Abstract
Malaria is the third most significant cause of infectious disease in the world. The search for new antimalarial chemotherapy has become increasingly urgent due to parasite resistance to classical drugs. Trioxaquines are synthetic hybrid molecules containing a trioxane motif (which is responsible for the antimalarial activity of artemisinin) linked to an aminoquinoline entity (which is responsible for the antiplasmodial properties of chloroquine). These trioxaquines are highly potent against young erythrocytic stages of Plasmodium falciparum and exhibit efficient activity in vitro against chloroquine-sensitive and -resistant strains of P. falciparum (50% inhibitory concentration, 4 to 32 nM) and are also active in vivo against P. vinckei petteri and P. yoelii nigeriensis in suppressive and curative murine tests. The trioxaquine DU1302 is one of these promising antimalarial agents. The present study confirms the absence of toxicity of this drug on cell lines and in a mice model. Moreover, DU1302 exhibits potent activity against gametocytes, the form transmitted by mosquitoes, as killing of the gametocytes is essential to limit the spread of malaria. The ease of chemical synthesis of this trioxaquine prototype should be considered an additional advantage and would make these drugs affordable without perturbations of the drug supply.
Due to the increasing resistance of Plasmodium falciparum to most of the current antimalarial drugs, it is now urgent that new effective, safe, and affordable antiplasmodial agents be found (25, 31, 38, 41).
The use of combination therapy has been recommended by the World Health Organization since 2001 (39), and a strong consensus exists that artemisinin-based combination therapies are the first choice of combination therapy for the treatment of malaria (13, 18). Unfortunately, artemisinin-based combination treatments are more expensive than treatments with regular drugs. In addition, the availability of artemisinin is unreliable (10, 16). In this regard, affordable synthetic peroxide-containing drugs, which offer increased potency (3, 21, 35), are under investigation.
The action of artemisinin derivatives, the major antiplasmodial drugs used to treat severe falciparum malaria, is due to the presence of a 1,2,4-trioxane group able to produce radicals that damage the parasite (26, 28); and our group has contributed to demonstration of the alkylating activities of artemisinin derivatives (14, 26, 27). Based on mechanistic considerations, we developed the “covalent bitherapy” concept to create new affordable drugs. These chimeric compounds, named trioxaquines, were obtained by covalent attachment of a trioxane entity, responsible for the activity of artemisinin, to an aminoquinoline entity, necessary for the accumulation of chloroquine in the parasite (6, 7).
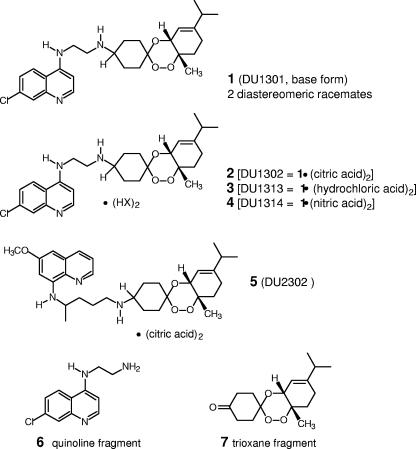
The first series of trioxaquines have been shown to exhibit potent in vitro antimalarial activities (7) and have been shown to be active against chloroquine-resistant and pyrimethamine-resistant human isolates of P. falciparum (4). The antimalarial activities of a second series of trioxaquines have been analyzed in order to study the influences of different structural parameters (6). From these preliminary data, the trioxaquine compound trioxaquine 2 (DU1302; Fig. 1) was found to be the most active in vitro against highly chloroquine-resistant P. falciparum strains and in mice at low doses after intraperitoneal (i.p.) or oral (p.o.) administration (6).
FIG. 1.
Chemical structures of trioxaquines.
Here, we report on a detailed study of the promising biological activities of the second series of trioxaquines (trioxaquines 1 to 5), including the determination of their site of action during the erythrocytic life cycle. We demonstrate that trioxaquines are also active against the gametocyte forms of Plasmodium.
MATERIALS AND METHODS
Synthesis of trioxaquine derivatives.
Trioxaquines 1 and 2 were synthesized from α-terpinene (1-methyl-4-isopropyl-1,3-cyclohexadiene), as described previously (6). In trioxaquine 1, the trioxane and cyclohexene ring are fused in cis, with the two contiguous chiral atoms having (R, S) or (S, R) configurations. In addition, the aminoquinoline and peroxide substituents can be in either the cis or the trans configuration with respect to the location of the cyclohexane ring. For these reasons, trioxaquine 1 exists as a 50-50 mixture of two diastereoisomeric racemates. The trioxaquine 1 is therefore a mixture of four stereoisomers, named trioxaquines 1-st1, 1-st2, 1-st3, and 1-st4. The separation of each of the four stereoisomers of trioxaquine 1 was achieved by high-pressure liquid chromatography of the chiral phase (in collaboration with Sanofi-Aventis). Under the conditions used, the four retention times for stereoisomers 1-st1, 1-st2, 1-st3, and 1-st4 were 13.9, 18.4, 28.6, and 38.5 min, respectively.
The protonation of trioxaquine 1 by citric acid, hydrochloric acid, and nitric acid by a previously described procedure (6) provided the salts trioxaquine 2 (DU1302; Fig. 1), trioxaquine 3 (DU1313; Fig. 1), and trioxaquine 4 (DU1314; Fig. 1), respectively. The salt trioxaquine 3 is therefore also a mixture of two diastereoisomeric racemates (trioxaquines 3-dia1 and 3-dia 2, respectively). The use of plastic utensils was preferred during this study.
Cultures of Plasmodium falciparum.
Five strains of P. falciparum, the two chloroquine-sensitive strains F32-Tanzania (concentration of drug that inhibited 50% of parasite growth [IC50] for chloroquine, 25 nM) and Nigerian (chloroquine IC50, 60 nM), kindly provided by H. J. Vial (2), Montpellier, France, and the three chloroquine-resistant strains FcB1-Columbia (chloroquine IC50, 115 nM), FcM29-Cameroon (chloroquine IC50, 180 nM), and W2-Indochina (chloroquine IC50, 560 nM), were cultured by a method modified from that of Trager and Jensen (33) in a 5% CO2 atmosphere at 37°C (34).
In vitro antiplasmodial activity.
All solutions of chloroquine diphosphate, artemisinin (Sigma Aldrich), and trioxaquines were prepared each day; and the trioxaquine solutions were checked to determine that they did not reprecipitate under these conditions. Parasite growth was estimated by [3H]hypoxanthine (Amersham Pharmacia Biotech, France) incorporation. The IC50 values were graphically determined by plotting the drug concentration versus the percentage of parasite growth inhibition at 48 h of incubation, as described previously (6). All results presented are the means of at least three independent experiments.
In vivo antiplasmodial efficacy against Plasmodium vinckei petteri and P. yoelii nigeriensis by suppressive test.
In vivo antimalarial activity against two murine strains, P. vinckei petteri and P. yoelii nigeriensis (both provided by I. Landau, Muséum National d'Histoire Naturelle de Paris, Paris, France) (37), was determined by the 4-day suppressive test of Peters and Robinson (19). P. vinckei petteri is fatal in mice from about 5 to 8 days (22, 24). Contrary to other murine Plasmodium strains, P. vinckei petteri is particularly adapted to the study of the suppressive and curative efficacies of antimalarial drugs (40), and P. yoelii nigeriensis is studied on the drug resistance phenomena (20).
Each group of animals, which consisted of five female Swiss albino mice (Janvier, Le genest Saint Isle, France), were infected i.p. on day 0 with 2 × 107 parasitized red blood cells. Thereafter, the trioxaquines and artemisinins were dissolved in dimethyl sulfoxide (DMSO), whereas chloroquine was dissolved in 0.9% NaCl solution. For four consecutive days, 100 μl of the drug solutions were administrated to the mice once daily either i.p., p.o., or subcutaneously (s.c.). All experiments included control groups: a sentinel group (the same batch of mice as treated mice but that were not infected and not treated), a drug-free group (mice that were infected with Plasmodium but that received no treatment), and an excipient group (mice that were infected with Plasmodium and that received no drug treatment but that did receive treatment with the excipient used for the drugs tested).
In parallel, three to five groups of mice were treated with different doses of the trioxaquine and three groups were treated with different doses of the artemisinin derivatives (artemether, artesunate, or artemisinin).
Parasitemia levels were determined on the day following the last treatment (day 4). The 50% effective dose (ED50) and ED90 values were the doses that led to 50% and 90% parasite growth inhibition, respectively, in comparison to the level in the nontreated control animals. For each dose, at least 5 animals were treated, and for each route of administration, 10 control animals (vehicle solution) were tested. The 50% curative dose (CD50) and CD90 values were the doses that led to a total cure (no parasite was detected after 60 days) in 50% and 90% of the treated mice, respectively.
In vivo therapeutic (established infection) treatment test.
The treatment of malaria in experimental mice with established infection was carried out by the approach of Ryley and Peters (29), in which treatment with a drug was initiated when the infection was already well established. All animals were first infected by 106 P. vinckei petteri parasites (day 0) and were left for 3 days before the beginning of treatment (day 4). As in the in vivo suppressive test, these experiments included control groups. Moreover, five groups were treated with different doses of trioxaquine 1 and five groups were treated with different doses of artesunate, the trioxane control. On day 4, artesunate and trioxaquine 1 were administered once daily in DMSO in a final volume of 100 μl. These animals were treated p.o. by gavage for 4 days from days 4 to 7 (12). Thin blood smears from each animal were prepared daily before and after treatment.
All procedures involving living animals were performed according to European regulations (EEC directive 86/609 dated 24 November 1986). All the experiments involving animals were carried out in the animal room of the Parasitology Department of Rangueil Hospital (Toulouse, France) under the control of the National Veterinary Services. The staff in charge of the animal experiments had received the appropriate training. All in vivo studies were approved by the French Institutional Animal Experimentation Ethic Committee (approvals MP/05/35/10/03 and MP/04/34/10/03 for the suppressive and therapeutic tests, respectively).
In vitro tests with gametocytes.
The trioxaquines, primaquine (Aldrich), artesunate (Sanofi-Aventis), and atovaquone (GlaxoSmithKline) were dissolved in DMSO; and chloroquine (BUFA, Holland) was dissolved in RPMI 1640 medium.
Gametocyte cultures of strain W2 were initiated as described by Ifediba and Vanderberg (11), but with the following minor variations. Cultures were treated with 50 mM N-acetyl-d-glucosamine (Sigma) for 3 or 5 days to remove asexual forms. Young (7-day-old) or old (13-day-old) gametocyte cultures were transferred to a 24-well plate, and dilutions of each drug were added. Each value corresponds to the mean of three independent experiments. After 48 h of incubation, thin blood films were prepared and stained with Giemsa (Diff-quick). The numbers of gametocytes per 6,000 to 10,000 erythrocytes were counted. In parallel, tests to determine the IC50 values for the asexual stages of W2 strain were performed on the same day with the same dilutions of drugs and by the method detailed in the section “In vitro antiplasmodial activity.” Counting of gametocytes was done microscopically with Giemsa smears, whereas activity against the asexual erythrocytic parasites was determined by the radioactive micromethod.
In vitro cytotoxicity.
The cytoxicities of trioxaquine 2, chloroquine, and artemisinin were estimated with a human colorectal tumor cell line (HCT cells). These HCT cells were grown with 10% fetal calf serum as a monolayer on cover glasses. When these cancer cells multiplied, they were separated with 0.05% trypsin-EDTA (Gibco, France). To determine the cytotoxicities of the compounds, 15 × 103 cells (in 100 μl) were distributed in each well of 96-well plates, and then 100 μl of culture medium containing a dilution of the drug was added to each well. Cell growth was estimated by determination of [3H]thymidine (Amersham) incorporation after 48 h of incubation. As described above for P. falciparum, the results were analyzed graphically by plotting the drug concentration versus the percentage of cell growth inhibition at 48 h of incubation. The security index thus determined is the ratio of the IC50 values for in vitro toxicity versus the in vitro activity against P. falciparum.
In vivo toxicity studies with healthy mice.
In vivo acute toxicity was expressed as the 50% lethal dose (LD50), which corresponds to the dose that results in the death of 50% of the mice, 60 days after drug injection. Acute toxicity was determined with the trioxaquines either after the administration of one i.p. injection or after the administration of four consecutive p.o. doses (as in the suppressive test of Peters and Robinson [19]) to two to five noninfected mice Swiss per dose.
The toxicity of trioxaquine 2 after only one i.p. injection was studied.
In two independent experiments, the toxicity of trioxaquine 1 at doses of either 100, 200, or 400 mg/kg of body weight/day administered p.o. by gavage for four consecutive days was studied. For each experiment, two to four mice were studied per dose and one mouse was killed per dose for each study day (days 5, 30, and 60). Postmortem examinations (weight, morphology, observation) of the liver, kidneys, intestine, stomach, heart, spleen, ovary, uterus, and brain were done comparatively with treated mice and control mice (treated only either with DMSO or with 0.9% NaCl solution) on days 5, 30, and 60. Sections of the stomachs from the mice were examined microscopically on days 5, 30, and 60 after hematoxylin-eosin staining. This experiment was done with four mice per group and was performed twice.
In vivo subacute toxicity studies with infected mice.
The subacute toxicity of trioxaquine 4 was studied in mice infected by P. vinckei petteri and treated for four consecutive days from day 0 to day 3. The mice were treated with 10, 25, or 50 mg/kg/day of trioxaquine 4 administered either p.o., i.p., or s.c. Five mice were used for each dose and each route of administration.
Stage of trioxaquine action with respect to erythrocytic life cycle.
For the experiments in which the efficacy of the drug against synchronized parasites was assessed, cultures were synchronized by a Plasmion-based method (15). For this protocol, dilutions of trioxaquine 2, chloroquine, and artemisinin were prepared each day.
To determine the in vitro efficacies of the three antimalarial drugs in culture, serial dilutions close to their IC50 values, determined previously, were prepared: for artemisinin, 7, 14, and 28 nM; for chloroquine, 120, 163, and 232 nM; and for trioxaquine 2, 11.5, 23, and 46 nM. After synchronization, the parasites were plated at the ring stage in eight 24-well plates. Each plate corresponded to one of the successive 6-h periods of the P. falciparum life cycle.
RESULTS
Trioxaquines inhibit Plasmodium growth in vitro.
The in vitro antimalarial activities of trioxaquines 1 to 4 (Fig. 1) are presented in Table 1. Trioxaquine 1 (a mixture of four stereoisomers) and the four separated and independently tested stereoisomers had similar IC50 values, which ranged from 19 to 32 nM for strain FcM29. In the same way, both diastereoisomeric racemates, trioxaquines 3-dia1 and 3-dia2, showed IC50 values in the same low-nanomolar range: 7 to 9 nM for trioxaquine 3-dia1 and 8 to 12 nM for trioxaquine 3-dia2. In comparison, the IC50s of trioxaquine 3 were 18 to 26 nM.
TABLE 1.
In vitro antimalarial activities of trioxaquine derivatives against P. falciparum strains and cytotoxicity for the HCT cell line
| Drug (mol wt) | Mean IC50 (nM)a
|
||||
|---|---|---|---|---|---|
| F32 (CQS) | Nigerian (CQS) | FcB1 (CQR) | FcM29 (CQR) | HCT cell line | |
| Trioxaquine 1 (486) | 5 (2)b | ND | 6 (3)b | 21 (10)b | ND |
| Trioxaquine 1-st1 (486) | ND | ND | 49 (7) | 29 (3) | ND |
| Trioxaquine 1-st2 (486) | ND | ND | 48 (8) | 32 (4) | ND |
| Trioxaquine 1-st3 (486) | ND | ND | 47 (9) | 23 (5) | ND |
| Trioxaquine 1-st4 (486) | ND | ND | 43 (8) | 19 (3) | ND |
| Trioxaquine 2 (870) | 4 (3) | 23 (3)b | 5 (4) | 18 (16) | 3 × 103 (130-600)c |
| Trioxaquine 3 (559) | 18 (6) | 18d | 26 (8) | 18 (8) | ND |
| Trioxaquine 3-dia1 (559) | 7 (0.5) | 9d | 7 (1) | 7 (3) | ND |
| Trioxaquine 3-dia2 (559) | 11 (3) | ND | 8 (1) | 12 (5) | ND |
| Trioxaquine 4 (612) | 25 (11) | 15 (3) | 21 (9) | 16 (7) | ND |
| Chloroquine (516) | 25 (6) | 60 (10) | 115 (50) | 180 (30) | 50 × 103 (270-2,000) |
| Artemisinin (282) | 6 (0.3) | 6 (1) | 5 | 8 (1) | >35 × 103 (4,370-7,000) |
| Artesunate (384) | ND | ND | 2.3 (3.1) | 1.6 (3.7) | ND |
All results are the means for three to nine independent experiments. CQS, chloroquine-sensitive strain; CQR, chloroquine-resistant strain; ND, not determined.
Values in parentheses are the standard error of the mean.
Values in parentheses are the security index, which is the ratio of the in vitro toxicity to the in vitro activity against P. falciparum.
Data are based on the results of only one experiment.
The activities of the different salts of trioxaquine 1 against P. falciparum were also evaluated; and it was found that they exhibited activities similar to that of the base form, trioxaquine 1 (IC50s, 5 to 21 nM): the IC50 values were 4 to 23 nM for the citrate salt (trioxaquine 2), 18 to 26 nM for the chloride salt (trioxaquine 3), and 15 to 25 nM for the nitrate salt (trioxaquine 4).
Moreover, the activities of the trioxaquines were independent of the chloroquine sensitivities of the P. falciparum strains tested: the activities (IC50s) of the trioxaquines ranged from 4 to 25 nM and from 5 to 32 nM for chloroquine-sensitive and chloroquine-resistant strains, respectively.
Trioxaquines 1 to 4 (IC50s, 5 to 32 nM) showed in vitro antimalarial activities similar to those obtained with artemisinin (IC50s, 5 to 8 nM) and artesunate (IC50s, 1.6 to 2.3 nM) under the same conditions. For the same strains, the corresponding IC50 values of chloroquine ranged from 25 to 180 nM.
To validate the concept of dual molecules, both trioxaquine precursors, quinoline 6 and trioxane 7 (Fig. 1), were tested separately. Whatever P. falciparum strains were used, the trioxane motif alone exhibited IC50 values ranging from 200 nM to 600 nM and the IC50 values of the quinoline entity alone ranged from 120 nM to 2 μM. Furthermore, both entities, trioxane and quinoline, tested together in the same wells showed IC50s ranging from 40 nM to 180 nM, whereas the trioxaquines had IC50s ranging from 4 nM to 32 nM. These data suggest (i) that the link between both pharmacophores of the trioxaquines is essential for their activity and (ii) that trioxaquines are more than simple trioxanes and that the linked aminoquinoline entity largely improves their antiplasmodial activities.
The poor solubility of trioxaquines in biological medium requires that the trioxaquines be dissolved, first in DMSO and then diluted in NaCl solution, as is the case for the artemisinin derivatives, to avoid precipitation and to obtain reproducible results. However, it should be noted that trioxaquines are very stable in the solid state for long periods of time. Chemical analyses of powders of the trioxaquines stored at room temperature for more than 3 years showed that they were unchanged and the trioxaquines had IC50 values identical to those before storage. This observation is particularly important for antiplasmodial compound candidates, which may need to be stored in hot areas where malaria is endemic for long periods of time without a loss of activity.
Trioxaquines inhibit Plasmodium growth in vivo.
In vivo, the antimalarial activities of the trioxaquines were initially tested by using a 4-day suppressive test with mice infected with the erythrocytic P. vinckei petteri parasite (Table 2). Given by the i.p. route, all trioxaquines tested exhibited antimalarial activities (ED50 range, 0.7 to 3 mg/kg/day) between those of artemisinin (ED50, 4 mg/kg/day) and artemether (ED50, 0.3 mg/kg/day).
TABLE 2.
In vivo antimalarial activities of different trioxaquines, artemisinin, artemether, and artesunate against P. vinckei determined by the suppressive test
| Drug (mol wt)a | i.p. route
|
p.o. route
|
s.c. route
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ED50b (mg/kg/day) | ED90b (mg/kg/day) | CD50 (mg/kg/day) | CD90 (mg/kg/day) | ED50 (mg/kg/day) | ED90 (mg/kg/day) | CD50 (mg/kg/day) | CD90 (mg/kg/day) | ED50 (mg/kg/day) | ED90 (mg/kg/day) | CD50 (mg/kg/day) | CD90 (mg/kg/day) | |
| Trioxaquine 1 (486) | 0.7 | 8 | 5 | 8 | 4 | 9 | 12 | 24 | 1.5 | 9 | 5 | 8 |
| Trioxaquine 2 (870) | 3 | 8 | 12 | >20 | 17 | 23 | >25 | >25 | 2.8 | 8 | >10 | >10 |
| Trioxaquine 2-dia1 (870) | 3 | 8 | 12 | >20 | NDc | ND | ND | ND | ND | ND | ND | ND |
| Trioxaquine 2-dia2 (870) | 2.5 | 7 | 7 | >20 | ND | ND | ND | ND | ND | ND | ND | ND |
| Trioxaquine 4 (612) | 0.7 | 8 | 3 | 9 | 16 | 34 | 24 | 46 | 2 | 6 | 3 | 8 |
| Artemisinin (282) | 4 | >5 | ND | ND | 17 | 30 | >25 | >25 | 3.2 | 8 | >10 | >10 |
| Artemether (298) | 0.3 | 0.7 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Artesunate (384) | ND | ND | ND | ND | 2.5 | 8 | 19 | 28 | 0.3 | 0.8 | 2.5 | 7.5 |
Treatments were applied for 4 days, once daily, by the i.p., p.o., or s.c. route. The molecular weights of the trioxaquines also include those of the counterions.
ED50s and ED90s are for day 4.
ND, not determined.
Trioxaquines were also administered p.o. and their activities were compared to those of artemisinin and artesunate. Artesunate is the most potent drug of the artemisinin family available (Table 2). The ED50 values were 17 mg/kg/day for artemisinin and 2.5 mg/kg/day for artesunate and ranged from 4 to 17 mg/kg/day for the trioxaquine derivatives. The most potent trioxaquine seemed to be trioxaquine 1, with a CD50 value (12 mg/kg/day) slightly better than those of artesunate (CD50, 19 mg/kg/day) and artemisinin (CD50, >25 mg/kg/day). With both antimalarial drugs, trioxaquine 1 and artesunate, all infected mice were completely cured when they were treated by the p.o. route, without any recrudescence detected by the use of doses less than the 30-mg/kg/day threshold.
The activities of these molecules when they were administered by the s.c. route were also evaluated (Table 2). The trioxaquines showed ED50 values ranging from 1.5 to 2.8 mg/kg/day, similar to that of artemisinin (ED50, 3.2 mg/kg/day). The CD values obtained with the trioxaquines indicated that these hybrid molecules have better curative effects in mice than artemisinin, and their activities approach that of artesunate.
In addition, the antimalarial activities of the trioxaquines was tested in mice infected with an additional murine strain, P. yoelii nigeriensis (Table 3). By p.o. administration, trioxaquine 2 (CD50 by the p.o. route, 22 mg/kg/day) showed better antimalarial activity than artemisinin (CD50 by the p.o. route, 75 mg/kg/day). Moreover, trioxaquine 2 at 50 mg/kg/day cured all the mice infected by this virulent strain (without any recrudescence at 60 days), whereas the dose of artemisinin required to achieve a complete cure was more than 100 mg/kg/day by the p.o. route.
TABLE 3.
In vivo antimalarial activities of trioxaquine 2, artemisinin, and chloroquine against P. yoelii nigeriensis determined by the suppressive test
| Druga | p.o. route
|
s.c. route
|
||||||
|---|---|---|---|---|---|---|---|---|
| ED50 (mg/kg/day) | ED90 (mg/kg/day) | CD50 (mg/kg/day) | CD90 (mg/kg/day) | ED50 (mg/kg/day) | ED90 (mg/kg/day) | CD50 (mg/kg/day) | CD90 (mg/kg/day) | |
| Trioxaquine 2 | 15 | 23 | 22 | 44 | 2 | 5 | 3 | 12 |
| Artemisinin | 16 | 27 | 75 | >100 | 5 | 23 | 7 | 23 |
| Chloroquine | NDb | ND | ND | ND | 3 | 5 | 3 | 5 |
The treatments were applied for 4 days, once daily, p.o. or s.c.
ND, not determined.
In parallel, by s.c. administration, the activity of trioxaquine 2 (CD50 by the s.c. route, 3 mg/kg/day) also seemed to be better than that of artemisinin (CD50 by the s.c. route, 7 mg/kg/day) and similar to that of chloroquine (CD50 by the s.c. route, 3 mg/kg/day).
Trioxaquines cure Plasmodium infection in vivo with treatment postinfection.
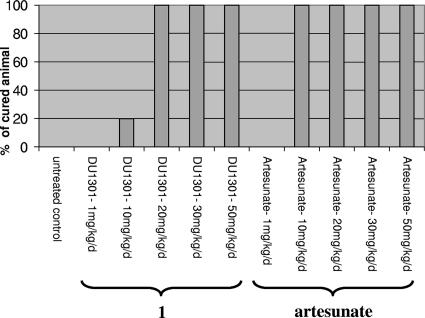
Trioxaquine 1 was also tested in vivo in mice when the infection was already well established. These conditions are more similar to actual therapeutic conditions (Fig. 2). A 4-day p.o. treatment was started on day 4 after infection, when the infected mice already had a level of parasitemia of 4% ± 2%. Under these conditions, the potent antimalarial activities of trioxaquine 1 at 13 mg/kg/day and artesunate at 5 mg/kg/day by p.o. administration were confirmed, curing all the mice without any recrudescence after 60 days.
FIG. 2.
In vivo antimalarial activities of trioxaquine 1 and artesunate against P. vinckei petteri when mice were infected on day 0 and treated by the p.o. route once daily for 4 days from days 4 to 7 (doses are in mg/kg/day). The level of parasitemia on day 4 was 4%.
The activities of the trioxaquines against gametocytes are highly potent.
The activities of trioxaquines 2 and 5 against gametocyte cultures of P. falciparum were tested and compared to those of four reference drugs, namely, artesunate, primaquine, chloroquine, and atovaquone (Table 4). Trioxaquines 2 and 5 and artesunate were the most potent drugs against the gametocyte forms of P. falciparum, independent of the young (stages II and III) or old (stages IV and V) targeted stages of gametocytes (the mosquito-transmissible form). This result indicates the key role of the trioxane moiety of antimalarial peroxide-containing drugs that exhibit a high degree of efficacy against highly chloroquine-resistant strain W2, both asexual stages (IC50 range, 6 to 43 nM) and gametocytes (IC50 range, 46 to 108 nM). The three trioxane-containing drugs are much more active against gametocytes (range, 15- to 1,100-fold) than the three other nonperoxidic antimalarial drugs (primaquine, chloroquine, and atovaquone).
TABLE 4.
In vitro activities of trioxaquines 2 and 5 and four reference antimalarial drugs against young and old gametocytes and in parallel against W2 asexual-stage parasite culture
| Drug | IC50 (nM)a
|
||
|---|---|---|---|
| Gametocytes stages II and III | Gametocytes stages IV and V | Asexual stage | |
| Trioxaquine 2 | 69 (29) | 67 (5) | 28 (7) |
| Trioxaquine 5 | 57 (13) | 46 (18) | 43 (11) |
| Artesunate | 72 (21) | 108 (11) | 6 (2) |
| Primaquine | 4,580 (2,200) | 1,540 (914) | 1,320 (300) |
| Chloroquine | 300 (1,500) | 10,700 (3,900) | 560 (190) |
| Atovaquone | 24,000 (10,700) | 50,400 (29,000) | 4.6 (2) |
IC50 values of drugs against gametocytes of P. falciparum strain W2 and against asexual-stage erythrocytic parasites from P. falciparum strain W2. Each value corresponds to the mean of at least three independent experiments. Standard errors of the means are indicated in parentheses.
Evaluation of cytotoxicity of trioxaquine 2.
The cytotoxicities of trioxaquine 2, chloroquine, and artemisinin for the HCT cell line were determined (Table 1). Trioxaquine 2 appeared to be a very promising compound, with the in vitro security index ranging from 130 to 600, which is similar to that observed for chloroquine (the security index is defined in footnote c of Table 1).
Evaluation of in vivo toxicities of trioxaquines 1, 2, and 4.
The acute toxicities of the trioxaquines were evaluated by using healthy mice that were treated and observed for 60 days. By i.p. injection, trioxaquines 2 and 4 showed acute toxicity after the administration of a single dose, with the LD50 being 70 mg/kg/day. These relatively weak toxicities indicated promising therapeutic indices (TIs; TI = LD50/ED50) of 23 for trioxaquine 2 and 100 for trioxaquine 4.
By p.o. administration, mice were treated for four consecutive days with different doses of trioxaquine 1: 100 mg/kg/day, 200 mg/kg/day, or 400 mg/kg/day. No toxicity was observed for these treatments, with the total administered dose corresponding to 1,600 mg/kg for the last dose. The postmortem examination showed no difference between treated and control mice (control mice given NaCl or DMSO) on days 5, 30, and 60, except for stomach weight, and that difference was detected only for the 400-mg/kg/day dose given four times.
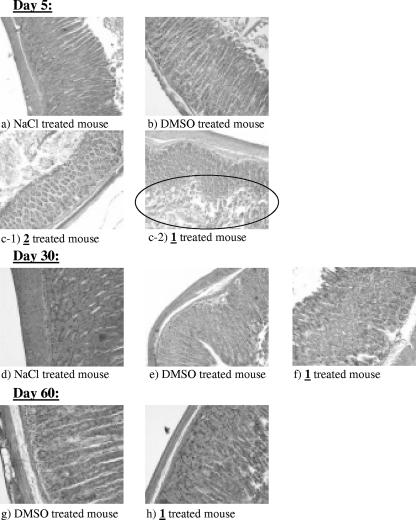
Sections of the stomachs of these mice treated four times with 400 mg/kg/day of trioxaquine 1 were then examined on days 5, 30, and 60 after hematoxylin-eosin staining (Fig. 3). On each of these days no changes in the glandular cells of the mouse stomach were observed in the control mice treated either with 0.9% NaCl solution (Fig. 3a and d) or with the vehicle DMSO (Fig. 3b, e, and g). However, for the mice treated with trioxaquine 1, limited areas of the stomachs exhibited injured glandular cells on day 5 (Fig. 3c-2), whereas the majority of the area of the stomach had no such damage (Fig. 3c-1). On day 30 (Fig. 3f) and day 60 (Fig. 3h), all areas of the stomachs of these mice treated with a total of 1,600 mg/kg of trioxaquine 1 were completely repaired with regard to the glandular cell aspect and the stomachs were similar to stomachs of the control mice. The reversibility of such a localized response to a drug is often observed at high doses administered by the p.o. route. There was thus no irreversible toxicity for the 400-mg/kg/day dose administered four times by the p.o. route, and the TI of trioxaquine 1 (>1,600/4 mg/kg/day) was thus greater than 400.
FIG. 3.
Sections on days 5, 30, and 60 of the stomachs of mice treated p.o. for four consecutive days (days 1, 2, 3, and 4) either with 400-mg/kg/day doses of trioxaquine 1 or with the DMSO or NaCl vehicle.
Trioxaquine 4 was also injected by the s.c. route in the backs of healthy mice for four consecutive days at 100 mg/kg/day (i.e., a total injected dose of 400 mg/kg). Besides a small necrosis at the injection point, no effects on mouse behavior or on postmortem examinations were observed. On day 15, the observed necrosis had disappeared, and no other damage was observed during the subsequent 60 days. The TI of trioxaquine 4 administered by the s.c. route is greater than 200.
The subacute toxicity of trioxaquine 4 was evaluated for P. vinckei petteri-infected mice treated for four consecutive days and showed (i) no toxicity in mice infected and treated p.o. at 50 mg/kg/day, (ii) no toxicity in infected mice treated i.p. with 25 mg/kg/day but lethal toxicity in infected mice treated i.p. with 50 mg/kg/day (i.e., 70 times the ED50 value of trioxaquine 4 by i.p. administration), and (iii) temporary (only during the treatment) and reversible necrosis but no mortality in infected mice treated by the s.c. route with 50 mg/kg/day. These favorable subacute toxicity data obtained for infected mice indicated a favorable therapeutic ratio as regards the antimalarial activities of trioxaquines.
Trioxaquines act on the early stages of the erythrocytic Plasmodium life cycle.
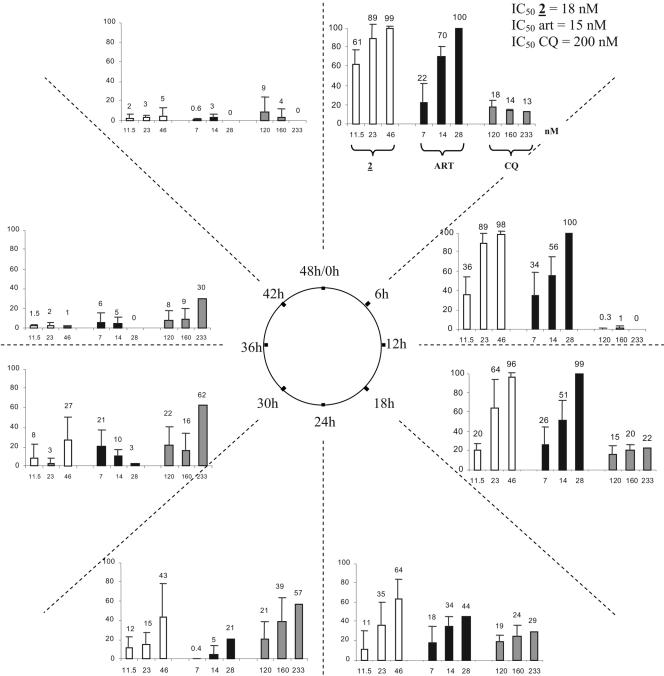
We studied the stage specificity of the action of trioxaquine 2 in comparison to those of chloroquine and artemisinin on the erythocytic life cycle of P. falciparum (Fig. 4). Synchronous cultures of chloroquine-resistant P. falciparum strain FcB1-Knobs+ (15) were exposed to different concentrations of the compounds tested. As expected for that strain, chloroquine (IC50, 200 nM) was less active than artemisinin (IC50, 15 nM) and trioxaquine 2 (IC50, 18 nM).
FIG. 4.
Parasite growth inhibition (in percent) by trioxaquine 2, artemisinin (ART), and chloroquine (CQ) at different doses (in nM), according to the periods of the Plasmodium erythrocytic cycle. The stage-specific activities of the different drugs were tested during the eight successive 6-h periods of the Plasmodium falciparum 48-h life cycle against chloroquine-resistant strain FcB1. Gray, black, and white bars, three concentrations of trioxaquine 2 (11.5, 23, and 46 nM, respectively), artemisinin (7, 14, and 28 nM, respectively) and chloroquine (120, 160, and 233 nM, respectively). The experiments with trioxaquine 2 were performed four times, and the experiments with the two other drugs were performed three times.
By using drug doses close to the IC50 values, the maximum activity of chloroquine was observed between the 24th and the 36th hours of the parasite life cycle, whereas artemisinin and trioxaquine 2 had similar stage specificity profiles, with the maximum inhibition of parasite growth occurring at the beginning of the cycle (0 to 18 h, corresponding to the ring and trophozoite stages).
DISCUSSION
The in vitro activities of trioxaquines 1 to 4 are independent of the salts used, with the citrate salt (trioxaquine 2), the chloride salt (trioxaquine 3), and the nitrate salt (trioxaquine 4) exhibiting IC50 values close to those of the free base (trioxaquine 1). The different counterions had no influence on the in vitro antiplasmodial activities of the trioxaquines.
Trioxaquines 1 to 4 are a 50-50 mixture of two diastereomeric racemates. Whatever the diastereomer (trioxaquine 3-dia1 or 3-dia2) or the salt of trioxaquine 3 tested, they were equally active against the chloroquine-sensitive and chloroquine-resistant strains of P. falciparum, as already described for the two diastereomers of trioxaquine 2 (6). This result was confirmed by an independent evaluation of the four separated stereoisomers of trioxaquine 1 (trioxaquines 1-st1 to 1-st4), with the IC50 values of four stereoisomers being in the same range as that of trioxaquine 1. In addition, because the antimalarial activities of the trioxaquines were independent of their stereochemistry, this result suggests that their target is not chiral. These results were also confirmed in vivo with the two separated diastereomers, trioxaquines 2-dia1 and 2-dia2, which independently exhibited activities in the same range as that of trioxaquine 2 (the 50-50 mixture of both diastereoisomers).
The in vitro antimalarial activities of all trioxaquines were promising (IC50s, 4 to 32 nM), and the trioxaquines were as potent as artemisinin and had potencies comparable to those of other antimalarial compounds, such as synthetic endoperoxides (IC50s, 7 to 940 nM) (3), synthetic yingzhaosu (IC50s, 56 to 380 nM) (32), trioxolanes (IC50s, 1 to 140 nM) (35), ferroquine (IC50s, 14 to 42 nM) (8), and quaternary ammonium salts (IC50s, 0.03 to 129 nM) (2). As expected, the trioxaquines were active independently of the sensitivities of the P. falciparum strains to chloroquine. This fact is particularly important for new drugs that will be used in areas where malaria is endemic and where chloroquine resistance is widespread (17, 38).
The in vivo activities of the trioxaquines (Tables 2 and 3 and Fig. 2) compared to those of other trioxane-containing antimalarial drugs are promising. Based on the level of curative doses, trioxaquines, in particular, trioxaquine 1, are more active than artemisinin and artemether and exhibit activities similar to that of artesunate. Based on the data in Table 2, the bioavailability after p.o. administration is important, since the ED50 values obtained by the p.o. route differed by only a factor of 3 to 4 from those obtained by the s.c. or the i.p. route.
Excellent in vivo results for the trioxaquines were obtained by suppressive tests (Tables 2 and 3) as well as postinfection tests (Fig. 2). These results seem to indicate that trioxaquines should be efficient as a curative treatment even when the level of parasitemia is high and the physiopathological disorders associated with malaria are present.
Moreover, the trioxaquines showed promising results independently of the strains of murine Plasmodium tested. Trioxaquine 2 showed greater activity in mice infected by P. yoelii nigeriensis, a virulent parasite strain, than artemisinin. Despite previous claims (30), trioxaquines are both active against and curative of infections with the P. yoelii nigeriensis strain (Table 3).
The activities of both trioxaquines 2 and 5 (IC50s, 46 to 69 nM) were very potent against gametocytes, showing slightly greater activity than artesunate (IC50s, 72 to 108 nM). The difference in the quinoline moiety between trioxaquine 2 (with a 4-aminoquinoline) and trioxaquine 5 (with an 8-aminoquinoline) had no effect on activities against both the asexual and the sexual stages of P. falciparum strain W2. In the future, the use of trioxaquines for the treatment of malaria could effectively reduce the gametocyte population and, consequently, lower transmission rates. We have confirmed the efficacy of artesunate agianst gametocytes, which has already been shown in clinical trials (23, 36). It is important to note that atovaquone, which is often used nowadays in the association atovaquone-proguanil (Malarone), presented very strong in vitro activity against asexual erythrocytic P. falciparum cultures but had no activity against gametocytes. Moreover, the activity of primaquine against gametocytes is weak in vitro, whereas the capacity of primaquine to accelerate gametocyte clearance in P. falciparum malaria has been described in vivo (23).
The potent antimalarial activity of trioxaquine 2 is associated with a good security index in vitro, similar to those observed for chloroquine (5) and ferroquine (8). Weak toxicities were observed in vivo by the i.p. route for trioxaquines 2 and 4, with promising TIs of 23 and 100, respectively. These TIs are very encouraging compared to those of other antimalarials currently studied, such as G25, which has a therapeutic index of 7 (1). Moreover, no subacute toxicity after p.o. administration of high doses (400 mg/kg/day four times) of trioxaquines was observed, comparable to the values for a trioxolane derivative (300 mg/kg/day five times) (35). In addition, treatment with the trioxaquines by the p.o. and s.c. routes for four consecutive days showed no cumulative toxicity.
The stage specificity action of trioxaquine 2 on the erythrocytic life cycle was studied in parallel with the actions of artemisinin and chloroquine with synchronized parasites (15). Like artemisinin, trioxaquine 2 was effective at pharmacological doses at the beginning of the erythrocytic cycle, i.e., on the ring and the trophozoite stages, during the first 24-h period. On the contrary, the activity of chloroquine began only in the second half of the cycle, on the late trophozoite stage, during a relatively short period (24 to 36 h). These results demonstrate that trioxaquines behave like artemisinin, which is particularly relevant because of the presence of widespread chloroquine resistance and the absence of artemisinin resistance in the field (9).
In conclusion, the data reported here indicate that trioxaquines, based on the covalent bitherapy concept, are very potent antiplasmodial drugs against both the asexual and the sexual (gametocytes) stages. The activities of trioxaquines against gametocytes, which would limit the transmission of the malaria parasite, are potentially very promising. With such a biological profile, trioxaquines should be considered a model for new candidates in the arsenal of drugs able to fight malaria by treating individual symptoms and limiting parasite transmission.
Acknowledgments
This work has been supported by the CNRS, Palumed, and the Région Midi-Pyrénées. Palumed and Sanofi-Aventis are currently working on the development of trioxaquines.
F.B.-V. is “chargée de recherche” at INSERM (Institut National de la Santé et de la Recherche Médicale). We thank C. Musso-Rigal (CHU) for advice and discussions on histological studies. A. Pradines (Sanofi-Aventis) is gratefully acknowledged for the separation of the chiral phase by high-pressure liquid chromatography. J. C. Rives (CHU), A. Erraud (Palumed), and C. Duboé (Palumed) are acknowledged for technical assistance. We also thank Sanofi-Aventis for the generous gift of artesunate. F. Coslédan (Palumed) is gratefully acknowledged for fruitful discussions on trioxaquines.
The authors have no conflicts of interest concerning the work reported in this paper.
Footnotes
Published ahead of print on 22 January 2007.
REFERENCES
- 1.Ancelin, M. L., M. Calas, A. Bonhoure, S. Herbute, and H. J. Vial. 2003. In vivo antimalarial activities of mono- and bis quaternary ammonium salts interfering with Plasmodium phospholipid metabolism. Antimicrob. Agents Chemother. 47:2598-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ancelin, M. L., M. Calas, V. Vidal-Sailhan, S. Herbute, P. Ringwald, and H. J. Vial. 2003. Potent inhibitors of Plasmodium phospholipid metabolism with a broad spectrum of in vitro antimalarial activities. Antimicrob. Agents Chemother. 47:2590-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachi, M. D., E. E. Korshin, R. Hoos, A. M. Szpilman, P. Ploypradith, S. Xie, T. A. Shapiro, and G. H. Posner. 2003. A short synthesis and biological evaluation of potent and nontoxic antimalarial bridged bicyclic beta-sulfonyl-endoperoxides. J. Med. Chem. 46:2516-2533. [DOI] [PubMed] [Google Scholar]
- 4.Basco, L. K., O. Dechy-Cabaret, M. Ndounga, F. S. Meche, A. Robert, and B. Meunier. 2001. In vitro activity of DU-1102, a new trioxaquine derivative, against Plasmodium falciparum isolates. Antimicrob. Agents Chemother. 45:1886-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benoit-Vical, F., A. Robert, and B. Meunier. 1999. Potentiation of artemisinin activity against chloroquine-resistant Plasmodium falciparum strains by using heme models. Antimicrob. Agents Chemother. 43:2555-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dechy-Cabaret, O., F. Benoit-Vical, C. Loup, A. Robert, H. Gornitzka, A. Bonhoure, H. Vial, J. F. Magnaval, J. P. Seguela, and B. Meunier. 2004. Synthesis and antimalarial activity of trioxaquine derivatives. Chemistry 10:1625-1636. [DOI] [PubMed] [Google Scholar]
- 7.Dechy-Cabaret, O., F. Benoit-Vical, A. Robert, and B. Meunier. 2000. Preparation and antimalarial activities of trioxaquines, new modular molecules with a trioxane skeleton linked to a 4-aminoquinoline. Chem. Biochem. 4:281-283. [DOI] [PubMed] [Google Scholar]
- 8.Delhaes, L., H. Abessolo, C. Biot, L. Berry, P. Delcourt, L. Maciejewski, J. Brocard, D. Camus, and D. Dive. 2001. In vitro and in vivo antimalarial activity of ferrochloroquine, a ferrocenyl analogue of chloroquine against chloroquine-resistant malaria parasites. Parasitol. Res. 87:239-244. [DOI] [PubMed] [Google Scholar]
- 9.Delhaes, L., F. Benoit-Vical, D. Camus, M. Capron, and B. Meunier. 2003. Chloroquine and artemisinin: six decades of research—what next? IDrugs 6:674-680. [PubMed] [Google Scholar]
- 10.Dondorp, A. M., P. N. Newton, M. Mayxay, W. Van Damme, F. M. Smithuis, S. Yeung, A. Petit, A. J. Lynam, A. Johnson, T. T. Hien, R. McGready, J. J. Farrar, S. Looareesuwan, N. P. Day, M. D. Green, and N. J. White. 2004. Fake antimalarials in Southeast Asia are a major impediment to malaria control: multinational cross-sectional survey on the prevalence of fake antimalarials. Trop. Med. Int. Health 9:1241-1246. [DOI] [PubMed] [Google Scholar]
- 11.Ifediba, T., and J. P. Vanderberg. 1981. Complete in vitro maturation of Plasmodium falciparum gametocytes. Nature 294:364-366. [DOI] [PubMed] [Google Scholar]
- 12.Isah, A. B., Y. K. Ibrahim, and E. O. Iwalewa. 2003. Evaluation of the antimalarial properties and standardization of tablets of Azadirachta indica (Meliaceae) in mice. Phytother. Res. 17:807-810. [DOI] [PubMed] [Google Scholar]
- 13.Ittarat, W., A. L. Pickard, P. Rattanasinganchan, P. Wilairatana, S. Looareesuwan, K. Emery, J. Low, R. Udomsangpetch, and S. R. Meshnick. 2003. Recrudescence in artesunate-treated patients with falciparum malaria is dependent on parasite burden not on parasite factors. Am. J. Trop. Med. Hyg. 68:147-152. [PubMed] [Google Scholar]
- 14.Laurent, S. A.-L., A. Robert, and B. Meunier. 2005. C-10 modified artemisinin derivatives: efficient heme-alkalating agents. Angew. Chem. Int. Ed. 44:2060. [DOI] [PubMed] [Google Scholar]
- 15.Lelievre, J., A. Berry, and F. Benoit-Vical. 2005. An alternative method for Plasmodium culture synchronization. Exp. Parasitol. 109:195-197. [DOI] [PubMed] [Google Scholar]
- 16.Mutabingwa, T. K. 2005. Artemisinin-based combination therapies (ACTs): best hope for malaria treatment but inaccessible to the needy! Acta Trop. 95:305-315. [DOI] [PubMed] [Google Scholar]
- 17.Mutabingwa, T. K., D. Anthony, A. Heller, R. Hallett, J. Ahmed, C. Drakeley, B. M. Greenwood, and C. J. Whitty. 2005. Amodiaquine alone, amodiaquine+sulfadoxine-pyrimethamine, amodiaquine+artesunate, and artemether-lumefantrine for outpatient treatment of malaria in Tanzanian children: a four-arm randomised effectiveness trial. Lancet 365:1474-1480. [DOI] [PubMed] [Google Scholar]
- 18.Olliaro, P. 2005. Drug resistance hampers our capacity to roll back malaria. Clin. Infect. Dis. 41:S247-S257. [DOI] [PubMed] [Google Scholar]
- 19.Peters, W., and B. Robinson. 1998. Section IV, p. 756-771. In O. Zack and M. Sande (ed.), Malaria. Academic Press, London, United Kingdom.
- 20.Peters, W., and B. L. Robinson. 2000. The chemotherapy of rodent malaria. LVIII. Drug combinations to impede the selection of drug resistance, Part. 2: the new generation-artemisinin or artesunate with long-acting blood schizontocides. Ann. Trop. Med. Parasitol. 94:23-35. [DOI] [PubMed] [Google Scholar]
- 21.Posner, G. H., M. H. Parker, J. Northrop, J. S. Elias, P. Ploypradith, S. Xie, and T. A. Shapiro. 1999. Orally active, hydrolytically stable, semisynthetic, antimalarial trioxanes in the artemisinin family. J. Med. Chem. 42:300-304. [DOI] [PubMed] [Google Scholar]
- 22.Powers, K. G., R. L. Jacobs, W. C. Good, and L. C. Koontz. 1969. Plasmodium vinckei: production of chloroquine-resistant strain. Exp. Parasitol. 26:193-202. [DOI] [PubMed] [Google Scholar]
- 23.Pukrittayakamee, S., K. Chotivanich, A. Chantra, R. Clemens, S. Looareesuwan, and N. J. White. 2004. Activities of artesunate and primaquine against asexual- and sexual-stage parasites in falciparum malaria. Antimicrob. Agents Chemother. 48:1329-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reece, S. E., A. B. Duncan, S. A. West, and A. F. Read. 2005. Host cell preference and variable transmission strategies in malaria parasites. Proc. Biol. Sci. 272:511-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ridley, R., and Y. Toure. 2004. Winning the drugs war. Nature 430:942-943. [DOI] [PubMed] [Google Scholar]
- 26.Robert, A., F. Benoit-Vical, C. Claparols, and B. Meunier. 2005. The antimalarial drug artemisinin alkylates heme in infected mice. Proc. Natl. Acad. Sci. USA 102:13676-13680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robert, A., J. Cazelles, and B. Meunier. 2001. First characterization of the alkylation product of heme by the antimalarial drug artemisinin. Angew. Chem. Int. Ed. 40:1954-1957. [DOI] [PubMed] [Google Scholar]
- 28.Robert, A., O. Dechy-Cabaret, J. Cazelles, and B. Meunier. 2002. From mechanistic studies on artemisinin derivatives to new modular antimalarial drugs. Accounts Chem. Res. 35:167-174. [DOI] [PubMed] [Google Scholar]
- 29.Ryley, J. F., and W. Peters. 1970. The antimalarial activity of some quinolone esters. Ann. Trop. Med. Parasitol. 64:209-222. [DOI] [PubMed] [Google Scholar]
- 30.Singh, C., H. Malik, and S. K. Puri. 2004. Synthesis and antimalarial activity of a new series of trioxaquines. Bioorg. Med. Chem. 12:1177-1182. [DOI] [PubMed] [Google Scholar]
- 31.Snow, R. W., C. A. Guerra, A. M. Noor, H. Y. Myint, and S. I. Hay. 2005. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434:214-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szpilman, A. M., E. E. Korshin, H. Rozenberg, and M. D. Bachi. 2005. Total syntheses of yingzhaosu A and of its C(14)-epimer including the first evaluation of their antimalarial and cytotoxic activities. J. Org. Chem. 70:3618-3632. [DOI] [PubMed] [Google Scholar]
- 33.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 34.Van Huyssen, W., and K. H. Rieckmann. 1993. Disposable environmental chamber for assessing the drug susceptibility of malaria parasites. Trop. Med. Parasitol. 44:329-330. [PubMed] [Google Scholar]
- 35.Vennerstrom, J. L., S. Arbe-Barnes, R. Brun, S. A. Charman, F. C. K. Chiu, J. Chollet, Y. Dong, A. Dorn, D. Hunziker, H. Matile, K. McIntosh, M. Padmanilayam, J. Santo Tomas, C. Scheurer, B. Scorneaux, Y. Tang, H. Urwyler, S. Wittlin, and W. N. Charman. 2004. Identification of an antimalarial synthetic trioxolane drug development candidate. Nature 430:900-904. [DOI] [PubMed] [Google Scholar]
- 36.von Seidlein, L., P. Milligan, M. Pinder, K. Bojang, C. Anyalebechi, R. Gosling, R. Coleman, J. I. Ude, A. Sadiq, M. Duraisingh, D. Warhurst, A. Alloueche, G. Targett, K. McAdam, B. Greenwood, G. Walraven, P. Olliaro, and T. Doherty. 2000. Efficacy of artesunate plus pyrimethamine-sulphadoxine for uncomplicated malaria in Gambian children: a double-blind, randomised, controlled trial. Lancet 355:352-357. [DOI] [PubMed] [Google Scholar]
- 37.Vuong, P. N., F. Richard, G. Snounou, F. Coquelin, L. Renia, F. Gonnet, A. G. Chabaud, and I. Landau. 1999. Development of irreversible lesions in the brain, heart and kidney following acute and chronic murine malaria infection. Parasitology 119:543-553. [DOI] [PubMed] [Google Scholar]
- 38.White, N., F. Nosten, S. Looareesuwan, W. Watkins, K. Marsh, R. Snow, G. Kokwaro, J. Ouma, T. Hien, M. Molyneux, T. Taylor, C. Newbold, T. Ruebush, M. Danis, B. Greenwood, R. Anderson, and P. Olliaro. 1999. Averting a malaria disaster. Lancet 353:1965-1967. [DOI] [PubMed] [Google Scholar]
- 39.WHO. 2001. Antimalarial drug combination therapy: report of a WHO technical consultation. Report WHO/CDS/RBM/2001.35. World Health Organization, Geneva, Switzerland.
- 40.Wiesner, J., D. Henschker, D. B. Hutchinson, E. Beck, and H. Jomaa. 2002. In vitro and in vivo synergy of fosmidomycin, a novel antimalarial drug, with clindamycin. Antimicrob. Agents Chemother. 46:2889-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wongsrichanalai, C., A. L. Pickard, W. H. Wernsdorfer, and S. R. Meshnick. 2002. Epidemiology of drug-resistant malaria. Lancet Infect. Dis. 2:209-218. [DOI] [PubMed] [Google Scholar]