154 M KCl and assayed for total protein using a BCL kit with a BSA standard. Sucrase activity was determined to assess functional viability of the organ culture after each experiment and histologic sections obtained to assess structural integrity.
RNA Isolation and Northern Blot Analysis.
Human fetal/neonatal small intestinal samples were rinsed and rapidly frozen in liquid nitrogen. Total RNA was extracted from epithelial cells or whole intestinal preparations by a single-step method of RNA isolation using acid guanidinum thiocyanate-phenol-chloroform extraction (18). RNA from each intestinal resection or cell line sample was denatured in 50% formamide at 65°C, subjected to electrophoresis on a 1% agarose gel containing formaldehyde, and transferred onto a Gene Screen-Plus membrane (Dupont) (19,20) and probed with IL-8 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA labeled by a random-primer labeling method (18–20). The membrane was hybridized according to the method of Church and Gilbert (19), washed, and exposed to films. Using a densitometer, the bands were quantitated and expressed as a IL-8:GAPDH ratio. GAPDH is a ubiquitous gene used here to control for sample variation.
IL-8 Analysis.
Levels of IL-8 were measured in culture supernatants using ELISA kits (R & D Systems). The culture supernatants were collected after each experiment, centrifuged at 800 rpm, and frozen at −20°C. IL-8 was quantified in each supernatant in duplicate. Calorimetric results were read on a Titertek Multiscan II 96-well plate reader at a wavelength of 450 nm. Values were normalized to total protein in cells or organ cultures.
Immunohistochemistry.
Before and after each experiment, organ culture specimens were obtained for morphologic examination to determine structural viability. Tissues were fixed in 4% paraformaldehyde at 4°C for 2 h before and after treatment. Then the tissue was further processed in 40% sucrose overnight at 4°C before embedded in OCT (Miles Laboratories) and frozen in absolute alcohol in dry ice. Six micrometer sections, prepared with a cryostat, were spread on glass slides and air dried before being processed for immunohistochemistry. Sections were fixed in paraformaldehyde and then incubated with rabbit polyclonal antibodies (Zymed) directed against human IL-8 diluted 1:1,000 in PBS containing 2% BSA. Peroxidase-conjugated goat anti-rabbit IgG1 at 1:50 dilution and incubated for 45 min (Boehriger Mannheim) was used as a secondary antibody. Isotype-specific rabbit IgG1 raised against nonspecific proteins were used at the same concentration as a negative control. The sections were photographed with 1000 ASA film in a Zeiss fluorescence microscope as described (20, 21).
Disaccharidase Analysis.
After each experiment, homogenates of intestinal organ cultures also were used to determine the level of sucrase activity as described previously (21, 22). Protein concentrations were measured using a BCL kit (Pierce) with BSA as standard. Sucrase activity was expressed as micromoles of substrate hydrolyzed per hour per milligram of total protein.
Statistics.
Results are presented as the mean ± SE. The effects of age and treatment on IL-8 secretion were analyzed by two-way analysis of variance. After overall significance was determined, posthoc tests for individual variables were performed by a two-tailed unpaired t test. Differences with a P value of <0.05 were considered significant.
Results
LPS and IL-lβ Interaction with Immature Fetal (H4) and Mature (Caco-2) Cell Cultures.
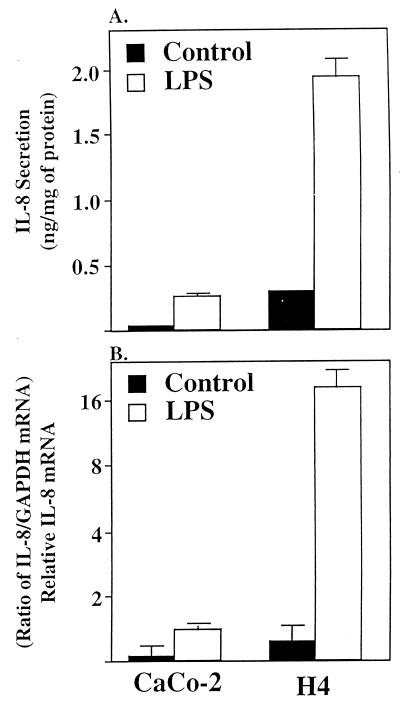
Fig. 1A summarizes data from at least four independent experiments performed in duplicate before and after exposure to LPS. The basal expression of IL-8 was low in both cell lines. However, H4 cells expressed IL-8 at a level 3- to 4-fold higher than Caco-2 cells. After exposure to LPS, a significantly (P < 0.001) elevated level of IL-8 in the media from both cell lines was noted. However, the level of IL-8 in media from H4 cells was 8-fold higher than media from Caco-2 cells.
Figure 1.
Depiction of IL-8 secretion (A) and IL-8 mRNA induction (B) in confluent Caco-2 and fetal human enterocytes (H4 cells) in response to LPS (50 μg/ml) or media alone as a control. Secreted IL-8 is expressed as ng/mg of total cellular protein. IL-8 mRNA was quatitated by densitometry and normalized to the relative level of GAPDH mRNA. Results are given as means ± SEM. The number of independent experiments for each data point ranged from four to five and an absence of an error bar indicates the SEM is smaller than the symbol.
Fig. 1B summarizes data depicting the level of IL-8 mRNA in these cells in response to LPS. In both cell lines, basal expression was minimal, and in Caco-2 cells more than 2 wk of exposure was needed to obtain a signal for IL-8 mRNA with a double intensifying screen. In response to LPS, Caco-2 cells showed a modest but significant rise of IL-8 mRNA. However, with a H4 cells, an extensive (15-fold) increased level of IL-8 mRNA was detected after a correction for experimental variation with relative levels of GAPDH mRNA.
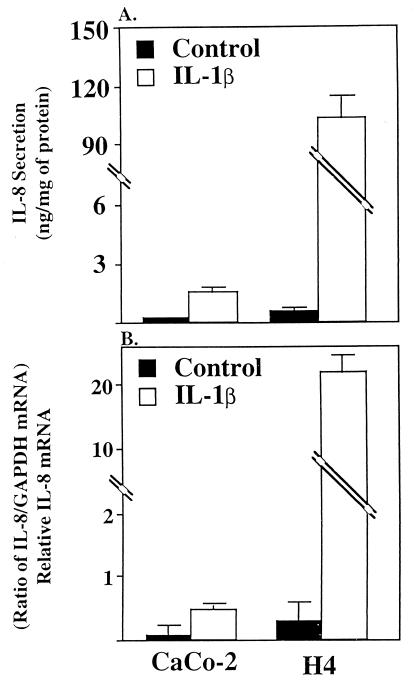
Fig. 2A summarizes data generated by four independent experiments performed in duplicate before and after IL-1β exposure. As noted above, the basal expression of IL-8 was very low in both cell lines, especially in Caco-2 cells, but 3- to 4-fold higher in H4 cells. Exposure to IL-1β resulted in a highly significantly elevated (20-fold) level of IL-8 in Caco-2 cells and (400-fold) in H4 cells (P < 0.0001).
Figure 2.
Depiction of IL-8 secretion (A) and IL-8 mRNA induction (B) in confluent Caco-2 and fetal human enterocytes (H4 cells) in response to IL-1β (1 ng/ml) or media alone as a control. Secreted IL-8 is expressed as ng/mg of total cellular protein. IL-8 mRNA was quatitated by densitometry and normalized to the relative level of GAPDH mRNA. The results are given as means ± SEM. The number of independent experiments for each data point ranged from four to five and as absence of error bar indicates the SEM is smaller than the symbol.
Fig. 2B summarizes data on the level of IL-8 mRNA in these cells in response to IL-1β. In both cell lines, basal expression was minimal, especially in Caco-2 cells. In response to IL-1β, Caco-2 cells showed a modest but significant level of IL-8 mRNA. However, with H4 cells, a strikingly increased level (40-fold) of IL-8 mRNA was detected after the correction.
LPS and IL-1β Interaction with Fetal and Infant/Neonatal Intestinal Organ Cultures.
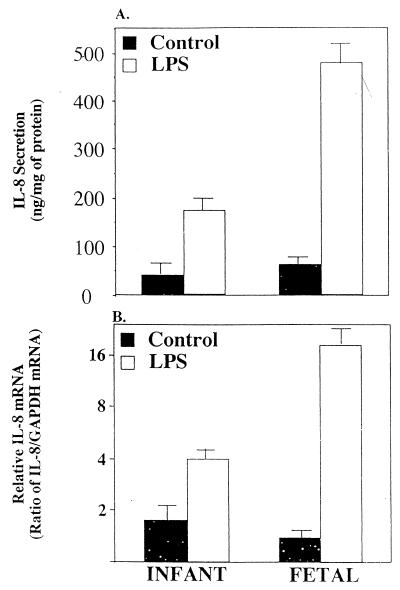
To confirm the striking difference in LPS and IL-1β stimulation of IL-8 responsiveness between H4 and Caco-2 cell lines, organ cultures of fetal and infant/child small intestinal biopsies were used to compare the IL-8 response to these stimuli (Fig. 3). There was no difference in IL-8 secretion in response to LPS or IL-1β stimulation when infant and young children's biopsies were used and thus they were grouped together. Basal IL-8 secreted by the infant/child control and fetal tissues were not significantly different (P > 0.6). However after LPS treatment IL-8 secretion increased significantly by 3- and 10-fold in infant/child (P < 0.05) and fetal and organ culture (P < 0.03), respectively (Fig. 3A). The level of sucrase activity in organ cultures was not significantly different before or after LPS stimulation regardless of the age or treatment of the organ culture (P > 0.7) and morphologic appearance was unchanged (data not shown) suggesting that the integrity of the organ cultures were not compromised during the experiments. Fig. 3B indicates that the level of IL-8 mRNA induced by LPS in these organ cultures was comparable to that of secreted IL-8. Tissues from both groups expressed a low basal IL-8 mRNA. However after stimulation infant tissue increased only 2-fold whereas in fetal tissue increased 16-fold.
Figure 3.
Depiction of IL-8 secretion (A) and IL-8 mRNA induction (B) in infant and fetal intestinal organ culture in response to LPS (50 μg/ml) or media alone as a control. Secreted IL-8 is expressed as ng/mg of total tissue protein. IL-8 mRNA was quatitated by densitometry and normalized to the relative level of GAPDH mRNA. The results are given as means ± SEM. The number of independent experiments for each data point ranged from three to four.
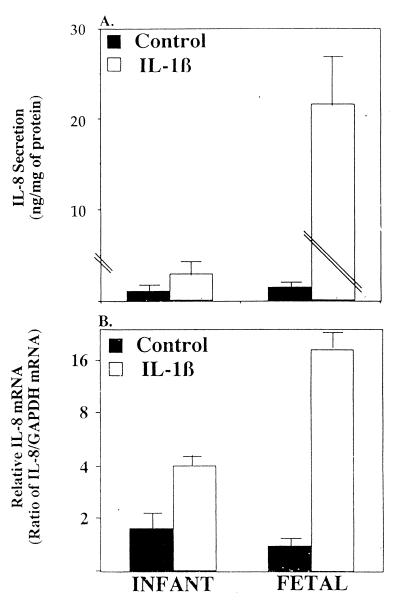
Treatment with IL-1β (Fig. 4) resulted in a significant increase in IL-8 in infant/child (3-fold; P < 0.002) and in fetal (200-fold; P < 0.001) organ cultures, respectively (Fig. 4A). The level of secreted IL-8 was reflected in a similar difference in the level of IL-8 mRNA (Fig. 4B) A 3- to 4-fold increase in mRNA was observed in the infant/child organ culture compared to a 30-fold increase in the fetal culture. Again sucrase activity and morphologic appearance were not significantly different before or after stimulation with IL-1β regardless of the age of the organ culture or stimulation (P > 0.7).
Figure 4.
Depiction of IL-8 secretion (A) and IL-8 mRNA induction (B) in infant and fetal intestinal organ culture in response to IL-1β (1 ng/ml) or media alone as a control. Secreted IL-8 is expressed as ng/mg of total tissue protein. IL-8 mRNA was quatitated by densitometry and normalized to the relative level of GAPDH mRNA. The results are given as means ± SEM. The number of independent experiments for each data point ranged from three to four.
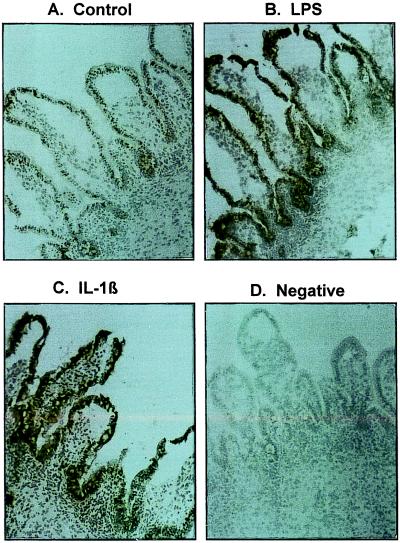
To determine the cellular source of IL-8 produced by these organ cultures, immunohistochemical analysis was performed by using rabbit polyclonal antibodies (Zymed) against human IL-8. Analysis was performed in triplicate. Fig. 5 illustrates a representative section showing basal levels (Fig. 5A) of IL-8 synthesis in organ culture. When stimulated with either LPS or IL-1β, the staining for IL-8 was significantly increased in the epithelium of the organ cultures from fetal (Fig. 5 B and C) and from infant (data not shown) biopsies. There were a few lymphoid cells in the lamina propria that expressed IL-8 whereas the entire epithelium from the crypt to villus was positive for IL-8. These studies strongly suggest that the predominant source of IL-8 after LPS and IL-1β stimulation was the intestinal epithelium regardless of the source of the tissue.
Figure 5.
This figure depicts IL-8 immunoreactivity in microscopic sections from human organ cultures after treatment with control media (A), LPS (B), or IL-1β (C). An additional microscopic section from a LPS-treated organ culture section stained with a class specific control antibody is shown in D. It should be noted that crypt and differentiated villus epithelial compartments are strongly positive for IL-8 with a few lymphoid cells in the lamina propria showing a reaction whereas no immunoreactivity is noted in D (A–D, ×50X, Toluidine Blue).
Discussion
In this study using immature fetal and mature cell lines and organ culture techniques, we have presented strong direct evidence that the fetal (immature) human small intestine responds excessively to external inflammatory stimuli (endotoxin and IL-1β) compared to the small intestine from infants and children. In previous studies, fetal intestinal organ cultures have been used to determine the role of luminal trophic factors in the development of the human gut (23, 24). However, this study is the first time that this technique has been used to characterize and compare the intestinal epithelial response to luminal inflammatory stimuli by measuring IL-8 transcription and translation in enterocytes as an effector response. IL-8 is a neutrophil-attracting chemokine known to be produced by enterocytes in response to inflammatory stimuli and probably plays an important role in intestinal inflammation (25). Although an established fetal human intestinal primary cell line was most likely representative of the human immature enterocyte response to inflammatory stimuli, confluent Caco-2 cells, known to be differentiated in a manner similar to that of mature human small intestinal villous cells (16), was not considered an ideal comparison since these cells are derived from a human colonic epithelial malignancy. Therefore, because we also used organ culture techniques to confirm the original observation in cell lines and the response was comparable, we suggest that this combination of observations provide compelling evidence to support our hypothesis.
NEC, a disease of prematurity, is characterized by an extensive inflammation and necrosis of the distal small bowel and proximal colon leading to perforation, peritonitis, and Gram-negative sepsis. It is the leading gastrointestinal cause of morbidity and mortality in premature infants (26). The disease develops shortly after oral feedings, usually infant formula, are introduced (4). In contrast, prematures given their mothers expressed breast milk have a much lower incidence of NEC and when it occurs a less severe disease (27). We know from previous studies that the nature of colonizing bacteria in newborn infants is influenced by whether they are breast-fed or not. Breast-fed infants develop intestinal flora that are predominately lactobacilli or bifidobacteria, whereas formula-fed newborns have a preponderance of enterobacteria and Gram-negative organisms (28). In previous animal studies from this laboratory, we have shown that glycosylation of microvillus membrane glycoconjugates (the microvillus membrane receptor for bacteria) is developmentally regulated (29) and predisposes the neonate to increased pathologic colonization (30). Recently, we have confirmed the same developmental regulation pattern in human fetuses and neonates (D. Dai, unpublished observations). In this study using human models for premature and mature small intestine, we have extended these observations to document another immaturity in the intestinal response presumably accounting for pathologic bacterial stimuli, namely an accentuated enterocyte chemokine response principally leading to the extensive inflammation and necrosis seen in NEC. Other inflammatory cytokines, platelet-activating factor and TNF are increased in the serum of premature infants with NEC compared to age-matched controls and full-term newborns (31, 32). In addition, when cortisone, a potent modulator of intestinal maturation, is given to mothers at risk for premature deliveries or to preterm infants to help accelerate lung maturation to prevent the expression of hyaline membrane disease, these infants were noted to have a lower incidence of NEC (33), suggesting that gut immaturity is involved. These observations strongly suggest that an inappropriate response of the immature gut to oral feedings and bacterial colonization represents a developmental risk factor for NEC.
Several studies have shown that enterocytes can constitutively express pro-inflammatory cytokines (IL-8, TNF, γ-INF, and IL-1) (34) and this response is up-regulated by inflammatory stimuli such as endotoxin and IL-1β (8, 35). IL-1β is also up-regulated in lymphocytes and enterocytes in response to endotoxin (36). This up-regulation of pro-inflammatory cytokines may be mediated by the transcription factor NF-κB (37). The intracellular signal transduction pathway for the IL-1β receptor expressed on the luminal surface of enterocytes has been shown to activate NF-κB and up-regulate IL-8 transcription. Recently, “toll-like receptors” (TLR1–4) have been reported on lymphoid cells (38, 39). These receptors which could be the natural receptor for the LPS complex (LPS bound to its co-factors lipopolysaccharide binding protein (LBP) and the glycosylphosphatidylinositol-anchoring membrane protein CD14) shares an intracellular domain comparable to that of the IL-1 receptor, which also activates NF-κB (36, 40). These observations may help explain the mechanism of LPS action on lymphocytes. In recent preliminary studies (R.D.F., unpublished observation), we have identified TLR-2 and -4 mRNA and TLR-2 protein on human enterocytes including the H4 fetal intestinal cell line and fetal organ cultures. If this observation is confirmed by more extensive studies, we may have an explanation for the endotoxin-activated intracellular response in enterocytes leading to an inflammatory cytokine response.
In this study, we have shown an enhanced IL-8 response by fetal enterocytes when stimulated with endotoxin and IL-1β compared to enterocytes from older children. In previous animal studies, we have reported that the immature compared to the mature enterocyte responses to cholera toxin and labile Escherichia coli toxin stimulation with an up-regulation of the GTP stimulatory protein α (Gsα) leading to an enhanced secretory response (41, 42). These observations suggest that the fetal human enterocyte may have signal transduction pathways in utero that are developmentally regulated and programmed to respond extensively to luminal stimuli resulting in the accentuated pathologic response noted in these studies. However, additional studies to quantitate receptor and signal transduction expression developmentally are needed to confirm this observation.
In these studies, a greater IL-8 response to both endotoxin and IL-1β were noted in fetal cells and biopsies compared to Caco-2 cells and biopsies from infants/children. The constitutive and after stimulation response to IL-1β in fetal enterocytes and the biopsies was much greater than that of the endotoxin response. Although we do not have an exact explanation for this observation, we can only speculate that receptor expression and/or the signal transduction activation response to IL-1β may be more highly developed in fetal intestine than is that for the endotoxin response. The endotoxin stimulus possibly via a toll-like receptor's signal transduction pathway leading to NF-κB activation is divergent from that of the IL-1β pathway and may not be as up-regulated as the IL-1β pathway. Alternatively, the capacity of LPS to complex with LBP and CD-14 may not be completely developed in the immature intestinal milieu and therefore may produce a lesser IL-8 enterocyte up-regulation when interacting with TLRs than the IL-1β receptors. Evidence exists that formation of the LPS-LPB-CD14 complex enhances the inflammatory response via toll receptors in lymphoid tissues (36, 43). These phenomenon also may dictate the endotoxin response in enterocytes. Again additional quantitative studies are needed to clarify this possible explanation
Compared to neutrophils and hepatocytes, the dose required for endotoxin to induce an IL-8 response in the mature enterocyte is between 100- to 1,000-fold higher. It is not surprising that these cells (enterocytes) are not very sensitive to endotoxins since they are exposed to Gram-negative bacteria and their endotoxins from the time of birth with the initial colonization of the gut. It is, therefore, conceivable that an increased tolerance to LPS and decreased responsiveness to IL-1β with age is developmentally controlled as an adaptation to the extrauterine environment of the intestine containing large quantities of bacteria and their toxins. It will therefore be of interest to determine in future studies which genes in the endotoxin/IL-1β signal transduction cascade are responsible for these ontogenic changes leading to increased IL-8 secretion reported in this study.
The capacity of intestinal epithelial cells to produce cytokines have been demonstrated and it is quite diverse compared to various lymphatic tissues (7–9). Among cytokines secreted by enterocytes a number of pro-inflammatory cytokines (e.g., IL-6, IL-8, TNF-α, GM-CSF, etc.) and anti-inflammatory cytokines (e.g., IL-10, IL-15, etc.) have been identified. However, they are limited in their repertoire. These cytokines have been implicated in recruiting various lymphoid elements during inflammation. In this study, we have begun to dissect out this balance by first examining IL-8 as a marker for pro-inflammatory signals generated by the epithelium in response to inflammatory stimuli. The balance of pro- and anti-inflammatory signals generated by intestinal epithelium is critical in mounting an effective acute response to inflammation and preventing chronic inflammation. However, an analysis of anti-inflammatory cytokines is beyond the scope of this study. Nevertheless, our studies demonstrate that there is a developmental regulatory component involved resulting in excessive induction of IL-8 by the epithelium in response to endotoxin and IL-1β. This may in part be responsible for NEC especially in the preterm infants. It is possible that a combination of enhanced pro-inflammatory cytokine response in conjunction with a muted anti-inflammatory cytokine (IL-4 and IL-10) response accounts for the increased incidence of NEC in premature infants. In a previous study of NEC patients, platelet activating factor, another pro-inflammatory cytokine, was increased and platelet-activating factor-acetylhydrolase, its natural degradation enzyme, was decreased (44) suggesting that an imbalance in pro-and anti-inflammatory factors may indeed contribute to the excessive inflammatory response in NEC patients. Additional studies are planed to determine this possibility.
In summary, using two technical approaches (cell and organ culture), we provide strong evidence that the immature fetal (premature) human enterocyte compared to the mature enterocyte of older children has an enhanced IL-8 response to inflammatory stimuli. This enhanced response may in part explain the pathophysiology of NEC. Additional studies are needed to further define the specific developmental step involved in the regulation of this process and the balance between pro- and anti-inflammatory stimuli in the intestinal response.
Acknowledgments
We thank the members of the Developmental Gastroenterology Laboratory for their encouragement during the course of the work, Lei Lu for her skillful assistance in immunochemistry, and Drs. Bobby Cherayil and Uzma Shah for critically reviewing the manuscript. This work was supported in part by National Institutes of Health Grants (R37 HD-12437, RO1 HD-31852, and PO1 DK-33506), a pilot feasibility grant from the Center for the Study of Inflammatory Bowel Diseases at the Massachusetts General Hospital (P30 DK33506), Core Facilities from the Harvard Clinical Nutrition Research Center (P30 DK40561), as well as a research grant from Wyeth Ayerst Nutritionals International.
Abbreviations
- NEC
necrotizing enterocolitis
- LPS
lipopolysaccharide
- TNF
tumor necrosis factor
- CMRL
1066 cell culture media-RL
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- TLR
toll-like receptors
References
- 1.Kliegman R M, Walker W A, Yolken R H. Pediatr Res. 1983;34:701. doi: 10.1203/00006450-199312000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Neu J. Pediatr Clin North Am. 1996;43:409. doi: 10.1016/S0031-3955(05)70413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacKendrick W, Caplan M. Pediatr Clin North Am. 1993;40:1047. doi: 10.1016/s0031-3955(16)38622-9. [DOI] [PubMed] [Google Scholar]
- 4.Foglia R P. Curr Prob Surg. 1994;32:757–823. doi: 10.1016/s0011-3840(05)80014-0. [DOI] [PubMed] [Google Scholar]
- 5.Stoll B J. Clin Perinatol. 1994;21:205–216. doi: 10.1016/S0095-5108(18)30341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kosloske A M. Acta Paediatr Suppl. 1994;396:2–7. doi: 10.1111/j.1651-2227.1994.tb13232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ulevitch, R. J. & Tobias, P. S. Ann. Rev. Immunol.13, 437–457. [DOI] [PubMed]
- 8.Eckmann L, Jung H C, Schurer-Maly C, Panja A, Morzycka-Wroblewska E, Kagnoff M F. Gastroenterology. 1993;105:1689–1697. doi: 10.1016/0016-5085(93)91064-o. [DOI] [PubMed] [Google Scholar]
- 9.DeFranco A L, Crowley M T, Finn A, Weinstein L. Prog Clin Biol Res. 1998;397:119–136. [PubMed] [Google Scholar]
- 10.Israel E J. Acta Paediatr Suppl. 1994;396:27–32. doi: 10.1111/j.1651-2227.1994.tb13238.x. [DOI] [PubMed] [Google Scholar]
- 11.Schiffrin E J, Walker W A, Carter E A, Benjamin J, Freiberg E, Israel E. J Pediatr Gastroenterol Nutr. 1993;17:271–275. [PubMed] [Google Scholar]
- 12.Lake A M, Walker W A. Clin Gastroenterol. 1977;6:463–480. [PubMed] [Google Scholar]
- 13.Sanderson I R, Ezzell R M, Kedinger M, Erlanger M, Xu Z, Pringault E, Leon-Robine S, Louvard D, Walker W A. Proc Natl Acad Sci USA. 1996;93:7717–7722. doi: 10.1073/pnas.93.15.7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacDonald T T, Spencer J J. J Exp Med. 1988;167:1341–1349. doi: 10.1084/jem.167.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trier J S. N Engl J Med. 1976;295:150–155. doi: 10.1056/NEJM197607152950308. [DOI] [PubMed] [Google Scholar]
- 16.Pinto M, Robine-Leon S, Appay M, Kedinger M, Triadou N, Dussaulx E, Laeroix B, Simon-Assmann P, Maffen K, Fogh J, Zweibaum A. Biol Cell. 1983;47:323–330. [Google Scholar]
- 17.Autrup H. Methods Cell Biol. 1980;21B:385–401. doi: 10.1016/s0091-679x(08)60694-9. [DOI] [PubMed] [Google Scholar]
- 18.Cmczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 19.Church G M, Gibert W. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandrasena G I, Sunitha C L, Nanthakumar N N, Henning S J. Cell Mol Biol. 1992;38:243–254. [PubMed] [Google Scholar]
- 21.Nanthakumar N N, Henning S J. Am J Physiol. 1993;264:G306–G311. doi: 10.1152/ajpgi.1993.264.2.G306. [DOI] [PubMed] [Google Scholar]
- 22.Antonowicz I, Lebenthal E. Gastroenterology. 1977;72:1299. [PubMed] [Google Scholar]
- 23.Playford R J, Woodman A C, Clark P, Watanapa P, Vessey D, Deprez P H, Williamson R C N, Calam J. Lancet. 1993;341:843–848. doi: 10.1016/0140-6736(93)93057-8. [DOI] [PubMed] [Google Scholar]
- 24.Menard D, Corriveau L, Arsenault P. J Pediatr Gastroenterol Nutr. 1990;10:13–20. doi: 10.1097/00005176-199001000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Luster A D. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 26.Israel E, Schiffrin E J, Carter E A, Freiberg E, Walker W A. Gastroenterology. 1990;99:133–138. doi: 10.1016/0016-5085(90)91158-3. [DOI] [PubMed] [Google Scholar]
- 27.Duffy C C, Zeilezmy M A, Carrion V, Griffiths E, Dryja D, Hilty M, Rock C, Morin F. Dig Dis Sci. 1997;42:359–365. doi: 10.1023/a:1018826204819. [DOI] [PubMed] [Google Scholar]
- 28.Bakner S E, Scott P H, Wharton B A. Arch Dis Child. 1989;64:1678–1674. doi: 10.1136/adc.64.12.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozaki C K, Chu S W, Walker W A. Biochem Biophys Acta. 1989;991:243–247. doi: 10.1016/0304-4165(89)90111-6. [DOI] [PubMed] [Google Scholar]
- 30.Schiffrin E J, Walker W A, Carter E A, Benjamin J, Freiberg E, Israel E. J Pediatr Gastroenterol Nutr. 1993;17:271–275. [PubMed] [Google Scholar]
- 31.Marecorft J A, Spitz L, Hamilton P A, Holmes S J. J Pediatr Surg. 1994;129:798–800. doi: 10.1016/0022-3468(94)90374-3. [DOI] [PubMed] [Google Scholar]
- 32.Moya F R, Eguchi H, Zhao B, Furukawa M, Steir J, Osono M, Ogawa Y, Johnston J M. J Pediatr Gastroenterol Nutr. 1994;19:236–239. doi: 10.1097/00005176-199408000-00015. [DOI] [PubMed] [Google Scholar]
- 33.Bauer C R, Morrison J C, Poole K, Korones S B, Boehm J J, Rigatto H, Zachman R D. Pediatrics. 1984;73:682. [PubMed] [Google Scholar]
- 34.Stadnyk A W. FASEB J. 1994;8:1041–1047. doi: 10.1096/fasebj.8.13.7926369. [DOI] [PubMed] [Google Scholar]
- 35.Yang S K, Eckmann L, Panja A, Kagnoff M F. Gastroenterology. 1997;113:1214–1223. doi: 10.1053/gast.1997.v113.pm9322516. [DOI] [PubMed] [Google Scholar]
- 36.Modlin R L, Brightbill H D, Godowski P J. N Engl J Med. 1999;340:1834–1835. doi: 10.1056/NEJM199906103402312. [DOI] [PubMed] [Google Scholar]
- 37.Muzio M, Natoli G, Saccani S, et al. J Exp Med. 1998;187:2097–2101. doi: 10.1084/jem.187.12.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Medzhitov R, Preston-Hurlburt P, Janeway C A. Nature (London) 1997;338:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 39.Yang R-B, Mark M R, Gray A, et al. Nature (London) 1998;395:284–288. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- 40.Kirschning C J, Wesche H, Ayres T M, Rothe M. J Exp Med. 1998;188:2091–2097. doi: 10.1084/jem.188.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chu S W, Walker W A. Gastroenterology. 1993;104:916–925. doi: 10.1016/0016-5085(93)91032-d. [DOI] [PubMed] [Google Scholar]
- 42.Sanderson I R, Xu Z, Chu S W, Xie Q Y, Levine L, Walker W A. Gut. 1996;38:853–858. doi: 10.1136/gut.38.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fusunyan R D, Quinn J J, Ohno Y, MacDermott R P, Sanderson I R. Pediatr Res. 1998;43:84–89. doi: 10.1203/00006450-199801000-00013. [DOI] [PubMed] [Google Scholar]
- 44.Harris M C, Costarmo A J, Sullivan J S, Dulkerian S, et al. J Pediatr. 1994;124:105–111. doi: 10.1016/s0022-3476(94)70264-0. [DOI] [PubMed] [Google Scholar]