Abstract
The in vivo effectiveness of a series of conformationally restricted polyamine analogues alone and selected members in combination with dl-α-difluoromethylarginine against Cryptosporidium parvum infection in a T-cell receptor alpha-deficient mouse model was tested. Polyamine analogues were selected from the extended bis(ethyl)-sym-homospermidine or bis(ethyl)-spermine backbone having cis or trans double bonds at the center of the molecule. The cis isomers were found to have significantly greater efficacy in both preventing and curing infection in a mouse model than the trans polyamine analogues when tested in a T-cell receptor alpha-deficient mouse model. When tested in combination with dl-α-difluoromethylarginine, the cis-restricted analogues were found to be more effective in preventing oocyst shedding. This study demonstrates the potential of polyamine analogues as anticryptosporidial agents and highlights the presence of multiple points in polyamine synthesis by this parasite that are susceptible to inhibition resulting in growth inhibition.
Cryptosporidium parvum is an intracellular protozoan parasite causing enteric infection and severe diarrheal disease in various mammals, including humans (4). Cryptosporidiosis is the most common diarrheic disease of calves in the United States. In a survey of 1,103 separate dairy herds, 48% of the calves aged between 7 and 21 days were found to be infected with C. parvum (6, 9). The importance of C. parvum as a human pathogen was first recognized among immunocompromised hosts, most notably AIDS patients (3, 13). Since then, several major outbreaks of C. parvum infection in association with contaminated water supplies have been detected (5, 20). Despite intensive research in recent years, effective treatment regimens for C. parvum infection are still unavailable (25). The polyamines spermidine and spermine are cationic molecules that have several indispensable cellular functions that include maintenance of the correct nucleic acid conformation (14), membrane structure (21), antioxidant properties by preventing lipid peroxidation (24), and regulation of membrane-bound enzymes (35). Mammals and most protozoa convert arginine into ornithine, which is then acted upon by ornithine decarboxylase (ODC) for polyamine biosynthesis (1, 30). However, initiation of polyamine synthesis in C. parvum occurs via a pathway most commonly used by plants and certain bacteria (10) in which arginine is converted to agmatine by the action of arginine decarboxylase (ADC). Although arginine decarboxylase and agmatine have been demonstrated in mammalian tissue (16, 19), they are present in only minor concentrations, and apart from the report of agmatine as a neurotransmitter in the brain of mammals (11), no function has been attributed to agmatine, and no report of agmatine as a precursor of mammalian polyamines has been published. Additionally, C. parvum has a reverse polyamine biosynthetic pathway, mediated by spermidine/spermine N1-acetyltransferase (SSAT) and polyamine oxidase, which is 10-fold more active than the forward pathway (10, 36). Mammalian cells also possess a spermine oxidase, which oxidizes spermine to spermidine directly (29, 31); this enzyme is absent in C. parvum. Hence, the hypothesis of this study is that the use of inhibitors directed toward parasite ADC and SSAT will effectively block polyamine intercoversion and thereby compromise parasite growth and development. The host polyamine forward pathway utilizes ODC and is thereby unaffected by inhibitors of ADC, and the reverse pathway can operate via spermine oxidase and hence will still function even if mammalian SSAT activity is adversely affected by the conformationally restricted polyamine analogues. These differences in parasite compared to host cell polyamine synthesis are outlined in Fig. 1 and present several targets within the polyamine pathway for chemotherapeutic intervention. In this study, several cis- and trans-conformationally restricted polyamine analogues (Table 1) based upon the 4-4-4 or 3-4-3 repetitive backbone were used alone and/or in combination with dl-α-difluoromethylarginine (DFMA) or dl-α-difluoromethylornithine (DFMO) to determine the utility of Cryptosporidium polyamine metabolism as a target for chemotherapy of disease caused by this parasite.
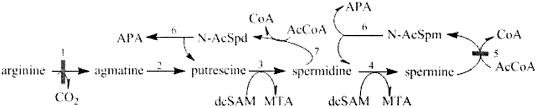
FIG. 1.
Pathway of polyamine metabolism by C. parvum. C. parvum differs from mammalian cells in several ways. The initial step of the forward pathway is catalyzed by arginine decarboxylase (step 1) instead of ornithine decarboxylase. The agmatine formed is converted into putrescine by agmatine iminohydrolase (step 2). In common with the mammalian pathway, aminopropyl groups are subsequently added to form spermidine and spermine by spermidine synthase (step 3) and spermine synthase (step 4), respectively. The reverse pathway in the parasite operates at a higher rate than the forward pathway and involves the acetylation of host-derived spermine by spermidine/spermine N1-acetyltransferase (step 5), and the N1-acetylspermine (N-AcSpm) formed is oxidized to spermidine and aminopropionaldehyde (APA) by polyamine oxidase (step 6). Spermidine can also be metabolized to putrescine and APA by the consecutive action of spermidine N1-acetyltransferase (N-AcSpd) (step 7) and a polyamine oxidase (step 6). In the mammalian cell, spermine can also be directly oxidized to spermidine by spermine oxidase, which is absent in C. parvum. Blocking C. parvum SSAT and ADC using DFMA and CGC-11157 (horizontal gray bar) will effectively compromise parasite polyamine metabolism with minimal effect upon the host, which lacks ADC and has a functional spermine oxidase, allowing the reverse polyamine pathway to function. dcSAM, decarboxylated S-adenosylmethionine; MTA, methylthioadenosine.
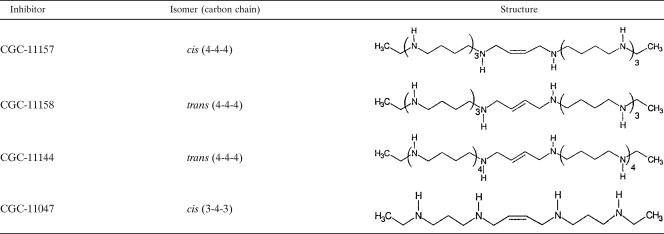
TABLE 1.
Structure of polyamine analogues used in this study
MATERIALS AND METHODS
TCR-α-deficient mouse model.
Breeding pairs of T-cell receptor alpha (TCR-α)-deficient mice were purchased from Jackson Laboratories (Bar Harbor, ME). A breeding colony was established and maintained at Iowa State University (Ames, IA) for the generation of mice for experiments. Mice received tap water and autoclaved rodent chow (Harlan Teklad, Madison, WI) ad libitum. For the challenge inoculum, purified oocysts were isolated from feces collected from calves experimentally inoculated with C. parvum oocysts by a method described previously (32). Oral challenge of mice consisted of 104 oocysts in 100 μl of 0.15 M phosphate-buffered saline (PBS). Mice were challenged with C. parvum oocysts at 1 week of age by gavage using a 24-gauge animal feeding needle. To assess C. parvum colonization, fecal pellets were collected either by placing individual mice into beakers until they defecated or from the distal colon after mice were euthanized. Fresh fecal pellets were then smeared onto glass slides, stained with carbol fuchsin, and examined microscopically (magnification, ×400) for the presence of C. parvum oocysts. Samples were scored as positive (oocysts detected) or negative (oocysts not detected). At the end of the experiment, mice were euthanized, and intestinal sections from the distal ileum and cecum were fixed in 10% formalin and embedded in paraffin. Histologic sections were cut at a thickness of 4 μm, stained with hematoxylin and eosin, and examined microscopically for C. parvum and intestinal lesions. Infectivity scores were as follows: 0, no C. parvum organisms detected; 1, less than 10 oocysts per field; 2, greater than 10 oocysts per field. Scores were determined upon examination of individual tissue sections; means were calculated for each treatment group, and data are presented as group means ± standard errors of the means. Data were analyzed by one-way analysis of variance followed by Tukey-Kramer multiple-comparison tests (mean infectivity scores), or two-by-two contingency tables were formulated and data were analyzed by Fisher's exact test (percent infected). Data were considered significant if P values of <0.05 were obtained.
Prevention studies.
To determine if test compounds were capable of preventing C. parvum infection, groups (six to eight mice) of suckling TCR-α-deficient mice received one of the following treatment regimens: PBS (negative control); 0.10 mg (20 mg/kg of body weight) of DFMA, CGC-11157, or an equal combination of both; 0.1 mg (20 mg/kg) of DFMO alone or in combination with an equal dose of CGC-11157; 0.25 mg (50 mg/kg) of CGC-11047; or 0.5 mg (100 mg/kg) of paromomycin (positive control). Treatment was once daily for 2 days prior to infection with 2 × 104 oocysts. Treatment was continued for a further 5 days. All mice were euthanized 1 week after the final treatment. Ileal and cecal sections were examined for C. parvum and any associated histopathology.
Curative studies.
The ability of polyamine analogues to effectively treat an established C. parvum infection was tested using 7-day-old TCR-α-deficient mice infected with 2 × 104 oocysts. One week after C. parvum challenge, fecal samples from each of the infected groups were checked to verify that mice were infected. Infected mice (six per group) were treated once daily for 7 days with one of the following regimens: PBS (negative control); 0.1 mg (20 mg/kg) of either CGC-11157, CGC-11158, DFMA, DFMO, or an equal combination of CGC-11157 plus DFMA, CGC-11157 plus DFMO, or CGC-11158 plus DFMA; 0.67 mg (134 mg/kg) of CGC 11047; or 0.5 mg (100 mg/kg) paromomycin (positive control). A second group (5 to 13 mice per group) of mice was treated twice daily for 7 days with 0.08 mg (16 mg/kg) or 1.6 mg (32 mg/kg) of CGC-11158 or CGC-11144. All mice were euthanized 1 week after the final treatment. Ileal and cecal sections were examined for C. parvum and any associated histopathology. Viability of recovered oocysts was determined by the ability to establish an in vitro infection of an HCT-8 cell line (26).
Toxicity of polyamine analogues on intestinal cells.
Mice (Swiss-Webster; Ace Animals, Boyertown, PA) were dosed with CGC-11157 either orally using 0.6 mg (20 mg/kg) via a 24-gauge feeding needle or by intraperitoneal (i.p.) injection using 0.06 mg (2 mg/kg). The dosing regimen was once per day for 4 days. The weights of the mice were recorded daily, and mice were sacrificed by CO2 asphyxiation at 48, 72, and 96 h. The intestines were removed, washed in 0.1 M phosphate-buffered saline (pH 6.7), and homogenized in 40 mM Tris-HCl (pH 6.7). The tissue was homogenized on ice using a Potter-Elvehjem homogenizer (45 strokes), and the homogenate was analyzed for activity of ODC and SSAT. ODC activity was measured by trapping the 14CO2 released from l-[14C]ornithine (42.5 mCi/mmol) by using a reaction mixture containing 0.2 M acetate (pH 6.5), 60 μM pyridoxal 5′-phosphate, and 3 mg/ml bovine serum albumin in a final volume of 0.5 ml. The reaction was initiated by adding 125 μg of protein to the mixture, and the mixture was incubated for 30 min in a shaking water bath at 37°C. The reaction was stopped by the addition of 1 ml of 40% (wt/vol) trichloroacetic acid to the mixture, and the reaction mixture was incubated for a further 30 min. The 14CO2 released was trapped using benzethonium hydroxide-soaked filter paper, which was removed and placed into Omnifluor for determinations of radioactivity using a Beckman TriCarb 1600 CA liquid scintillation counter (22).
SSAT activity was determined by measuring the incorporation of radioactivity from labeled acetyl coenzyme A (acetyl-CoA) into monoacetylspermine in 0.5 mM Bicine (pH 8.0), 16.5 μM [1-14C]acetyl-CoA (60 μCi mmol−1), 1 mM acetyl-CoA, 2 mM spermine, and 100 μg protein in a final volume of 100 μl for 60 min at 37°C. The reaction was stopped with 20 μl ice-cold 0.5 M hydroxylamine, the mixture was boiled for 3 min and microcentrifuged (12,500 × g) for 1 min, and 50 μl was applied to cellulose phosphate discs (Whatman P-81). The filters were washed thoroughly with distilled water and finally flushed with methanol prior to drying and immersion in Omnifluor. Radioactivity was determined using a Beckman TriCarb 1600 CA liquid scintillation counter (12).
Polyamine analogues.
The synthesis of oligoamines was carried out as described previously. Briefly, N1-monoethyl tetramides in which the amino groups were protected by a mesitylenesulfonyl residue (18, 27) were dimerized by reactions with (E)- or (Z)-2-butene-1,4-diyl-bis(mesitylenesulfonate) to give the corresponding octamide. The protecting group was then removed, and the oligoamine was isolated as the hydrochloride. The mixture was kept at 20°C for 18 h; further workup was performed according to previously published procedures (17). Conformationally restricted polyamine analogues were obtained as the hydrochloride at typical yields of 89%. DFMA was supplied by Marrion Merrell Dow. Paromomycin was purchased from Sigma Chemical Co. (St. Louis, MO).
RESULTS
Ability of polyamine analogues to prevent an infection.
The utility of several polyamine analogues as anticryptosporidial agents was tested using a TCR-α-deficient mouse model lacking a subset of immune cells necessary for the clearance of C. parvum (32, 33).
The effect of CGC-11157 on mouse intestinal cells was determined by dosing mice with 20 mg/kg per os (p.o.). Fifty percent of the animals (n = 8) pretreated with 20 mg/kg/day of CGC-11157, a cis-restricted extended-chain analogue based on a 4-4-4 structure (Table 1), did not develop the disease. Similar results were obtained using animals treated with 20 mg/kg/day of DFMA, a specific inhibitor of arginine decarboxylase. An additive effect was obtained by pretreatment with the combination of 20 mg/kg/day each of CGC-11157 and DFMA, which was 100% effective in preventing an infection (Table 2). CGC-11047, a cis-restricted extended-chain polyamine analogue based on a 3-4-3 structure (Table 1), was effective in preventing infection by C. parvum at 50 mg/kg/day (Table 2). The CGC-11047 analogues are based on the 3-4-3 structure of the parent spermine, and these analogues cause the overexpression of SSAT in mammalian cells, resulting in polyamine depletion due to the excretion of the acetylated polyamine (2). These analogues do not have this effect on the parasite SSAT; therefore, we propose that these 3-4-3 analogues are effective in preventing parasitemia by the depletion of host cell of polyamines and by acting as functionless equivalents of natural polyamines.
TABLE 2.
In vivo abilities of polyamine analogues to prevent an infectiona
| SSAT inhibitor | ADC/ODC inhibitor | No. of animals shedding oocysts/total no. of animals | Mean infectivity score + SEM (no. of determinations) |
|---|---|---|---|
| None | None | 8/8 | 1.36 ± 0.12 (8) |
| None | DFMA | 4/8 | 0.69 ± 0.14 (8) |
| None | DFMO | 8/8 | 1.29 ± 1.29 (8) |
| CGC-11157 | None | 4/8 | 0.54 ± 0.16 (8) |
| CGC-11157 | DFMA | 0/8 | 0.08 ± 0.02b (8) |
| CGC-11157 | DFMO | 4/8 | 0.71 ± 0.12 (8) |
| CGC-11047 | None | 6/8 | 1.01 ± 0.14 (8) |
| CGC-11047 | DFMA | 4/8 | 0.86 ± 0.21 (8) |
| Paromomycin (positive control) | 3/6 | 0.78 ± 0.14 (6) |
Mice (six or eight per group) were treated once daily with 20 mg/kg of the test compound either alone or in equal combinations for 2 days prior to infection with 2 × 104 oocysts. Treatment was continued for 5 days using the same dose. Paromomycin was used at 100 mg/kg. Ten days posttreatment, mice were evaluated for oocyst shedding.
Significantly different from controls (P < 0.05).
Ability of polyamine analogues to cure an existing infection.
Treatment of infected TCR-α-deficient mice with 20 mg/kg/day of CGC-11157 prevented oocyst shedding in 50% of the animals (n = 6); by comparison, treatment with 20 mg/kg/day DFMA prevented oocyst shedding in 33% of the animals (n = 6). As noted in the prevention studies mentioned above, the combination of CGC-11157 and DFMA resulted in an additive effect, preventing oocyst shedding in 85% of the animals (n = 6) (Table 3). In addition, the oocysts collected from the single animal shedding at the end of this study lacked the capacity to cause an in vitro infection of an HCT-8 monolayer. No toxicity was noted in sections taken from the intestines of animals treated with this dose of CGC-11157 and DFMA. CGC-11158, the trans isomer of CGC-11157, was ineffective at curing the infection at similar dose levels (Table 3), and in combination with DFMA, no enhancement of DFMA alone was observed (Table 3). Consistent with the report that C. parvum lacks ODC, the use of 20 mg/kg body weight of DFMO for 7 days had no effect upon parasitemia, and in combination with CGC-11157, no enhancement in the effect of the latter compound was observed (Table 3). The cis-restricted 3-4-3 analogue CGC-11047 at a dose that was effective in curing 50% of the animals treated with CGC-11157 was ineffective in curing the mouse model. In combination with DFMA, this analogue cured 50% of the animals, which was slightly better than the use of DFMA alone. The use of higher doses of the polyamine analogues alone was partially effective in curing the C. parvum infection in the mouse model, but no dose tested was better than the combination of DFMA with CGC-11157, including the use of paromomycin at five times the single drug dose. The analogue CGC-11047 was also less effective than CGC-11157 in curing infection in the mouse model; using 13-fold more compound than CGC-11157 resulted in 86% of the animals not shedding oocysts (n = 14) (Table 4). The effective dosage regimen of CGC-11047 resulted in toxic signs including depression, weight loss, and hyperplastic changes of the intestinal mucosa, possibly due to the upregulation of host cell SSAT and the resultant excretion of acetylated polyamines.
TABLE 3.
In vivo abilities of polyamine analogues to cure an existing infectiona
| SSAT inhibitor | ADC/ODC inhibitor | No. of animals shedding oocysts/total no. of animals | Mean infectivity score ± SEM |
|---|---|---|---|
| None | None | 6/6 | 1.44 ± 0.10 |
| None | DFMA | 4/6 | 0.58 ± 0.11 |
| None | DFMO | 6/6 | 1.68 ± 0.09 |
| CGC-11157 | None | 3/6 | 0.62 ± 0.13 |
| CGC-11157 | DFMA | 1/6b | 0.12 ± 0.08c |
| CGC-11157 | DFMO | 3/6 | 0.71 ± 0.19 |
| CGC-11158 | None | 6/6 | 1.68 ± 0.16 |
| CGC-11158 | DFMA | 4/6 | 0.74 ± 0.21 |
| CGC-11047 | None | 6/6 | 1.03 ± 0.24 |
| CGC-11047 | DFMA | 3/6 | 0.71 ± 0.14 |
Mice were infected with 2 × 104 oocysts 7 days prior to treatment with test compounds. Treatment was once per day for 7 days using 20 mg/kg of body weight. Combination studies used 20 mg/kg of body weight of both compounds once per day for 7 days. Seven days posttreatment, mice were evaluated for oocyst shedding. Six animals were used per group, and mean infectivity scores ± standard errors of the means of six determinations are presented. Control animals were infected but not treated.
Oocysts failed to infect an HCT-8 monolayer.
Significantly different from controls (P < 0.05).
TABLE 4.
In vivo abilities of polyamine analogues to cure an existing infectiona
| Test compound | Dose (mg/kg body wt) (dosing regimen) | No. of animals with fecal oocysts/total no. of animals | Mean infectivity score ± SEM |
|---|---|---|---|
| Control | 0 | 7/7 shedding | 1.68 ± 0.23 |
| CGC-11157 | 16 (2/day, 7 days) | 4/8 shedding | 0.39 ± 0.12 |
| CGC-11157 | 32 (2/day, 7 days) | 2/8 shedding | 0.12 ± 0.07 |
| CGC-11158 | 16 (2/day, 7 days) | 12/13 shed | 1.12 ± 0.10 |
| CGC-11158 | 32 (2/day, 7 days) | 4/5 shedding | 1.18 ± 0.09 |
| CGC-11047 | 134 (1/day, 7 days) | 1/6 shedb | 0.12 ± 0.13c |
| CGC-11144 | 16 (2/day, 7 days) | 5/9 shedding | 1.10 ± 0.10 |
| CGC-11144 | 32 (2/day, 7 days) | 3/8 sheddingb | 0.16 ± 0.08c |
| Paromomycin | 100 (1/day, 7 days) | 6/8 shedding | 0.96 ± 0.14 |
The in vivo abilities of polyamine analogues to cure an existing infection were tested in mice infected with 2 × 104 oocysts 7 days prior to treatment with test compounds. Treatment was done once per day for 7 days using the stated dose. Seven days posttreatment, mice were evaluated for oocyst shedding.
Oocysts failed to infect an HCT-8 monolayer.
Significantly different from controls (P < 0.05).
Effect of polyamine analogues on mouse intestinal epithelia.
The activities of ODC and SSAT, two key regulatory enzymes of polyamine metabolism in mammalian cells, were measured in intestinal tissues removed from mice treated for 48 h, 72 h, and 96 h with either orally (p.o.) administered 20 mg/kg CGC-11157 or 2 mg/kg CGC-11157 administered by the i.p. route. The SSAT determinations remained constant over 96 h, with a range from 0.034 to 0.09 in intestinal tissues of the CGC-11157-treated mice. The average SSAT values for all of the samples from i.p. and p.o. treated mice were 0.052 ± 0.025 nmol/min/mg of protein (six determinations) and 0.055 ± 0.030 nmol/min/mg of protein (six determinations), which is comparable to that of the untreated control tissue, 0.033 ± 0.013 nmol/min/mg of protein (two determinations) (Table 5). By comparison, determinations of ODC activity in intestinal tissue from animals treated both p.o. and i.p. with CGC-11157 indicate that there was no change in activity for the first 72 h, but after this, ODC activity was markedly increased (Table 5). The sharp increase in ODC activity is reflected in the ODC/SSAT ratio for the tissue, which remained constant between 0.97 and 1.55 for the 48-h and 72-h samples but increased sharply, to 2.9 to 4.0 at 96 h.
TABLE 5.
Activities of SSAT and ODC in the intestinal epithelia of animals treated with CGC-11157 for various timesa
| Treatment | Activity (nmol/min/mg protein) ± SD
|
ODC/SSAT ratio | |
|---|---|---|---|
| SSAT | ODC | ||
| Control | 0.033 ± 0.013 | 0.049 ± 0.022 | 1.48 |
| i.p., 48 h | 0.081 ± 0.026 | 0.079 ± 0.047 | 0.97 |
| i.p., 72 h | 0.034 ± 0.011 | 0.034 ± 0.011 | 1.00 |
| i.p., 96 h | 0.040 ± 0.015 | 0.160 ± 0.169 | 4.00 |
| p.o., 48 h | 0.090 ± 0.016 | 0.089 ± 0.070 | 0.99 |
| p.o., 72 h | 0.036 ± 0.010 | 0.056 ± 0.029 | 1.55 |
| p.o., 96 h | 0.040 ± 0.014 | 0.116 ± 0.048 | 2.90 |
Dosing was once per day. i.p. indicates 0.2 mg of CGC-11157 administered via intraperitoneal injection; p.o. indicates 2 mg of CGC-11157 administered per os.
DISCUSSION
Polyamine analogues have previously been demonstrated to have utility in treating infections in a mouse model, such as [N1,N12]bis(ethyl)-cis-6,7-dehydrospermine (CGC-11047; previously SL-11047, a 3-4-3 analogue), which cured 12 of 14 animals with a dose of 134 mg/kg (34). It was found that CGC-11047 is more effective in prevention than in curing an established infection, which we propose is due to the ability of this group of analogues to upregulate host cell SSAT, resulting in the overproduction of acetylated polyamines (17) and their resultant excretion into the lumen of the gut from where it is voided, making it unavailable to the parasite. By comparison, the 4-4-4 analogues such as CGC-11157 do not induce host cell SSAT (18, 27), and their effect is directly on the parasite SSAT, confirming previous studies that demonstrated that parasite SSAT is significantly inhibited by this group of inhibitors (36). Our results of SSAT activity in control and CGC-11157-treated mouse intestinal tissues agree with previous results demonstrating that SSAT activity does not change with exposure to this compound (Table 5). Interestingly, though, ODC activity is significantly higher in 96-h samples treated with CGC-11157 compared to controls. The route of administration (per os or intraperitoneal) did not affect the observed result (Table 5). The ratio of ODC/SSAT activity remained relatively constant at 0.97 to 1.55 in 48- and 72-h intestinal samples but increased to 3.0- to 4.0-fold after 96 h of treatment with CGC-11157 (Table 5). These results indicate that while the cis 4-4-4 conformationally restricted polyamine analogues may not cause the induction of mouse SSAT, they are acting as slow inhibitors of this enzyme, and this results in a compensatory increase in mouse intestinal ODC.
These results indicate the central importance of polyamine metabolism to the survival and infectivity of C. parvum. In the majority of eukaryotes, SSAT functions to acetylate polyamines for the purpose of facilitating the excretion of excess spermidine and spermine (30). In contrast, we propose that SSAT in C. parvum, and possibly other parasitic lower eukaryotes, functions to acetylate host-derived polyamines and alleviates the necessity for de novo synthesis of these molecules. This enzyme therefore functions differentially in these lower eukaryotes by acting as the lead enzyme in scavenging polyamines. The critical role of this enzyme in the polyamine metabolism of C. parvum is supported by calculating the flow through the pathway, where it can be calculated that the rate of spermidine synthesis from spermine is 25-fold greater from SSAT than from arginine. The role of parasite-produced agmatine may be to act as a regulator of polyamine metabolism by inducing SSAT activity. Agmatine has been shown to act as a modulator of polyamine content in mammalian cells by inducing SSAT (28), a feature which is enhanced by low oxygen tensions (28); in this regard, it is significant that C. parvum sporozoites infect the crypts of the intestinal epithelium, a microaerophilic environment.
The possible role of host cell arginase in converting DFMA into DFMO (23), which could block host ODC synthesis and thereby lower host cell polyamine availability to the parasite, is unlikely because we have previously shown that DFMO has no effect upon growth of the parasite in vitro (10), and we demonstrate in this study that DFMO alone at 20 mg/kg is incapable of curing or preventing C. parvum infection in a mouse model. We therefore propose that the effect of DFMA is therefore upon parasite ADC, causing a reduction in parasite polyamine content.
The results of this study indicate the utility of using dual inhibitors of both the polyamine forward synthesis and backward synthesis pathways. This study also clearly demonstrates the greater activity of cis conformationally restricted 4-4-4 polyamine analogues compared to the trans-isomeric analogues, a feature unique to C. parvum SSAT (36). We have previously demonstrated that cis-(4-4-4)-polyamine analogues are potent inhibitors of C. parvum SSAT and have long residence times in the enzyme active site (34), and additionally, DFMA is an irreversible inhibitor of C. parvum ADC (10); hence, the combined actions of these agents will be to not only effectively block de novo spermidine synthesis from arginine but also prevent back-synthesis from spermine, which is present in high concentrations in the gut (7, 8, 15).
Acknowledgments
The research was supported in part by grants from the National Institutes of Health, NCDDG AI40320 (N.Y.), NIAID AI45739 (B.J.F.), and NIAID AI43931 (W.R.W.), and by the Iowa Livestock Health Advisory Council.
We thank Mitchell Palmer for assistance with histopathological analysis of mouse tissues.
Footnotes
Published ahead of print on 22 January 2007.
REFERENCES
- 1.Bacchi, C. J., and N. Yarlett. 2002. Polyamine metabolism as a chemotherapeutic target in protozoan parasites. Mini Rev. Med. Chem. 2:553-563. [DOI] [PubMed] [Google Scholar]
- 2.Casero, R. A., Jr., and A. E. Pegg. 1993. Spermidine/spermine N1-acetyltransferase—the turning point in polyamine metabolism. FASEB J. 7:653-661. [PubMed] [Google Scholar]
- 3.Crawford, F. G., and S. H. Vermund. 1988. Human cryptosporidiosis. Crit. Rev. Microbiol. 16:113-159. [DOI] [PubMed] [Google Scholar]
- 4.Fayer, R., C. A. Speer, and J. P. Dubey. 1997. The general biology of Cryptosporidium, p. 1-41. In R. Fayer (ed.), Cryptosporidium and cryptosporidiosis. CRC Press, Boca Raton, FL.
- 5.Franzen, C., and A. Muller. 1999. Cryptosporidia and microsporidia—waterborne diseases in the immunocompromised host. Diagn. Microbiol. Infect. Dis. 34:245-262. [DOI] [PubMed] [Google Scholar]
- 6.Garber, L. P., H. S. Salman, H. S. Hurd, T. Keefe, and J. L. Schlater. 1994. Potential risk factors for Cryptosporidium infection in dairy calves. J. Am. Vet. Med. Assoc. 205:86-91. [PubMed] [Google Scholar]
- 7.Gomez, M., and P. Hellstrand. 1999. Endogenous polyamines modulate Ca2+ channel activity in guinea pig intestinal smooth muscle. Pflugers Arch. 438:445-451. [DOI] [PubMed] [Google Scholar]
- 8.Greco, S., L. Hugueny, P. George, P. Perrin, P. Louisot, and M. C. Biol. 2000. Influence of spermine on intestinal maturation of the glycoprotein glycosylation process in neonatal rats. Biochem. J. 345:69-75. [PMC free article] [PubMed] [Google Scholar]
- 9.Harp, J. A., P. Jardon, E. R. Atwill, M. Zylstra, S. Checel, J. P. Goff, and C. DeSimone. 1996. Field testing and prophylactic measures against Cryptosporidium parvum infection in calves in a California dairy herd. Am. J. Vet. Res. 57:1586-1588. [PubMed] [Google Scholar]
- 10.Keithly, J. S., G. Zhu, S. J. Upton, K. M. Woods, M. P. Martinez, and N. Yarlett. 1997. Polyamine biosynthesis in Cryptosporidium parvum and its implications for chemotherapy. Mol. Biochem. Parasitol. 88:35-42. [DOI] [PubMed] [Google Scholar]
- 11.Li, G., S. Regunathan, C. J. Barrow, J. Eshraghi, R. Cooper, and D. J. Reis. 1994. Agmatine: an endogenous clonidine-displacing substance in the brain. Science 263:966-969. [DOI] [PubMed] [Google Scholar]
- 12.Libby, P. R., M. Henderson, R. J. Bergeron, and C. W. Porter. 1989. Major increases in spermidine/spermine N1-acetyltransferase activity by spermine analogues and their relationship to polyamine depletion and growth inhibition in L1210 cells. Cancer Res. 49:6226-6231. [PubMed] [Google Scholar]
- 13.Majewska, A. C., P. Sulima, A. Werner, G. Baralkiewicz, J. Juszczyk, and N. J. Pieniazek. 1999. Cryptosporidosis in HIV-positive patients. Wiad. Parazytol. 45:125-128. [PubMed] [Google Scholar]
- 14.Mathews, H. R. 1993. Polyamines, chromatin structure and transcription. BioEssays 15:561-567. [DOI] [PubMed] [Google Scholar]
- 15.Osborne, D. L., and E. R. Seidel. 1990. Gastrointestinal luminal polyamines: cellular accumulation and enterohepatic circulation. Am. J. Physiol. 258:G576-G584. [DOI] [PubMed] [Google Scholar]
- 16.Raasch, W., S. Regunathan, G. Li, and D. G. Reis. 1995. Agmatine, the bacterial amine, is widely distributed in mammalian tissues. Life Sci. 56:2319-2330. [DOI] [PubMed] [Google Scholar]
- 17.Reddy, V. K., A. Valasinas, A. Sakar, H. S. Basu, L. J. Marton, and B. J. Frydman. 1998. Conformationally restricted analogues of 1N,12N-bisethylspermine: synthesis and growth inhibitory effects on human tumor cell lines. J. Med. Chem. 41:4723-4732. [DOI] [PubMed] [Google Scholar]
- 18.Reddy, V. K., A. Sarkar, A. Valasinas, L. J. Marton, H. S. Basu, and B. J. Frydman. 2001. cis-Unsaturated analogues of 3,8,13,18,23-pentaazapentacosane (BE-4-4-4-4): synthesis and growth inhibitory effects on human prostate cancer cell lines. J. Med. Chem. 44:404-417. [DOI] [PubMed] [Google Scholar]
- 19.Regunathan, S., and D. J. Reis. 2000. Characterization of arginine decarboxylase in rat brain and liver: distinction from ornithine decarboxylase. J. Neurochem. 74:2201-2208. [DOI] [PubMed] [Google Scholar]
- 20.Rose, J. B., J. T. Lisle, and M. LeChevallier. 1997. Waterborne cryptosporidiosis: incidence, outbreaks and treatment strategies, p. 93-109. In R. Fayer (ed.), Cryptosporidium and cryptosporidiosis. CRC Press, Boca Raton, FL.
- 21.Schuber, F. 1989. Influence of polyamine on membrane function. Biochem. J. 260:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seely, J. E., and A. E. Pegg. 1983. Ornithine decarboxylase (mouse kidney). Methods Enzymol. 94:158-161. [DOI] [PubMed] [Google Scholar]
- 23.Slocum, R. D., A. J. Bitonti, P. P. McCann, and R. P. Feirer. 1988. DL-alpha-difluoromethyl [3,4-3H]arginine metabolism in tobacco and mammalian cells. Inhibition of ornithine decarboxylase activity after arginase mediated hydrolysis of DL-alpha-difluoromethylarginine to DL-alpha-difluoromethylornithine. Biochem. J. 255:197-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tadolini, B. 1988. Polyamine inhibition of lipid peroxidation. Biochem. J. 249:33-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tzipori, S., and H. Ward. 2002. Cryptosporidosis: biology, pathogenesis and disease. Microbes Infect. 4:1047-1058. [DOI] [PubMed] [Google Scholar]
- 26.Upton, S. J., M. Tilley, M. V. Nesterenko, and D. B. Brillhart. 1994. A simple and reliable method for producing in vitro infections of Cryptosporidium parvum (apicomplexa). FEMS Microbiol. Lett. 118:45-50. [DOI] [PubMed] [Google Scholar]
- 27.Valasinas, A., A. Sakar, V. K. Reddy, L. J. Marton, H. S. Basu, and B. J. Frydman. 2001. Conformationally restricted analogues of 1N,14N-bisethylhomospermine (BE-4-4-4): synthesis and growth inhibitory effects on human prostate cancer cells. J. Med. Chem. 44:390-403. [DOI] [PubMed] [Google Scholar]
- 28.Vargiu, C., C. Cabella, S. Belliardo, C. Cravancola, M. A. Grillo, and S. Colombatto. 1999. Agmatine modulates polyamine content in hepatocytes by inducing spermidine/spermine acetyltransferase. Eur. J. Biochem. 259:933-938. [DOI] [PubMed] [Google Scholar]
- 29.Vujcics, S., P. Liang, P. Diegelman, D. L. Kramer, and C. W. Porter. 2003. Genomic identification and biochemical characterization of the mammalian polyamine oxidase involved in polyamine back-conversion. Biochem. J. 370:19-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallace, H. M., A. V. Fraser, and A. Hughes. 2003. A perspective of polyamine metabolism. Biochem. J. 376:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, Y., T. Murray-Stewart, W. Devereux, A. Hacker, B. J. Frydman, P. Woster, and R. A. Casero, Jr. 2003. Properties of purified recombinant human polyamine oxidase, PAOh1/SMO. Biochem. Biophys. Res. Commun. 304:605-611. [DOI] [PubMed] [Google Scholar]
- 32.Waters, W. R., and J. A. Harp. 1996. Cryptosporidium parvum infection in T-cell receptor (TCR)-α- and TCR-δ-deficient mice. Infect. Immun. 64:1854-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waters, W. R., T. A. Reinhardt, and J. A. Harp. 1997. Oral administration of putrescine inhibits Cryptosporidium parvum infection of neonatal C57BL-6 mice and is independent of nitric oxide synthesis. J. Parasitol. 83:746-750. [PubMed] [Google Scholar]
- 34.Waters, W. R., B. J. Frydman, L. J. Marton, A. Valasinas, V. K. Reddy, J. A. Harp, M. J. Wannemuehler, and N. Yarlett. 2000. [1N,12N]Bis(ethyl)-cis-6,7-dehydrospermine: a new drug for the treatment and prevention of Cryptosporidium parvum infection of mice deficient in T-cell receptor alpha. Antimicrob. Agents Chemother. 44:2891-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright, R. K., B. A. Buehler, S. N. Schott, and O. Rennert. 1978. Spermine and spermidine, modulators of the cell surface enzyme adenylate cyclase. Pediatr. Res. 12:830-833. [DOI] [PubMed] [Google Scholar]
- 36.Yarlett, N., G. Wu, W. R. Waters, J. A. Harp, M. J. Wannemuehler, M. Morada, D. Athanasopoulos, M. P. Martinez, S. J. Upton, L. J. Marton, and B. J. Frydman. Cryptosporidium parvum spermidine/spermine N1-acetyltransferase exhibits different characteristics to the host enzyme. Mol. Biochem. Parasitol., in press. [DOI] [PubMed]