Abstract
The reformulation of amphotericin B (AMB) into a lipid complex (AMB lipid complex [ABLC]) or liposomal carrier (liposomal AMB [L-AMB]) changes the rate and extent of drug distribution to the lung. The importance of pharmacokinetic differences among the various lipid AMB formulations in the treatment of invasive pulmonary aspergillosis (IPA) remains unknown. We compared the kinetics of AMB lung accumulation and fungal clearance of ABLC- and L-AMB-treated mice with acute IPA. BALB/c mice were immunosuppressed with cyclophosphamide and cortisone before intranasal inoculation with 1.5 × 106 Aspergillus fumigatus 293 conidia. ABLC or L-AMB was administered in daily intravenous doses (1, 5, or 10 mg/kg of body weight), starting 12 h after infection and continuing until day 5. At predetermined times (0, 24, 72, and 120 h), mice were euthanized, and lungs were harvested for determinations of lung fungal burdens (quantitative PCR) and total AMB lung tissue concentrations. Both ABLC and L-AMB were effective at reducing lung fungal burdens at doses of ≥5 mg/kg/day. Clearance of A. fumigatus during the first 24 h was associated with AMB tissue concentrations of >4 μg/g. At 5 mg/kg/day, ABLC produced a more rapid fungal clearance than did L-AMB, but at the end of therapy, fungal burden reductions were similar for both formulations and were not improved with higher dosages. These data suggest that ABLC delivers active AMB to the lung more rapidly than does L-AMB, resulting in faster Aspergillus clearance in an experimental model of IPA. However, pharmacodynamic differences between the two formulations were less apparent when mice were dosed at 10 mg/kg/day.
Despite the availability of new treatment options, lipid formulations of amphotericin B (AMB) continue to play a central role in the management of invasive pulmonary aspergillosis (IPA) due to their broad spectrum and low potential for cross-resistance with other antifungals (5). Currently, the following three lipid formulations are approved for the treatment of IPA in patients who have failed or are intolerant to other therapies: AMB colloidal dispersion, AMB lipid complex (ABLC), and liposomal AMB (L-AMB). All three AMB formulations differ in terms of their lipid composition and particle size, resulting in different pharmacokinetic characteristics when the drugs are administered in vivo. For example, L-AMB consists of small unilamellar particles (60 to 70 nm) that avoid uptake by cells of the reticuloendothelial system (RES) (7, 8, 14, 25). Hence, intravenous administration of L-AMB results in sustained, high concentrations of encapsulated AMB in the serum, with a somewhat delayed distribution of free drug to tissues. Conversely, intravenous administration of ABLC results in relatively low serum AMB concentrations due to the rapid RES cell uptake of the large lipid complex (1,600 to 11,000 nm) (1). Extensive RES cell uptake of ABLC is thought to account for the more rapid distribution of ABLC to certain organs, such as the lungs, than that of other formulations (18, 19). The clinical relevance of these pharmacokinetic differences between L-AMB and ABLC, however, remains unknown.
It is generally accepted that any delay in the initiation of antifungal therapy for IPA contributes to a poorer clinical response (2, 11). In neutropenic hosts, unimpeded growth of Aspergillus hyphae in the lung results in hemorrhage and coagulative tissue necrosis with limited blood flow (27). Hence, it is unlikely that significant amounts of drug are delivered to infarcted tissue containing the sequestered Aspergillus hyphae. Using a neutropenic rat model of IPA, Becker and colleagues found that delaying the start of L-AMB therapy (10 mg/kg of body weight/day) as little as 8 h increased animal mortality (6). Interestingly, L-AMB treatment was significantly more effective if a dose of conventional AMB-deoxycholate (1 mg/kg/day) was administered at the start of L-AMB treatment, suggesting that the availability of active AMB in the lung at early stages of infection was a critical factor in animal survival (6). The investigators did not test other lipid formulations, such as ABLC, which is known to rapidly distribute to the lung after intravenous administration.
The goals of the current investigation were (i) to compare the kinetics of AMB accumulation in lung tissue following intravenous treatment with ABLC and L-AMB and (ii) to determine whether differences in the rates of tissue AMB accumulation between the two lipid formulations correlated with different rates of Aspergillus fumigatus clearance in an experimental model of acute IPA.
(Part of this work was presented previously [R. E. Lewis, G. Liao, J. Hou, G. Chamilos, R. A. Prince, and D. P. Kontoyiannis, Abstr. 46th Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-1689, 2006].)
MATERIALS AND METHODS
Reagents.
Cortisone acetate and cyclophosphamide were obtained from Sigma-Aldrich (St. Louis, MO). The commercial formulation of L-AMB (Ambisome) was obtained from Amerisource-Bergen (Chesterbrook, PA). The human clinical formulation of ABLC (Abelcet) was generously provided by Enzon Pharmaceuticals (Bridgewater, NJ).
Animals.
Eight-week-old female BALB/c mice (18 to 25 g; Charles River Laboratories) were used in all experiments. Mice were housed in sterilized filter-top cages and had access to sterile food and water ad libitum. All mice were cared for in accordance with the highest standards for humane and ethical care, as approved by the Institutional Animal Care and Use Committee.
Inoculum preparation.
Aspergillus fumigatus 293, the strain used for genome sequencing (21), was grown on potato dextrose agar for 7 days prior to collection of conidia. Conidia were harvested from the slant with 0.1% Tween 20 in phosphate-buffered saline and passed through a syringe with sterile glass wool to remove hyphal fragments. The resulting suspension was then centrifuged for 5 min at 15,000 × g, the supernatant was discarded, and the number of conidia was determined by hemocytometer counting. The final concentration was adjusted to 5 × 107 conidia. Harvested conidia were determined to be >98% viable based on plating of a serially diluted inoculum on Sabouraud dextrose agar. Susceptibility testing was performed using AMB epsilometer strips (AB Biodisk, Solna, Sweden) and established methods for determining the mean fungicidal concentration (MFC) for filamentous fungi (24).
Immunosuppression and infection.
Immune suppression was achieved by intraperitoneal (i.p.) injections of cyclophosphamide (75 mg/kg) 4 days and 1 day prior to infection. This regimen results in total polymorphonuclear neutrophil depletion until 96 h after infection (16). In addition, animals received a single 300-mg/kg i.p. dose of cortisone acetate suspension prepared in phosphate-buffered saline with 0.2% Tween 20 1 day prior to infection (31). Doxycycline HCl (Sigma) was added to the drinking water (0.5 mg/ml) as antibacterial prophylaxis. Additionally, doxycycline-drinking water-soaked mouse chow was placed in the corner of each cage and exchanged daily to reduce animal dehydration.
Prior to inoculation, animals were anesthetized with a single i.p. injection (200 μl) of ketamine (80 mg/kg) plus xylazine (10 mg/kg) and placed on warming pads prior to intranasal inoculation with 1.5 × 106 A. fumigatus conidia. Animal inoculation was performed by slowly instilling a 30-μl droplet on both nares, and the mice were allowed to inhale the inoculum in an upright position until normal breathing resumed (16). After inoculation, animals were returned to the warming pad and observed until full recovery. This protocol results in reproducible infection of the lungs, with untreated animals succumbing to the infection 96 to 120 h after inoculation (16).
Antifungal treatment and sample collection.
Groups of immunosuppressed mice (20 per treatment arm) received intravenous antifungal therapy with L-AMB or ABLC, at a dose of 1, 5, or 10 mg/kg, diluted in sterile 5% dextrose water and administered once daily by lateral tail vein injection. Control animals were administered 5% dextrose water alone. In selected experiments, L-AMB and ABLC regimens were also administered as a single intravenous dose of either 5 or 10 mg/kg. All antifungal regimens were started 12 h after inoculation and continued daily for 4 days. At serial time points after infection (0, 24, 72, and 120 h), five mice were euthanized by CO2 narcosis, and blood was immediately collected into a heparinized syringe by cardiac puncture. Blood was then transferred to a sterile capped tube and centrifuged (10,000 × g) for 10 min. Plasma supernatants were subsequently transferred to a beta-glucan (BG)-free cryovial and stored at −80°C until analysis. Lungs were then aseptically removed and stored at −80°C until analysis.
Tissue fungal burden.
Pulmonary fungal burdens were determined by real-time quantitative PCR by previously reported methods (9, 30). Briefly, DNA samples isolated from homogenized lungs were assayed in duplicate by use of an ABI PRISM 7000 sequence detection system (Applied Biosystems, Foster City, CA), using primers and a dually labeled fluorescent hybridization probe specific for the Aspergillus 18S rRNA gene (9). The cycle threshold of each sample was interpolated from a seven-point standard curve of cycle threshold values prepared by spiking uninfected mouse lungs with known amounts of conidia (101 to 107) from A. fumigatus 293. An internal standard was amplified in separate reactions to correct for the percent difference in DNA recovery (30, 31). Results are reported as conidial equivalents (CE) of A. fumigatus DNA.
AMB tissue concentrations.
Determinations of total AMB tissue concentrations in infected lungs were performed by high-performance liquid chromatography (HPLC), using a modification of previously published assays (15, 20, 26). Briefly, AMB and a spiked internal standard (1-amino-4-nitro-naphthalene [ANNP]) were isolated from tissue homogenates by acetonitrile precipitation of proteins, followed by centrifugation. A 50-μl aliquot of the extracted supernatant was injected through a C18 guard column into a Nova-Pak C18 column (3.9 mm by 150 mm by 4 μm). AMB and ANNP (internal standard) were eluted at a flow rate of 1 ml per minute with a gradient program (acetonitrile from 30 to 45% plus 2.5 mM EDTA from 70% to 55% in 8 min) and were detected at 406 nm. The calibration curve was linear over a range of 0.25 to 10 μg/g in tissue. Mean inter- and intra-assay coefficients of variation over the range of the standard curve were <10%. The lower limit of accurately detectable AMB in tissue was 0.25 μg/g.
Plasma BG concentration.
Plasma BG concentrations in infected animals treated with L-AMB or ABLC were determined using a commercially available assay according to the manufacturer's instructions (Fungitell; Associates of Cape Cod). Plasma samples (5 μl) were pretreated for 10 min at 37°C with an alkaline reagent (20 μl; 0.125 M KOH-0.6 M KCl) to inactivate serine proteases as well as inhibitors in mouse plasma and to enhance the reactivity to activated factor G (28). After the addition of the BG assay reagent, the microtiter plate was inserted into a preincubated microplate spectrophotometer (Powerwave X Select; Biotech Instruments, Winooski, VT), and a kinetic assay was run at 405 nm, with 490-nm correction, using KC4 software (Biotech) on unknowns from infected animals or BG standards (15 to 250 pg/ml) provided by the manufacturer.
Statistical analysis.
All data were expressed as means ± standard errors of the means and were compared by the Mann-Whitney test or one-way analysis of variance, with Tukey's posttest for multiple comparisons where appropriate. Differences were considered statistically significant when P values were <0.05. Total AMB tissue concentrations associated with inhibition of A. fumigatus growth (stasis) or a 1-log10 reduction in fungal burden at 24 h were determined by fitting a four-parameter logistic model (Hill equation), using computer curve-fitting software (Prism 4; GraphPad Software, Inc., San Diego, CA), to the experimental data. Goodness of fit was assessed by determining R2 and the standard error of the 50% effective concentration.
RESULTS
Isolate susceptibility testing.
Susceptibility testing performed in triplicate with the test isolate A. fumigatus 293 revealed an AMB MIC of 0.25 μg/ml and an MFC of 2 μg/ml. MIC and MFC results were consistent throughout the study and with previously published in vitro studies that utilized the same isolate (17).
Kinetics of fungal burden reduction.
Plots of tissue fungal burden at 0, 24, 72, and 120 h versus total concentrations of AMB in lung tissue are presented in Fig. 1. After inoculation, the mean baseline fungal burden in the lung was 5.86 × 105 (range, 1.31 × 105 to 6.60 × 106) A. fumigatus DNA CE across all treatment groups. In control animals (treated with 5% dextrose water), fungal burden increased 1 log10 by 72 h and were associated with the onset of animal mortality at 72 to 120 h, consistent with previous studies (9, 31).
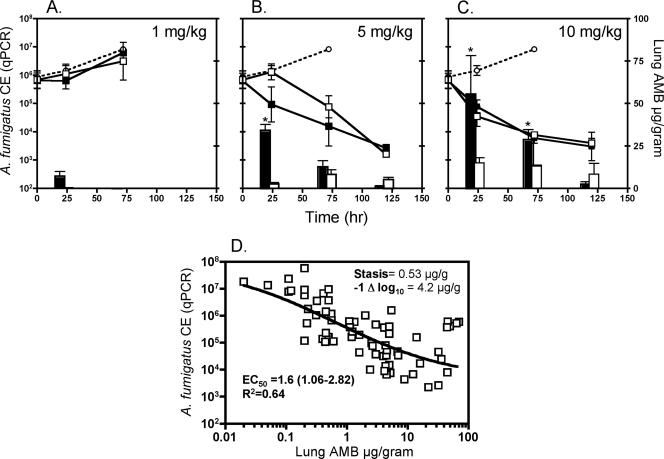
FIG. 1.
Differences in kinetics of AMB lung accumulation and fungal clearance between ABLC and L-AMB in a murine model of IPA. ○, control; ▪, ABLC; □, L-AMB. Line plots represent tissue A. fumigatus fungal burden, expressed as CE, versus time. Each data point is the mean ± standard error for five animals. Total lung AMB tissue concentrations (μg/gram) versus time are plotted on the z axis. Each bar represents the mean tissue concentration for five animals. (A) 1-mg/kg/day dosing; (B) 5-mg/kg/day dosing; (C) 10-mg/kg/day dosing. (D) Relationship of 24-hour A. fumigatus tissue fungal burden and total AMB tissue concentrations from all treatment groups (n = 60 mice). The line depicts the concentration-effective relationship determined by fitting a four-parameter logistic model (Hill equation) to the data, using the GraphPad Prism 4.0 software package. Mean best-fit values and 95% confidence intervals were calculated by the software. *, P < 0.05 for ABLC versus L-AMB tissue concentrations, as determined by the two-sided Mann-Whitney test.
Animals treated with either L-AMB or ABLC at 1 mg/kg/day exhibited increasing fungal burden until 72 h, similar to untreated (control) animals. Mortality was associated with fungal burden in excess of approximately 5 × 106 CE occurring among all three groups between 72 and 120 h (Fig. 1A). Therefore, experiments could not be completed until the 120-h time point. AMB could be detected in lung tissue only for ABLC-treated animals at 24 h (mean, 4.2 ± 1.77 μg/g) but was undetectable at later time points, despite daily dosing, and for all time points in L-AMB-treated animals.
Animals treated with L-AMB or ABLC at 5 mg/kg/day exhibited significant reduction in fungal burden versus control animals at 72 h (P < 0.05). Clear differences in the pattern of fungal burden reduction were observed between the two lipid AMB formulations, with ABLC producing immediate reductions in fungal burden by the first 24 h that were not seen with L-AMB until 72 h (Fig. 1B). Analysis of AMB tissue concentrations by HPLC confirmed the higher concentrations of AMB in ABLC-treated animals at 24 h than in L-AMB-treated animals (34.2 μg/g versus 2.09 μg/g; P < 0.05). By 72 h, the mean tissue fungal burdens (1.35 × 105 versus 3.23 × 105 A. fumigatus 293 CE) and AMB tissue concentrations (12.7 μg/g versus 8.21 μg/g) were similar for ABLC- and L-AMB-treated animals dosed at 5 mg/kg/day (Fig. 1B). The absolute difference in fungal burdens between the two dosing regimens at 24 h did not quite reach statistical significance (P = 0.06).
ABLC or L-AMB treatment at 10 mg/kg/day achieved higher concentrations of AMB in the lungs at 24 h (55.4 μg/g versus 15.0 μg/g; P < 0.05) and faster reductions in A. fumigatus tissue burdens in L-AMB- but not ABLC-treated animals than those for the 5-mg/kg/day treatment regimens (Fig. 1C). The rates of fungal clearance were similar between the treatment groups receiving L-AMB at 10 mg/kg/day and ABLC at 10 mg/kg/day. Despite improvements in the rate of fungal burden reduction at the 10-mg/kg dose for L-AMB, the extents of fungal burden reduction at the end of the experiment were similar for both formulations at the 5- and 10-mg/kg/day dosing levels.
Because early reductions in lung fungal burden appeared to correlate with a threshold concentration of AMB in the lung in the first 24 h, fungal burden data from all treatment groups at 24 h (n = 60 mice) were plotted in relation to total AMB tissue concentrations measured by HPLC (Fig. 1D). A four-parameter logistic model was then fitted to the data to predict threshold AMB tissue concentrations associated with growth stasis and a 1-log10 reduction in A. fumigatus DNA CE. Variability in fungal burdens across all treatment groups at 24 h could be explained largely by tissue concentrations of AMB (R2 = 0.64). The total AMB tissue threshold concentrations associated with growth stasis and a 1-log10 reduction in fungal burden were 0.53 μg/g and 4.20 μg/g, respectively. At a dosage of 5 mg/kg/day, 100% of ABLC-treated mice versus 20% of L-AMB-treated animals achieved or surpassed the tissue concentrations required for fungal clearance at 24 h (P = 0.04; two-sided Fisher's exact test). At a dosage of 10 mg/kg/day, both formulations consistently (100%) achieved tissue concentrations required for fungal clearance in the first 24 h.
Plasma BG concentrations.
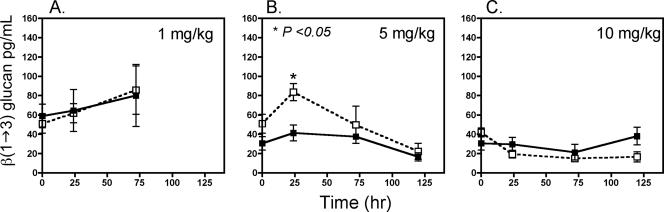
Plasma BG concentrations measured in infected neutropenic mice at baseline and at 24, 72, and 120 h correlated with A. fumigatus DNA CE fungal burden measurements determined by quantitative real-time PCR (Fig. 2). Animals treated with L-AMB or ABLC at 1 mg/kg/day exhibited an initial mean plasma BG concentration of 56.9 ± 7.1 pg/ml, which increased to 62.7 ± 8.5 pg/ml by 24 h and to 82.5 ± 19.4 pg/ml by 72 h (Fig. 1C). Similar to the fungal burden data, animals treated with L-AMB at 5 mg/kg/day experienced a higher mean peak BG concentration in the plasma at 24 h than did animals treated with 5 mg/kg/day of ABLC (84.7 ± 8.8 pg/ml versus 38.16 ± 8.1 pg/ml; P = 0.017); however, plasma BG concentrations were similar between the treatment groups at 72 and 120 h (Fig. 1B). No difference in mean plasma BG concentrations was noted between L-AMB- and ABLC-treated animals at a dose of 10 mg/kg/day (Fig. 1C).
FIG. 2.
Differences in concentrations of serum BG between ABLC- and L-AMB-treated mice with IPA. ▪, ABLC; □, L-AMB. The dotted line highlights the 60-pg/ml BG threshold considered by the manufacturer to be indicative of an invasive fungal infection. (A) 1-mg/kg/day dosing; (B) 5-mg/kg/day dosing; (C) 10-mg/kg/day dosing. *, P < 0.05 for ABLC versus L-AMB, as determined by the two-sided Mann-Whitney test.
Single-dose studies.
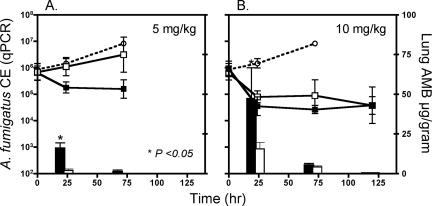
Because of the kinetic disparity in AMB delivery observed between the two formulations, we compared the durations of detectable drug concentrations in tissue and the persistence of antifungal effects after a single L-AMB or ABLC dose administered at 5 or 10 mg/kg (Fig. 3). Similar to the results of the multidose studies, a higher mean AMB lung concentration was observed at 24 h with ABLC than with L-AMB at a 5-mg/kg single dose (20.46 μg/g versus 3.28 μg/g; P < 0.05); however, tissue concentrations at subsequent time points were low or undetectable (<0.25 μg/g) in both the ABLC- and L-AMB-treated animals (Fig. 3A). A reduction in lung fungal burden was observed with ABLC-treated animals at 24 and 72 h (Fig. 1A). Similar to the results of the multidose studies using 1 mg/kg/day, animal deaths occurred with both groups between 72 and 120 h, suggesting an increase in fungal burden (data not shown on graph).
FIG. 3.
Differences in kinetics of AMB lung accumulation and fungal clearance between single-dose ABLC and L-AMB therapy in a murine model of IPA. ○, control; ▪, ABLC; □, L-AMB. Line plots represent tissue A. fumigatus fungal burdens, expressed as CE, versus time. Each data point is the mean ± standard error for five animals. Total lung AMB tissue concentrations (μg/gram) versus time are plotted on the z axis. Each bar represents the mean tissue concentration for five animals. (A) 5-mg/kg/day dosing; (B) 10-mg/kg/day dosing.
In the 10-mg/kg single-dose studies, tissue concentrations of AMB persisted to 72 h but were undetectable at 120 h (Fig. 3B). Fungal clearance was equivalent between L-AMB and ABLC, but reached a plateau at 24 to 72 h, failing to achieve the same fungal burden reduction observed at 120 h in the multidose studies (9.44 × 103 versus 6.43 × 104 A. fumigatus CE).
DISCUSSION
In this study, we compared the kinetics of AMB tissue accumulation and A. fumigatus clearance from the lungs in L-AMB- and ABLC-treated neutropenic mice with acute IPA. In agreement with previous studies of experimental pulmonary aspergillosis (6, 22), we found that treatment with the L-AMB formulation was associated with effective, albeit delayed delivery of AMB to the lung when dosed at 5 mg/kg/day that allowed the fungal burden to increase in the first 24 to 48 h of treatment. In contrast, ABLC-treated animals had detectable concentrations of AMB in the lungs in the first 24 h that surpassed the MFC of the test isolate and produced evidence of fungal clearance in the first 24 to 72 h. By 120 h, however, no difference in fungal burden was observed between the two formulations. Not surprisingly, the differences between these formulations have not been confirmed in intravenous models of infection, where L-AMB is effective immediately due to the high serum concentrations seen with intravenous dosing (10). Moreover, when dosed at 10 mg/kg/day, both formulations achieved high concentrations in the lung tissue in the first 24 h and produced similar patterns of fungal clearance. The enhanced rate of AMB distribution to the lung with 10 mg/kg/day versus 5 mg/kg/day may be a reflection of a dose-dependent uptake or a high-dose, first-pass effect in the lung that has been reported with other liposomal formulations (12, 13, 29).
Early clearance of A. fumigatus from the lung was associated with total AMB tissue concentrations that surpassed the MFC of the infecting isolate. Using logistic regression, we determined that AMB tissue concentrations of ≥4 μg/g were associated with a significant reduction (1 log10) in the lung fungal burden, as determined by quantitative real-time PCR. This threshold concentration is in agreement with results from Olson et al., who reported that tissue concentrations of AMB in the lungs of mice of >3 μg/g were required for therapeutic efficacy of L-AMB or ABLC treatment in a murine model of pulmonary aspergillosis (22). Unlike our study, the investigators did not report any L-AMB or ABLC treatment regimens (1 to 12 mg/kg/day) that achieved tissue concentrations of >10 μg/g (22). However, the investigators documented AMB biodistribution primarily in noninfected animals, which may be diminished in the absence of the hemorrhaging and residual inflammatory cell recruitment that are seen in infected animals (4). Indeed, in the study by Olson et al., tissue concentrations were disproportionately higher in infected animals treated at 15 mg/kg/day of ABLC or L-AMB (30 to 40 μg/g and 10 to 18 μg/g, respectively) than in uninfected animals who were treated with both lipid formulations at 12 mg/kg/day (10.07 μg/g and 3.16 μg/g, respectively) (22). Fungal burden, as determined by quantitative CFU cultures, were generally lower in ABLC-treated animals, with a 20-mg/kg/day ABLC regimen achieving the greatest reduction in fungal burden among all treatment groups (22).
One unexpected finding of our study was the pattern of decreasing tissue concentrations over the course of the experiment, despite daily dosing. Because the majority of animal studies that have examined the biodistribution of lipid AMB formulations in the lung used only single doses or single time points to assess plasma-to-tissue ratios of drug, we are unable to confirm if this observation is indeed unique to our study (3, 6, 22). Several factors could account for the decreased distribution of AMB during the course of repeated dosing in infected animals, including saturation of pathways involved in drug uptake in the lung and/or decreases in residual (lipid AMB-containing) phagocytic cell recruitment with decreasing fungal burdens. Another possibility is that our method for AMB extraction from tissue becomes less effective as infection persists. If this were the case, we would expect a consistent pattern of decreases in all dosing regimens, which was clearly not observed between the two lipid AMB formulations (Fig. 1B and C). Further studies with radiolabeled AMB would be required to address this issue.
When administered as a single dose of 5 or 10 mg/kg, neither L-AMB nor ABLC was as effective at reducing the fungal burden as multiple daily dosing (Fig. 3A and B). Indeed, AMB tissue concentrations were undetectable by 120 h, and fungal clearance reached a plateau after the first 24 to 48 h. These data raise concerns regarding whether dosing of lipid AMB formulations at extended intervals (i.e., greater than 3 to 5 days) would be prudent for the treatment of established IPA. However, higher-dose, infrequently administered regimens may still be effective for low-inoculum infections or in the setting of prophylaxis. Additional studies will be required to confirm the pharmacodynamics of extended interval dosing for the lipid AMB formulations.
Another novel aspect of this study is that we were able to demonstrate a good correlation between A. fumigatus tissue fungal burden measured by quantitative real-time PCR and serum concentrations of BG, including responses to antifungal therapy. The BG test has been reported to be a useful adjunctive diagnostic for aspergillosis in patients with acute myelogenous leukemia, with a sensitivity of 69.9% and a specificity of 87.1% for patients with proven infections when a 60-pg/ml cutoff value is used to define sample positivity (23). However, few preclinical studies have compared serial measurements of BG concentrations in relation to direct measurements of fungal burden in the lungs. We decided to use BG as a surrogate marker over galactomannan due to the greater dynamic range of the test, i.e., BG is reported quantitatively versus the semiquantitative galactomannan test. While the majority of the concentrations tested in this study fell below 60 pg/ml, it is notable that samples that exceeded this threshold were from animals with progressing IPA or delayed responses to antifungal therapy. Additional preclinical and clinical studies are warranted to further explore the utility of serial BG analysis as a surrogate for a response to drug therapy.
In conclusion, our comparative analysis of L-AMB and ABLC for the treatment of acute experimental IPA revealed notable differences in the patterns of early AMB lung distribution and fungal clearance when the formulations were dosed at 5 mg/kg/day but no significant differences in A. fumigatus clearance at a dose of 10 mg/kg/day. Differences in the rate of fungal clearance could be attributed largely to differences between respective formulations in delivering “fungicidal” concentrations of AMB to the lung tissue. While the importance of these findings remains to be determined for more slowly progressing forms of aspergillosis, our data suggest that the biopharmaceutical differences between the lipid formulations could have potentially important clinical implications for rapidly progressing fungal pneumonia (e.g., pulmonary zygomycosis). Currently, studies are under way to confirm these results with more rapidly invasive, less AMB-susceptible molds.
Acknowledgments
Research support for this study was provided by Enzon Pharmaceuticals. R.E.L. and D.P.K. received research support and consultancy fees from Merck & Co., Pfizer, Astellas, Enzon, and Schering-Plough. R.A.P. received research support from Merck & Co., Pfizer, Ortho McNeil, and Enzon.
Footnotes
Published ahead of print on 29 January 2007.
REFERENCES
- 1.Adedoyin, A., C. E. Swenson, L. E. Bolcsak, A. Hellmann, D. Radowska, G. Horwith, A. S. Janoff, and R. A. Branch. 2000. A pharmacokinetic study of amphotericin B lipid complex injection (Abelcet) in patients with definite or probable systemic fungal infections. Antimicrob. Agents Chemother. 44:2900-2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aisner, J., P. H. Wiernik, and S. C. Schimpff. 1977. Treatment of invasive aspergillosis: relation of early diagnosis and treatment to response. Ann. Intern. Med. 86:539-543. [DOI] [PubMed] [Google Scholar]
- 3.Andes, D., N. Safdar, K. Marchillo, and R. Conklin. 2006. Pharmacokinetic-pharmacodynamic comparison of amphotericin B (AMB) and two lipid-associated AMB preparations, liposomal AMB and AMB lipid complex, in murine candidiasis models. Antimicrob. Agents Chemother. 50:674-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balloy, V., M. Huerre, J. P. Latge, and M. Chignard. 2005. Differences in patterns of infection and inflammation for corticosteroid treatment and chemotherapy in experimental invasive pulmonary aspergillosis. Infect. Immun. 73:494-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes, P. D., and K. A. Marr. 2006. Aspergillosis: spectrum of disease, diagnosis, and treatment. Infect. Dis. Clin. N. Am. 20:545-561, vi. [DOI] [PubMed] [Google Scholar]
- 6.Becker, M. J., S. de Marie, M. H. Fens, W. C. Hop, H. A. Verbrugh, and I. A. Bakker-Woudenberg. 2002. Enhanced antifungal efficacy in experimental invasive pulmonary aspergillosis by combination of AmBisome with Fungizone as assessed by several parameters of antifungal response. J. Antimicrob. Chemother. 49:813-820. [DOI] [PubMed] [Google Scholar]
- 7.Bekersky, I., R. M. Fielding, D. E. Dressler, J. W. Lee, D. N. Buell, and T. J. Walsh. 2002. Pharmacokinetics, excretion, and mass balance of liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate in humans. Antimicrob. Agents Chemother. 46:828-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bekersky, I., R. M. Fielding, D. E. Dressler, J. W. Lee, D. N. Buell, and T. J. Walsh. 2002. Plasma protein binding of amphotericin B and pharmacokinetics of bound versus unbound amphotericin B after administration of intravenous liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate. Antimicrob. Agents Chemother. 46:834-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowman, J. C., G. K. Abruzzo, J. W. Anderson, A. M. Flattery, C. J. Gill, V. B. Pikounis, D. M. Schmatz, P. A. Liberator, and C. M. Douglas. 2001. Quantitative PCR assay to measure Aspergillus fumigatus burden in a murine model of disseminated aspergillosis: demonstration of efficacy of caspofungin acetate. Antimicrob. Agents Chemother. 45:3474-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clemons, K. V., and D. A. Stevens. 2004. Comparative efficacies of four amphotericin B formulations—Fungizone, amphotec (Amphocil), AmBisome, and Abelcet—against systemic murine aspergillosis. Antimicrob. Agents Chemother. 48:1047-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denning, D. W. 1996. Therapeutic outcome in invasive aspergillosis. Clin. Infect. Dis. 23:608-615. [DOI] [PubMed] [Google Scholar]
- 12.Groll, A. H., D. Mickiene, V. Petraitis, R. Petraitiene, R. M. Alfaro, C. King, S. C. Piscitelli, and T. J. Walsh. 2003. Comparative drug disposition, urinary pharmacokinetics, and renal effects of multilamellar liposomal nystatin and amphotericin B deoxycholate in rabbits. Antimicrob. Agents Chemother. 47:3917-3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groll, A. H., D. Mickiene, K. Werner, R. Petraitiene, V. Petraitis, M. Calendario, A. Field-Ridley, J. Crisp, S. C. Piscitelli, and T. J. Walsh. 2000. Compartmental pharmacokinetics and tissue distribution of multilamellar liposomal nystatin in rabbits. Antimicrob. Agents Chemother. 44:950-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janknegt, R., S. de Marie, I. A. Bakker-Woudenberg, and D. J. Crommelin. 1992. Liposomal and lipid formulations of amphotericin B. Clinical pharmacokinetics. Clin. Pharmacokinet. 23:279-291. [DOI] [PubMed] [Google Scholar]
- 15.Lewis, R. E., R. A. Prince, J. Chi, and D. P. Kontoyiannis. 2002. Itraconazole preexposure attenuates the efficacy of subsequent amphotericin B therapy in a murine model of acute invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 46:3208-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis, R. E., and N. P. Wiederhold. 2005. Murine model of invasive aspergillosis. Methods Mol. Med. 118:129-142. [DOI] [PubMed] [Google Scholar]
- 17.Lewis, R. E., N. P. Wiederhold, and M. E. Klepser. 2005. In vitro pharmacodynamics of amphotericin B, itraconazole, and voriconazole against Aspergillus, Fusarium, and Scedosporium spp. Antimicrob. Agents Chemother. 49:945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linden, P. K. 2003. Amphotericin B lipid complex for the treatment of invasive fungal infections. Expert Opin. Pharmacother. 4:2099-2110. [DOI] [PubMed] [Google Scholar]
- 19.Matot, I., and R. Pizov. 2000. Pulmonary extraction and accumulation of lipid formulations of amphotericin B. Crit. Care Med. 28:2528-2532. [DOI] [PubMed] [Google Scholar]
- 20.Ng, T. K., R. C. Chan, F. A. Adeyemi-Doro, S. W. Cheung, and A. F. Cheng. 1996. Rapid high performance liquid chromatographic assay for antifungal agents in human sera. J. Antimicrob. Chemother. 37:465-472. [DOI] [PubMed] [Google Scholar]
- 21.Nierman, W. C., A. Pain, M. J. Anderson, J. R. Wortman, H. S. Kim, J. Arroyo, M. Berriman, K. Abe, D. B. Archer, C. Bermejo, J. Bennett, P. Bowyer, D. Chen, M. Collins, R. Coulsen, R. Davies, P. S. Dyer, M. Farman, N. Fedorova, N. Fedorova, T. V. Feldblyum, R. Fischer, N. Fosker, A. Fraser, J. L. Garcia, M. J. Garcia, A. Goble, G. H. Goldman, K. Gomi, S. Griffith-Jones, R. Gwilliam, B. Haas, H. Haas, D. Harris, H. Horiuchi, J. Huang, S. Humphray, J. Jimenez, N. Keller, H. Khouri, K. Kitamoto, T. Kobayashi, S. Konzack, R. Kulkarni, T. Kumagai, A. Lafon, J. P. Latge, W. Li, A. Lord, C. Lu, W. H. Majoros, G. S. May, B. L. Miller, Y. Mohamoud, M. Molina, M. Monod, I. Mouyna, S. Mulligan, L. Murphy, S. O'Neil, I. Paulsen, M. A. Penalva, M. Pertea, C. Price, B. L. Pritchard, M. A. Quail, E. Rabbinowitsch, N. Rawlins, M. A. Rajandream, U. Reichard, H. Renauld, G. D. Robson, S. Rodriguez de Cordoba, J. M. Rodriguez-Pena, C. M. Ronning, S. Rutter, S. L. Salzberg, M. Sanchez, J. C. Sanchez-Ferrero, D. Saunders, K. Seeger, R. Squares, S. Squares, M. Takeuchi, F. Tekaia, G. Turner, C. R. Vazquez de Aldana, J. Weidman, O. White, J. Woodward, J. H. Yu, C. Fraser, J. E. Galagan, K. Asai, M. Machida, N. Hall, B. Barrell, and D. W. Denning. 2005. Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature 438:1151-1156. [DOI] [PubMed] [Google Scholar]
- 22.Olson, J. A., J. P. Adler-Moore, J. Schwartz, G. M. Jensen, and R. T. Proffitt. 2006. Comparative efficacies, toxicities, and tissue concentrations of amphotericin B lipid formulations in a murine pulmonary aspergillosis model. Antimicrob. Agents Chemother. 50:2122-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ostrosky-Zeichner, L., B. D. Alexander, D. H. Kett, J. Vazquez, P. G. Pappas, F. Saeki, P. A. Ketchum, J. Wingard, R. Schiff, H. Tamura, M. A. Finkelman, and J. H. Rex. 2005. Multicenter clinical evaluation of the (1→3) beta-d-glucan assay as an aid to diagnosis of fungal infections in humans. Clin. Infect. Dis. 41:654-659. [DOI] [PubMed] [Google Scholar]
- 24.Pfaller, M. A., D. J. Sheehan, and J. H. Rex. 2004. Determination of fungicidal activities against yeasts and molds: lessons learned from bactericidal testing and the need for standardization. Clin. Microbiol. Rev. 17:268-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Proffitt, R. T., A. Satorius, S. M. Chiang, L. Sullivan, and J. P. Adler-Moore. 1991. Pharmacology and toxicology of a liposomal formulation of amphotericin B (AmBisome) in rodents. J. Antimicrob. Chemother. 28(Suppl. B):49-61. [DOI] [PubMed] [Google Scholar]
- 26.Segarra, I., D. A. Movshin, and L. Zarif. 2002. Pharmacokinetics and tissue distribution after intravenous administration of a single dose of amphotericin B cochleates, a new lipid-based delivery system. J. Pharm. Sci. 91:1827-1837. [DOI] [PubMed] [Google Scholar]
- 27.Shibuya, K., M. Takaoka, K. Uchida, M. Wakayama, H. Yamaguchi, K. Takahashi, S. Paris, J. P. Latge, and S. Naoe. 1999. Histopathology of experimental invasive pulmonary aspergillosis in rats: pathological comparison of pulmonary lesions induced by specific virulent factor deficient mutants. Microb. Pathog. 27:123-131. [DOI] [PubMed] [Google Scholar]
- 28.Tamura, H., Y. Arimoto, S. Tanaka, M. Yoshida, T. Obayashi, and T. Kawai. 1994. Automated kinetic assay for endotoxin and (1→3)-beta-d-glucan in human blood. Clin. Chim. Acta 226:109-112. [DOI] [PubMed] [Google Scholar]
- 29.Walsh, T. J., J. L. Goodman, P. Pappas, I. Bekersky, D. N. Buell, M. Roden, J. Barrett, and E. J. Anaissie. 2001. Safety, tolerance, and pharmacokinetics of high-dose liposomal amphotericin B (AmBisome) in patients infected with Aspergillus species and other filamentous fungi: maximum tolerated dose study. Antimicrob. Agents Chemother. 45:3487-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiederhold, N. P., D. P. Kontoyiannis, J. Chi, R. A. Prince, V. H. Tam, and R. E. Lewis. 2004. Pharmacodynamics of caspofungin in a murine model of invasive pulmonary aspergillosis: evidence of concentration-dependent activity. J. Infect. Dis. 190:1464-1471. [DOI] [PubMed] [Google Scholar]
- 31.Wiederhold, N. P., V. H. Tam, J. Chi, R. A. Prince, D. P. Kontoyiannis, and R. E. Lewis. 2006. Pharmacodynamic activity of amphotericin B deoxycholate is associated with peak plasma concentrations in a neutropenic murine model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 50:469-473. [DOI] [PMC free article] [PubMed] [Google Scholar]