Abstract
Over a 12-year period, 68 Shigella strains (31 S. sonnei, 30 S. flexneri, 4 S. dysenteriae, and 3 S. boydii strains) were collected in a French University Hospital from the stools of patients who generally had a recent history of travel to various parts of the world (91%), particularly Africa (67%). These strains were often resistant (streptomycin, spectinomycin, trimethoprim, tetracycline, and sulfonamides, 66 to 84%; ampicillin and chloramphenicol, 34 to 38%; nalidixic acid, 4%) and even multiresistant (87%), and they generally carried integrons (81%) of class 1 (21%), class 2 (47%), or both (13%). Class 1 integrons were associated with ampicillin resistance due to the production of an OXA-30 β-lactamase in S. flexneri and S. dysenteriae. Class 2 integrons were associated with trimethoprim resistance in S. sonnei. Class 1 and class 2 integrons were inserted within transposons Tn21 and Tn7, respectively, themselves located on the bacterial chromosome, except in one strain. Class 1 integrons showed an atypical organization consisting of the insertion sequence IS1 at the 3′ end instead of the typical 3′ conserved segment and two blaOXA-30 and aadA1 gene cassettes, despite the absence of epidemiological relationships between the strains, and an apparently functional integrase. Class 2 integrons showed the same albeit classical organization with the three dfrA1, sat, and aadA1 gene cassettes. Occasionally, the 3′ end was deleted and the aadA1 gene cassette was unexpressed. Thus, integrons contributed only in part to the multidrug resistance of the Shigella strains. The highly conserved organization of integrons might be related to their location within mobile genetic superstructures.
The four Shigella species Shigella boydii, Shigella dysenteriae, Shigella flexneri, and Shigella sonnei are responsible for gastroenteritis that may progress to mucoid bloody diarrhea, also known as dysentery. The most severe forms, encountered with S. dysenteriae type 1 (the “Shiga bacillus”) in children under 5 years of age, lead to a mortality rate of 10 to 30% during outbreaks. Shigella spp. can be transmitted by contaminated food and water and through person-to-person contact. In developing countries with unsafe water supplies and substandard hygiene, shigellosis is widespread and causes extensive outbreaks. In industrialized countries, this disease has become rare, and it currently occurs as sporadic cases in migrant workers or those who travel to developing countries and is limited to epidemic episodes among children in daycare centers, individuals in custodial institutions, and homosexual men (27). Consequently, although shigellosis is a major public health concern, there is a great disparity between developing countries (over 163.2 million cases each year) and developed ones (1.5 million cases) (15). In France, 941 cases of shigellosis was recorded in 1999, and the annual incidence has been stable in the past five years (2).
Shigellosis is one of the acute enteric disease for which antimicrobial therapy is generally required to manage infection and reduce fecal excretion of the bacterium to prevent further transmission. Although Shigella spp. are intrinsically susceptible to all antibiotics that are active against gram-negative bacilli, under antibiotic pressure, they have progressively acquired resistances to commonly recommended drugs (12, 19, 25, 28, 38, 41). Resistance dissemination among Shigella spp. is facilitated by the ability of this genus to acquire mobile genetic elements such as plasmids or transposons (15). Moreover, these elements may harbor integrons that can integrate resistance gene cassettes by site-specific recombination and then provide an efficient means for cumulating resistance determinants (3, 10, 34). Integrons and their association with multidrug resistance have been studied in most Enterobacteriaceae and gram-negative rods (16, 17, 32, 36, 37, 43), and several surveys have analyzed the distribution of the integrons in strains of Shigella spp. However, these studies were generally confined to a single species (5, 9, 22, 24, 28) or class of integron (9, 13, 22, 25, 26), in a restricted geographic area (5, 9, 13, 22, 24, 25, 28, 30, 31, 38), and in the course of outbreaks (5, 22, 24, 28), and the genetic location and organization of these structures have been rarely described, especially for class 1 integrons (30, 39).
In this study, we examined the antibiotic resistance and the full integron content of all Shigella strains isolated over a 12-year period in a French University hospital for adults, where shigellosis essentially corresponds to unrelated episodes of traveler's diarrhea. The integrons' location and organization have been established and have revealed an unexpectedly high genetic stability over this prolonged period of time and despite the worldwide origin of the strains.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
All isolates of Shigella spp. collected between January 1990 and November 2002 at the Saint-André Hospital of Bordeaux, France, which specializes in gastrointestinal diseases, were included in this study. They were identified to the genus and species levels using the API 20E system (bioMérieux, Marcy l'Etoile, France) and antisera provided by Bio-Rad (Marnes la Coquette, France). Serotyping was performed by the Institut Pasteur of Paris. Clinical cases were retrospectively analyzed to obtain epidemiological data, particularly recent travel (less than 1 month). Escherichia coli TOP10 and DH5α were the recipient strains in transformation experiments. E. coli Ec1484R (7), Acinetobacter baumannii BM4431 (32), and E. coli DH5α (p22K9) (4) were used as controls for the amplification of the intI1, intI2 and intI3 genes, respectively, and E. coli Ec1484R and E. coli Ec223 (6) were used for the amplification of the blaOXA-1 and blaTEM genes, respectively. All bacterial strains were routinely cultured at 37°C on Mueller-Hinton (MH) agar (Diagnostics Pasteur, Marnes la Coquette, France) or in brain heart (Bio-Rad) or trypticase soy (Diagnostics Pasteur) broth.
Antibiotic susceptibility testing.
Antibiotic susceptibility of the 68 isolates was determined for 27 antimicrobial agents (Bio-Rad) by the disk diffusion method in MH agar medium according to French guidelines (http://www.sfm.asso.fr).
β-Lactamase extraction and isoelectric focusing.
β-Lactamases of the 26 isolates of Shigella spp. that are resistant to amino- and carboxypenicillins were released by ultrasonic treatment, and their pIs were determined by isoelectric focusing on a pH 3.5 to 10 ampholin polyacrylamide gel as described previously by Matthew et al. (23). Enzyme activities were detected by the iodine procedure in gel by using benzylpenicillin (75 μl/ml) as the substrate. β-Lactamases with known pIs, TEM-1 (pI 5.4) and OXA-1 (pI 7.4), were used as pI markers.
Transformation experiments and plasmid content analysis.
Plasmid DNA was extracted from the 55 Shigella strains harboring an integron and E. coli DH5α carrying recombinant plasmids using an alkaline lysis method (1) and the QIAGEN Plasmid DNA Midi kit (QIAGEN, Courtaboeuf, France). Plasmid content was analyzed by electrophoresis on a 1% agarose gel and subsequent exposure to UV light in the presence of ethidium bromide. Plasmid DNA extracts obtained from the clinical strains were electroporated into E. coli TOP10 or E. coli DH5α cells with selection on MH agar plates containing either ampicillin (AMP) (100 μg/ml) or trimethoprim (TMP) (20 μg/ml).
PCR, sequencing, and cloning experiments.
Total DNA of Shigella species strains was extracted as previously described (8). Gene detection was carried out under standard PCR conditions (35) using primers specific for the intI1, intI2, intI3, blaTEM, and blaOXA-1 genes or laboratory-designed primers (Table 1) and 0.1 μg of whole-cell DNA of clinical isolates. A combination of laboratory-designed primers also served for the determination of the integrons' genetic organization in clinical strains and from recombinant plasmids and for the detection of the free circular gene cassette aadA1 in cultures of S. flexneri Sf631 and E. coli DH5α containing plasmid pC631E (Table 1) grown overnight. Amplification of large fragments (more than 3 kb) was performed with the GeneAmp XL-PCR kit (Applied Biosystems, Courtaboeuf, France) according to the manufacturer's instructions. The amplicons were revealed by electrophoresis on a 1.5% agarose gel and exposure to UV light in the presence of ethidium bromide. For sequencing purposes, the PCR products, purified through Sephacryl S400 spin columns (Amersham Biosciences, Orsay, France), and recombinant plasmids pC631E and pC2023B were used as a template in a single-cycle reaction by using laboratory-designed primers and the DYEnamic ET dye terminator kit (Amersham Biosciences). Sequences were analyzed with an ABI 310 automatic sequencer (Perkin-Elmer, Courtaboeuf, France) using the Sequencing Analysis software and were compared to each other and to homologous sequences with the Sequence Navigator software. The nucleotide and the deduced protein sequences were analyzed by using software available over the Internet at the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov).
TABLE 1.
Primers used in this study
| Primer | Sequence (5′→3′) | Location | Reference or source |
|---|---|---|---|
| 5′ Conserved segment | GGCATCCAAGCAGCAAG | 5′CS integron 1 | 18 |
| intI 1L | ACATGTGATGGCGACGCACGA | intI1 | 32 |
| intI 1R | ATTTCTGTCCTGGCTGGCGA | intI1 | 32 |
| intI 2L | CACGGATATGCGACAAAAAGGT | intI2 | 32 |
| intI 2R | GTAGCAAACGAGTGACGAAATG | intI2 | 32 |
| intI 3L | GCCTCCGGCAGCGACTTTCAG | intI3 | 32 |
| intI 3R | ACGGATCTGCCAAACCTGACT | intI3 | 32 |
| OXA1F | CACAATACATATCAACTTCGC | blaOXA-1 | 29 |
| OXA1R | GTGTGTTTAGAATGGTGATCGC | blaOXA-1 | 29 |
| TEM-A2 | GTATCCGCTCATGAGACAATA | blaTEM-1 | 8 |
| TEM-EXT | TCTAAAGTATATATGAGTAAAC | blaTEM-1 | 8 |
| TnpM | GTCAGGGAAGACTCTATGACC | tnpM (Tn21) | This study |
| INT1FIN | CCTCACTAGTGAGAGGTAGGG | intI1 | This study |
| OXA1Fin | TATGGGAAAACTGGTGCAGGA | blaOXA-1 | This study |
| AAD2DI | CAGGAACCGGATCAAAGAG | aadA1 | This study |
| AADVD | AAGGTAGTCGGCAAATAATG | aadA1 | This study |
| INSB | CTTTGTCATGCAGCTCCACC | IS1 (insb) | This study |
| INSBR | GGTGGAGCATGACAAAG | IS1 (insb) | This study |
| ORFINV | CCAGACTGCTCCTGTATGACC | IS600 (ORFB) | This study |
| INT2INV | ACCTTTTTGTCGCATATCCGTG | intI2 | This study |
| DFRA1-FIN | CATCGAGCCGGAAGGTGATG | dfrA1 | This study |
| DFRA1R | GTTAGAGGCGAAGTCTTGGG | dfrA1 | This study |
| ORFXINV | CCATTTATGACGACCAATGC | ORFX | This study |
| AP 12h | CGGCCCCTGT | 44 | |
| ERIC1R | ATGTAAGCTCCTGGGGATTCAC | 42 | |
| ERIC2 | AAGTAAGTGACTGGGGTGAGCG | 42 |
The whole-cell DNA of isolates Sf631 and Ss2023 was totally digested by the EcoRI or BamHI enzyme, respectively, and ligated into the corresponding site of the pBK-CMV cloning vector. E. coli DH5α strains harboring recombinant plasmids pC631E and pC2023B were selected on MH agar plates containing 100 μg/ml of ampicillin and 50 μg/ml of kanamycin or 20 μg/ml of trimethoprim and 50 μg/ml of kanamycin, respectively.
Hybridization.
DNA-DNA hybridization was carried out as described previously by Sambrook et al. (35). To determine the number of each class of integron, the template was a Southern transfer of an agarose gel containing total DNA digested by the EcoRI restriction enzyme from the 23 isolates containing a class 1 integron and the HindIII restriction enzyme from the 41 isolates harboring a class 2 integron. Probes consisted of a 569-bp and a 789-bp PCR fragment generated from total DNA of isolates Sf631 and Ss2023, respectively, and corresponded to parts of the intI1 and intI2 genes. To assess the chromosomal location of the integrons, undigested total DNA and plasmid DNA for three representative strains and blaOXA-30- and dfrA1-specific probes were used. Nonradioactive labeling of the probe and signal detection were achieved by following the manufacturer's instructions (Roche, Applied Science, Meylan, France).
Molecular typing.
For the 19 isolates of S. flexneri and the 4 isolates of S. dysenteriae harboring an integron, random amplified polymorphic DNA assay and enterobacterial repetitive intergenic consensus PCR were performed with primers AP12h (44), 208, and 272 (21) and ERIC2 and ERIC1R (42), respectively. After a first cycle of denaturation for 10 min at 94°C, the 45 subsequent cycles of amplification consisted of a denaturation step for 1 min at 94°C, an annealing step for 1 min at 42°C, and an extension step for 1 min at 72°C, with a final extension step for 10 min at 72°C. The amplification products were analyzed by electrophoresis of 10-μl samples on 1.5% agarose gels in the presence of ethidium bromide.
Nucleotide sequence accession numbers.
The sequences of the 5.4-kb insert of recombinant plasmid pC631E and part of the 16.6-kb insert of plasmid pC2023B are available in the GenBank nucleotide database under accession numbers DQ923619 and DQ975275, respectively.
RESULTS
Distribution of Shigella strains according to species and geographic origin.
Over a 12-year period (1990 to 2002), 68 nonrepetitive isolates of Shigella spp. were collected at the Saint-André Hospital of Bordeaux exclusively from stools of individuals who presented symptoms of infectious diarrhea at the time of isolation. Most of the strains were S. sonnei (46%) and S. flexneri (44%) strains (Table 2). When their past history was available, it appeared that most of the patients (52/57; 91%) had recently traveled, often to developing countries, mainly Africa (38/57; 67%) (Table 2). The African strains, like the European strains (including five apparently autochthonous strains), belonged mainly to the S. sonnei and S. flexneri species, while the Asiatic strains were essentially S. sonnei and S. dysenteriae species. Finally, the three S. boydii strains were isolated from patients who had returned from Africa. Most often, the disease corresponded to isolated cases of shigellosis, except for two epidemic episodes concerning four isolates of S. sonnei collected from patients who had returned from travel to Gabon and three isolates of S. sonnei from patients who had returned from a humanitarian mission in the Ivory Coast.
TABLE 2.
Distribution of Shigella strains
| Continenta | No. of strainsb
|
||||
|---|---|---|---|---|---|
| S. sonnei | S. flexneri | S. dysenteriae | S. boydii | Total | |
| Africa | 19 | 15 | 1 | 3 | 38 |
| Europe | 4 | 5 | 9 | ||
| Asia | 4 | 1 | 3 | 8 | |
| America | 1 | 1 | 2 | ||
| ND | 3 | 8 | 11 | ||
| Total | 31 | 30 | 4 | 3 | 68 |
Africa includes Algeria (n = 3), Burkina-Faso (n = 2), Cameroon (n = 1), Egypt (n = 1), Gabon (n = 4), Guinea (n = 2), Ivory Coast (n = 4), Madagascar (n = 4), Mali (n = 2), Mauritania (n = 1), Morocco (n = 3), Nigeria (n = 1), Senegal (n = 7), Sudan (n = 1), Togo (n = 1), and unknown country (n = 1); Europe includes France (n = 5), Portugal (n = 1), Romania (n = 1), and Spain (n = 2); Asia includes India (n = 4), Indonesia (n = 1), Syria (n = 1), Turkey (n = 1), and Vietnam (n = 1); America refers to Cuba (n = 2).
Includes S. sonnei biotype g (n = 31); S. flexneri serotypes 2 (n = 10), 6 (Boyd88) (n = 6), 3 (n = 3), 1 (n = 2), y (n = 2), and 5 (n = 1) and undetermined (n = 6); S. dysenteriae serotypes 1 (n = 2) and 3 (n = 2); and S. boydii serotype 1 (n = 1). ND, not determined.
Antimicrobial resistance of Shigella strains.
Most Shigella strains (60/68; 88%) presented at least one acquired resistance phenotype (Table 3). The four species were affected but at variable frequencies: all S. sonnei and S. dysenteriae, 80% of the S. flexneri, and only one-third of the S. boydii strains were affected. The most frequent resistances were to streptomycin (STR), spectinomycin (SPT), TMP, tetracycline (TET), and sulfonamides (SUL) (66 to 84%), followed by AMP and chloramphenicol (CHL) (34 to 38%). Nalidixic acid-resistant strains (4%, two from India and one of unknown origin) were infrequent, and none of them were resistant to pefloxacin. All strains were fully susceptible to expanded-spectrum cephalosporins, kanamycin, gentamicin, tobramycin, netilmicin, and amikacin. Most strains (59/68; 87%) were resistant to at least three antibiotics (without taking spectinomycin into account). A total of 13 antibiotic resistance phenotypes were identified. The profile STR-SPT TET SUL TMP was most frequent in S. sonnei strains (18 strains), and AMP STR-SPT TET CHL SUL TMP was most frequent in S. flexneri strains (16 strains). Ampicillin resistance, analyzed by isoelectric focusing, PCR amplification, and sequencing, was due to the production of an OXA-30 β-lactamase (pI 7.4) in most S. flexneri and all S. dysenteriae strains and of a TEM-1 enzyme (pI 5.4) in a single S. flexneri and two S. sonnei strains.
TABLE 3.
Antimicrobial resistance of Shigella strains
| Species | No. of isolates (%) | No. of resistant isolates (%)a
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AMP | STR | SPT | TET | CHL | SUL | TMP | NAL | Total | ||
| S. sonnei | 31 (45.6) | 2 (6.5) | 31 (100) | 28 (90.3) | 23 (74.2) | 23 (74.2) | 31 (100) | 2 (6.5) | 31 (100) | |
| S. flexneri | 30 (44.1) | 20 (66.7) | 22 (73.3) | 19 (63.3) | 22 (73.3) | 19 (63.3) | 20 (66.7) | 18 (60) | 1 (3.3) | 24 (80) |
| S. dysenteriae | 4 (5.9) | 4 (100) | 3 (75) | 3 (75) | 4 (100) | 4 (100) | 1 (25) | 2 (50) | 4 (100) | |
| S. boydii | 3 (4.4) | 1 (33.3) | 1 (33.3) | 1 (33.3) | 1 (33.3) | 1 (33.3) | 1 (33.3) | |||
| Total | 68 (100) | 26 (38.2) | 57 (83.8) | 51 (75) | 50 (73.5) | 23 (33.8) | 45 (66.2) | 52 (76.5) | 3 (4.4) | 60 (88.2) |
NAL, nalidixic acid.
Integron content and antibiotic resistance of the Shigella strains.
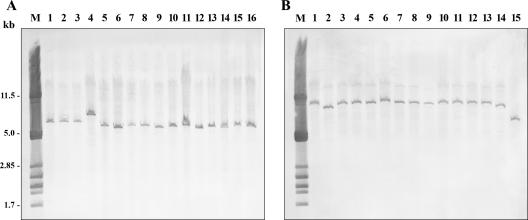
Most Shigella strains (55/68; 81%) were found to harbor integrons of one or two classes: 23 strains possessed a class 1 integron (42%), 41 strains possessed a class 2 integron (75%), and 9 strains had both classes of integron; no class 3 integrons were detected (Table 4). All the strains harboring an integron were multidrug resistant (two to six resistance markers), and only five resistant strains of S. flexneri did not contain any integron. None of the eight susceptible strains possessed an integrase gene. The distribution of the integrons varied according to the species and the resistance phenotype. The S. sonnei and S. boydii strains contained a single integron of class 2, while the S. flexneri and S. dysenteriae strains carried a class 1 integron alone or associated with a class 2 integron. All strains harboring a class 1 integron were resistant to ampicillin due to the production of an OXA-30 enzyme and to streptomycin-spectinomycin (except for S. dysenteriae strain Sd629), tetracycline, and chloramphenicol, and the most prevalent phenotype was AMP STR-SPT TET CHL SUL TMP (17/23, 74%). All of the strains containing a class 2 integron were resistant to trimethoprim and to streptomycin-spectinomycin (except for four strains), and the predominant phenotype was STR-SPT TET SUL TMP (19/41; 46%). Southern blot experiments carried out using the total DNA digested by EcoRI (class 1 integrons) or HindIII (class 2 integrons) produced, in all cases, a single band at a variable size (Fig. 1), indicating that a single copy of each class of integron was present.
TABLE 4.
Antimicrobial resistance of Shigella strains harboring an integrona
| Species (no. of strains) | Integrase 1 gene
|
Integrase 2 gene
|
Integrase 1 + 2 genes
|
Total no. of resistant strains | |||
|---|---|---|---|---|---|---|---|
| No. of strains | Resistance phenotype (no. of isolates) | No. of strains | Resistance phenotype (no. of isolates) | No. of strains | Resistance phenotype (no. of isolates) | ||
| S. sonnei (31) | 0 | 31 | STR-SPT TMP (8) | 0 | 31 | ||
| STR TET SUL TMP (1) | |||||||
| STR TET SUL TMP NAL (2) | |||||||
| STR-SPT TET SUL TMP (18) | |||||||
| AMP STR-SPT TET SUL TMP (2) | |||||||
| S. flexneri (30) | 12 | AMP STR-SPT TET CHL (3) | 0 | 7 | AMP STR-SPT TET CHL SUL TMP (7) | 19 | |
| AMP STR-SPT TET CHL SUL TMP (9) | |||||||
| S. dysenteriae (4) | 2 | AMP STR-SPT TET | 0 | 2 | AMP TET CHL TMP (1) | 4 | |
| CHL (2) | AMP STR-SPT TET CHL SUL TMP (1) | ||||||
| S. boydii (3) | 0 | 1 | STR-SPT TET SUL TMP (1) | 0 | 1 | ||
| Total (68) | 14 | 32 | 9 | 55 | |||
NAL, nalidixic acid.
FIG. 1.
Southern blotting and hybridization with intI1 (A)- or intI2 (B)-specific probes. Lane M, size marker consisting of λ phage DNA digested by PstI. (A) DNA of 14 strains of S. flexneri (lanes 1 to 14) and 2 strains of S. dysenteriae (lanes 15 and 16) digested with EcoRI. (B) DNA of 14 strains of S. sonnei (lanes 1 to 14) and 1 strain of S. boydii (lane 15) digested with HindIII.
Genetic location and structure of integrons.
Plasmid content analysis by electrophoresis in agarose gels yielded several bands for all Shigella strains harboring an integron. However, transformation experiments using these plasmid DNA extracts failed to give any transformants except for a single strain of S. flexneri (Sf2032), which contained a plasmid that was found to be conjugative and to harbor a class 2 integron. These results suggested a chromosomal location of the integrons in nearly all cases. Southern blot hybridization with undigested total DNA and plasmid DNA of three representative strains (Sf631, Ss2023, and Sf633 containing both classes of integrons) and using blaOXA-30- or dfrA1-specific probes also argued for the chromosomal location of these elements (data not shown).
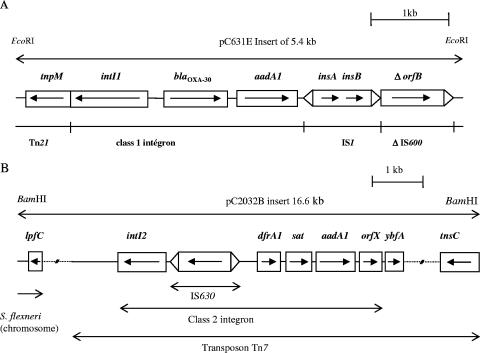
In order to determine the gene cassette arrays of the class 1 integrons, cloning of ampicillin resistance from S. flexneri strain Sf631 exhibiting the prevalent phenotype was undertaken. The obtained clone carried recombinant plasmid pC631E, and sequencing of the 5.4-kb insert revealed the presence of an atypical class 1 integron encompassing the intI1 gene, the attI recombination site, two cassettes including the blaOXA-30 and aadA1 genes associated with their attC recombination sites, and the insertion sequence IS1 instead of the conventional 3′ conserved segment end (Fig. 2). This element was inserted at its 5′ end downstream from the tnpM gene of the transposon Tn21 and at its 3′ end in the orfB of IS600. PCR experiments with the 22 remaining strains of Shigella containing the intI1 gene (18 S. flexneri and 4 S. dysenteriae isolates) showed the same atypical integron structure up to IS1, except for S. flexneri strain Sf2016, in which the aadA1 gene cassette and the IS1 element were absent. Likewise, for all strains, the insertion sites in Tn21 were identical.
FIG. 2.
Schematic representation of part of recombinant plasmid pC631E containing the atypical class 1 integron in strain Sf631 (A) and of recombinant plasmid pC2023B containing the class 2 integron in strain Ss2023 (B). The horizontal arrows indicate the translation orientation. The solid lines represent the sequenced fragments, with the different genes boxed, and the dotted lines indicate the unanalyzed sequences.
In order to determine the gene cassette arrays of the class 2 integrons, cloning of trimethoprim resistance from S. sonnei strain Ss2023, presenting the predominant phenotype, was achieved. The clone obtained carried recombinant plasmid pC2023B, and sequencing of a part of the 16.6-kb insert revealed the presence of a classical class 2 integron comprising the dfrA1, sat, and aadA1 gene cassettes and orfX, located in the Tn7 transposon (Fig. 2). However, in pC2023B, the class 2 integron was interrupted by IS630, inserted between intI2 and the attI2 recombination site, and there was a 42-bp deletion within orfX. PCR experiments with the 40 remaining strains of Shigella containing the intI2 gene (30 S. sonnei strains, 7 S. flexneri strains, 2 S. dysenteriae strains, and 1 S. boydii strain) showed the same organization of the class 2 integron as in Ss2023 (but without IS630 and the orfX deletion) for 34 strains (83%). The lack of orfX was observed once, and the absence of a fragment encompassing the aadA1 gene cassettes and orfX was detected in five cases (three streptomycin-resistant S. sonnei isolates, one S. flexneri isolate harboring a class 1 integron, and S. dysenteriae Sd629).
Epidemiological relationship between Shigella strains harboring class 1 integrons.
The surprising conservation of the genetic organization of the class 1 integrons led us to check the relationship between the carrying strains. Serotyping, plasmid profile analysis, and molecular typing by random amplified polymorphic DNA and enterobacterial repetitive intergenic consensus PCR (data not shown) demonstrated an absence of an epidemiological link between the isolates of S. flexneri or S. dysenteriae. Moreover, the set of primers amplifying the junction between IS1 and IS600 for the 23 strains containing the intI1 gene gave fragments of variable sizes (619 bp for Sf631 and another strain and ca. 750 bp for 10 strains), several fragments (five strains), or no amplicons at all (six strains), confirming that these strains were unrelated. Sequencing of the 750-bp fragment in an S. dysenteriae strain showed that the atypical integron was inserted within IS600, 125 bp upstream compared to Sf631.
Integrase and cassette gene expression.
With regard to class 1 integrons, integrase expression was tested by PCR detection (primers AAD2DI and AADVD) of the free circular gene cassette aadA1 in Sf631 and E. coli DH5α containing pC631E. An expected fragment of 550 bp was obtained only with E. coli strain DH5α harboring the recombinant plasmid, and sequencing confirmed the presence of the free circular gene cassette in three independent assays. The first blaOXA-30 gene cassette was always expressed in E. coli DH5α containing pC631E and in the clinical strains harboring the atypical class 1 integron, whereas the streptomycin-spectinomycin resistance conferred by the second gene cassette was lacking in S. dysenteriae strain Sd629 despite the presence of the aadA1 gene.
Concerning the class 2 integrons, the IS630 element between intI2 and attI2 in Sf2023 did not affect the expression of the gene cassette in E. coli DH5α containing pC2023B. When the genes were present, the clinical strains were resistant to trimethoprim and to streptomycin-spectinomycin, suggesting that the gene cassettes were regularly expressed. However, the conjugative plasmid of strain Ss2032 conferred only trimethoprim resistance despite the presence of the aadA1 gene, but this strain also harbored a chromosomally encoded class 1 integron likely responsible for its streptomycin-spectinomycin resistance.
DISCUSSION
Analysis of antibiotic resistance and its integron basis in Shigella spp. isolated in an industrialized country offers the opportunity to compare strains from various geographic origins, as observed in our study. The endemic species S. sonnei and S. flexneri were more prevalent than the epidemic species S. dysenteriae, as expected (2, 26). The S. boydii strains came from Africa rather than from India, as usually reported (27), but the geographic origin of the Shigella strains in industrialized countries is influenced by the preferential destinations of tourists and migrants.
Antibiotic resistance of Shigella spp. is usually frequent, particularly towards streptomycin, trimethoprim, tetracycline, and sulfonamides (14, 28). Data on ampicillin and chloramphenicol resistance rates and their distribution according to species vary greatly (5, 14, 22, 24, 25, 28, 31). Ampicillin, chloramphenicol, and cotrimoxazole are inexpensive antibiotics that have been used extensively for the treatment of shigellosis. Our study confirmed that they are now generally inefficient in the empirical treatment of this disease, even in industrialized countries. Quinolone-resistant strains were rare, in contrast with some recent Asiatic surveys (12, 14, 28), but they came from India.
Most Shigella strains harbored one or two classes of integron. Variable but often lower rates were reported elsewhere previously (5, 13, 26, 38). The apparent correlation between multidrug resistance and integron carriage was not related to the presence of these elements on conjugative plasmids, since they were chromosomally mediated in nearly all strains. When it has been documented, the genetic locations of antibiotic resistance genes and integrons in Shigella spp. have been found to be variable. Thus, class 2 integrons and blaOXA genes were usually located on the chromosome, while the blaTEM genes were commonly carried by conjugative plasmids (14, 28, 30, 31, 33, 38).
The distribution of the integrons correlated to some extent with the species and the resistance phenotype. Such a relationship has been inconstantly observed. Indeed, the predominance of class 2 integrons in S. sonnei has been previously observed, but in studies focusing on genetically related strains responsible for outbreaks (5, 9, 22, 24, 28, 30, 31), no class 2 integrons were detected in S. sonnei in one study (38), and integrons of class 1 have sometimes been reported in this species. For S. flexneri, the data concerning the integron content were still more discrepant: in a study from Brazil, class 2 integrons predominated (31); in other studies low (26) or high (25) class 1 integron rates were reported, and in the latter works, high frequencies of class 1 and 2 integrons were detected (30, 38). Finally, little information on the integron content of S. boydii and S. dysenteriae strains and on the absence of class 3 integrons in Shigella spp is available. The higher prevalence of the OXA-1-type β-lactamase in S. flexneri and S. dysenteriae and the higher prevalence of the TEM-1-type enzyme in S. sonnei have been reported previously (14, 25, 30, 31, 38), but the reason why the OXA-30 enzyme is widespread in some Shigella species although the TEM-1 enzyme is by far the most common β-lactamase responsible for ampicillin resistance in the taxonomically related species E. coli and in other Enterobacteriaceae remains unexplained.
Cloning and PCR experiments showed that almost all class 1 integrons of Shigella spp. exhibited the same atypical structure. The lower rates of class 1 integrons in S. flexneri mentioned previously in some studies (26, 31) might be due to the use of primers amplifying classical integrons with the 3′ conserved segment fragment. A similarly atypical class 1 integron on the chromosome of S. flexneri 2a strain YSH6000 isolated in Japan was described previously (33). This integron was located in the Shigella resistance locus (SRL) of 16.7 kb, which encompasses chloramphenicol and tetracycline resistance determinants within a 66-kb pathogenicity island (PAI) designated the SRL PAI (20). The insertion of the class 1 integrons within the SRL might explain the chloramphenicol and tetracycline resistances observed in all our S. flexneri and S. dysenteriae strains containing a class 1 integron. The sequence of the 5.4-kb insert of pC631E shared 98.8% identity with the corresponding sequence of YSH6000 (GenBank accession number AF326777), with the major difference being the deletion of a 57-bp fragment in the central region of IS600 in strain YSH6000. Similar fragments containing intI1, blaOXA-30, and aadA1 genes and part of the IS1 sequence have also been found in strains of various Shigella species from Japan and Australia (39) and the People's Republic of China (30). Thus, this chromosomal structure of resistance determinants previously observed in Asia and Oceania (30, 39) was also present in strains isolated in Africa, Europe, and Central America. The major sequence difference among our strains was the insertion of IS1 within orfB of IS600, suggesting that the integration of the IS1-flanked fragment into the PAI might be due to independent events in these strains, especially in different species such as S. flexneri and S. dysenteriae. Indeed, the PAI involves several insertion sequence-flanked elements that can be lost under specific conditions (40). Thus, it is possible that insertion sequence regions may be deleted or inserted independently at hot spots, such as orfB of IS600, as is the case for the integration of class 1 integrons within the transposons of the Tn3 family. The absence of a relationship between the strains led us to question about the functionality of the integrase to explain such conserved genetic organization. Preliminary experiments on the excision capability of the enzyme have suggested that IntI1 might be functional but repressed in its chromosomal location. Similarly, the absence of expression of the second gene cassette in one strain of S. dysenteriae, corroborated by previous reports (30), raises the question of a regulatory mechanism.
Class 2 integrons in Shigella strains, in contrast, had a classical organization (11) despite some variations, particularly a more frequent 3′-end deletion than in class 1 integrons. Since an internal stop codon within the intI2 gene renders the type 2 integrase nonfunctional, the cassette arrays in class 2 integrons are usually constant (5, 9, 24, 30, 31). The absence of expression of the aadA1 gene cassette was observed once, suggesting the existence of a regulatory process, as for class 1 integrons, but the literature does not provide much information on this aspect.
In conclusion, antibiotic resistance of Shigella spp. in industrialized countries appeared to be frequent and related to their epidemiology in developing countries. Most strains carried class 1 and/or class 2 integrons that contributed only in part to the multidrug resistance of Shigella spp., and their chromosomal location should limit resistance dissemination. This highly conserved organization of the class 1 integron in unrelated Shigella strains might be linked to the insertion and stabilization of these elements within mobile genetic superstructures such as SRL and PAI, depending on mechanisms that should be explored further, as the expression of the distal gene cassettes.
Acknowledgments
We are grateful to M. C. Ploy and A. Duarte for providing to us A. baumannii strain BM4431 and E. coli DH5α harboring plasmid p22K9, respectively.
This work was supported by grants from the Ministère de l'Education Nationale et de la Recherche (EA-525), Université de Bordeaux 2, Bordeaux, France.
Footnotes
Published ahead of print on 22 January 2007.
REFERENCES
- 1.Birnboim, H. C., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouvet, P., and P. Grimond. 2001. Données de surveillance 1999 du Centre National de Référence des Salmonella et Shigella. Bull. Epidémiol. Hebd. 12:49-52. [Google Scholar]
- 3.Collis, C. M., G. Grammaticopoulos, J. Briton, H. W. Stokes, and R. M. Hall. 1993. Site-specific insertion of gene cassettes into integrons. Mol. Microbiol. 9:41-52. [DOI] [PubMed] [Google Scholar]
- 4.Correia, M., F. Boavida, F. Grosso, M. J. Salgado, L. M. Lito, J. M. Cristino, S. Mendo, and A. Duarte. 2003. Molecular characterization of a new class 3 integron in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 47:2838-2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeLappe, N., F. O'Halloran, S. Fanning, G. Corbett-Feeney, T. Cheasty, and M. Cormican. 2003. Antimicrobial resistance and genetic diversity of Shigella sonnei isolates from western Ireland, an area of low incidence of infection. J. Clin. Microbiol. 41:1919-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubois, V., C. Arpin, P. Noury, C. Andre, L. Coulange, and C. Quentin. 2005. Prolonged outbreak of infection due to TEM-21-producing strains of Pseudomonas aeruginosa and enterobacteria in a nursing home. J. Clin. Microbiol. 43:4129-4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubois, V., C. Arpin, C. Quentin, J. Texier-Maugein, L. Poirel, and P. Nordmann. 2003. Decreased susceptibility to cefepime in a clinical strain of Escherichia coli related to plasmid- and integron-encoded OXA-30 β-lactamase. Antimicrob. Agents Chemother. 47:2380-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubois, V., L. Poirel, C. Marie, C. Arpin, P. Nordmann, and C. Quentin. 2002. Molecular characterization of a novel class 1 integron containing blaGES-1 and a fused product of aac3-Ib/aac6′-Ib′ gene cassettes in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 46:638-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gassama-Sow, A., M. H. Diallo, C. S. Boye, B. Garin, J. M. Sire, A. I. Sow, and A. Aidara-Kane. 2006. Class 2 integron-associated antibiotic resistance in Shigella sonnei isolates in Dakar, Senegal. Int. J. Antimicrob. Agents 27:267-270. [DOI] [PubMed] [Google Scholar]
- 10.Hall, R. M., and C. M. Collis. 1995. Mobile gene cassettes and integrons: capture and spread of genes by site-specific recombination. Mol. Microbiol. 15:593-600. [DOI] [PubMed] [Google Scholar]
- 11.Hansson, K., L. Sundström, A. Pelletier, and P. H. Roy. 2002. IntI2 integron integrase in Tn7. J. Bacteriol. 184:1712-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirose, K., J. Terajima, H. Izumiya, K. Tamura, E. Arakawa, N. Takai, and H. Watanabe. 2005. Antimicrobial susceptibility of Shigella sonnei isolates in Japan and molecular analysis of S. sonnei isolates with reduced susceptibility to fluoroquinolones. Antimicrob. Agents Chemother. 49:1203-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iversen, J., D. Sandvang, A. Srijan, P. D. Cam, and A. Dalsgaard. 2003. Characterization of antimicrobial resistance, plasmids, and gene cassettes in Shigella spp. from patients in Vietnam. Microb. Drug Resist. 9:S17-24. [DOI] [PubMed] [Google Scholar]
- 14.Jeong, Y. S., J. C. Lee, H. Y. Kang, H. S. Yu, E. Y. Lee, C. H. Choi, S. H. Tae, Y. C. Lee, D. T. Cho, and S. Y. Seol. 2003. Epidemiology of nalidixic acid resistance and TEM-1- and TEM-52-mediated ampicillin resistance of Shigella sonnei isolates obtained in Korea between 1980 and 2000. Antimicrob. Agents Chemother. 47:3719-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotloff, K. L., J. P. Winickoff, B. Ivanoff, J. D. Clemens, D. L. Swerdlow, P. J. Sansonetti, G. K. Adak, and M. M. Levine. 1999. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull. W. H. O. 77:651-666. [PMC free article] [PubMed] [Google Scholar]
- 16.Leverstein-van Hall, M. A., A. T. Box, H. E. Blok, A. Paauw, A. C. Fluit, and J. Verhoef. 2002. Evidence of extensive interspecies transfer of integron-mediated antimicrobial resistance genes among multidrug-resistant Enterobacteriaceae in a clinical setting. J. Infect. Dis. 186:49-56. [DOI] [PubMed] [Google Scholar]
- 17.Leverstein-van Hall, M. A., H. E. M. Blok, A. R. T. Donders, A. Paauw, A. C. Fluit, and J. Verhoef. 2003. Multidrug resistance among Enterobacteriaceae is strongly associated with the presence of integrons and is independent of species or isolate origin. J. Infect. Dis. 187:251-259. [DOI] [PubMed] [Google Scholar]
- 18.Levesque, C., L. Piche, C. Larose, and P. H. Roy. 1995. PCR mapping of integrons reveals several novel combinations of resistance genes. Antimicrob. Agents Chemother. 39:185-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lima, A. A., N. L. Lima, M. C. Pinho, E. A. Barros, Jr., M. J. Teixeira, M. C. Martins, and R. L. Guerrant. 1995. High frequency of strains multiply resistant to ampicillin, trimethoprim-sulfamethoxazole, streptomycin, chloramphenicol, and tetracycline isolated from patients with shigellosis in northeastern Brazil during the period 1988 to 1993. Antimicrob. Agents Chemother. 39:256-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luck, S. N., S. A. Turner, K. Rajakumar, H. Sakellaris, and B. Adler. 2001. Ferric dicitrate transport system (Fec) of Shigella flexneri 2a YSH6000 is encoded on a novel pathogenicity island carrying multiple antibiotic resistance genes. Infect. Immun. 69:6012-6021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahenthiralingam, E., M. E. Campbell, J. Foster, J. S. Lam, and D. P. Speert. 1996. Random amplified polymorphic DNA typing of Pseudomonas aeruginosa isolates recovered from patients with cystic fibrosis. J. Clin. Microbiol. 34:1129-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mammina, C., M. Pontello, A. Dal Vecchio, and A. Nastasi. 2005. Identification of Shigella sonnei biotype g isolates carrying class 2 integrons in Italy (2001 to 2003). J. Clin. Microbiol. 43:2467-2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matthew, M., A. M. Harris, M. J. Marshall, and G. W. Ross. 1975. The use of analytical isoelectric focusing for detection and identification of β-lactamases. J. Gen. Microbiol. 88:169-178. [DOI] [PubMed] [Google Scholar]
- 24.McIver, C. J., P. A. White, L. A. Jones, T. Karagiannis, J. Harkness, D. Marriott, and W. D. Rawlinson. 2002. Epidemic strains of Shigella sonnei biotype g carrying integrons. J. Clin. Microbiol. 40:1538-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navia, M. M., L. Capitano, J. Ruiz, M. Vargas, H. Urassa, D. Schellemberg, J. Gascon, and J. Vila. 1999. Typing and characterization of mechanisms of resistance of Shigella spp. isolated from feces of children under 5 years of age from Ifakara, Tanzania. J. Clin. Microbiol. 37:3113-3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Navia, M. M., J. Ruiz, and J. Vila. 2004. Molecular characterization of the integrons in Shigella strains isolated from patients with traveler's diarrhea. Diagn. Microbiol. Infect. Dis. 48:175-179. [DOI] [PubMed] [Google Scholar]
- 27.Niyogi, S. K. 2005. Shigellosis. J. Microbiol. 43:133-143. [PubMed] [Google Scholar]
- 28.Oh, J. Y., H. S. Yu, S. K. Kim, S. Y. Seol, D. T. Cho, and J. C. Lee. 2003. Changes in patterns of antimicrobial susceptibility and integron carriage among Shigella sonnei isolates from southwestern Korea during epidemic periods. J. Clin. Microbiol. 41:421-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ouellette, M., G. C. Paul, A. M. Philippon, and P. H. Roy. 1988. Oligonucleotide probes (TEM-1, OXA-1) versus isoelectric focusing in β-lactamase characterization of 114 resistant strains. Antimicrob. Agents Chemother. 32:397-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan, J. C., R. Ye, D. M. Meng, W. Zhang, H. Q. Wang, and K. Z. Liu. 2006. Molecular characteristics of class 1 and class 2 integrons and their relationships to antibiotic resistance in clinical isolates of Shigella sonnei and Shigella flexneri. J. Antimicrob. Chemother. 58:288-296. [DOI] [PubMed] [Google Scholar]
- 31.Peirano, G., Y. Agerso, F. M. Aarestrup, and D. dos Prazeres Rodrigues. 2005. Occurrence of integrons and resistance genes among sulphonamide-resistant Shigella spp. from Brazil. J. Antimicrob. Chemother. 55:301-305. [DOI] [PubMed] [Google Scholar]
- 32.Ploy, M. C., F. Denis, P. Courvalin, and T. Lambert. 2000. Molecular characterization of integrons in Acinetobacter baumannii: description of a hybrid class 2 integron. Antimicrob. Agents Chemother. 44:2684-2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajakumar, K., D. Bulach, J. Davies, L. Ambrose, C. Sasakawa, and B. Adler. 1997. Identification of a chromosomal Shigella flexneri multi-antibiotic resistance locus which shares sequence and organizational similarity with the resistance region of the plasmid NR1. Plasmid 37:159-168. [DOI] [PubMed] [Google Scholar]
- 34.Recchia, G. D., and R. M. Hall. 1995. Gene cassettes: a new class of mobile element. Microbiology 141:3015-3027. [DOI] [PubMed] [Google Scholar]
- 35.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 36.Severino, P., and V. D. Magalhaes. 2002. The role of integrons in the dissemination of antibiotic resistance among clinical isolates of Pseudomonas aeruginosa from an intensive care unit in Brazil. Res. Microbiol. 153:221-226. [DOI] [PubMed] [Google Scholar]
- 37.Seward, R. J. 1999. Detection of integrons in worldwide nosocomial isolates of Acinetobacter spp. Clin. Microbiol. Infect. 5:308-318. [DOI] [PubMed] [Google Scholar]
- 38.Toro, C. S., M. Farfan, I. Contreras, O. Flores, N. Navarro, G. C. Mora, and V. Prado. 2005. Genetic analysis of antibiotic-resistance determinants in multidrug-resistant Shigella strains isolated from Chilean children. Epidemiol. Infect. 133:81-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turner, S. A., S. N. Luck, H. Sakellaris, K. Rajakumar, and B. Adler. 2003. Molecular epidemiology of the SRL pathogenicity island. Antimicrob. Agents Chemother. 47:727-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turner, S. A., S. N. Luck, H. Sakellaris, K. Rajakumar, and B. Adler. 2001. Nested deletions of the SRL pathogenicity island of Shigella flexneri 2a. J. Bacteriol. 183:5535-5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vasilev, V., R. Japheth, R. Yishai, and N. Andorn. 2004. Antimicrobial resistance of Shigella flexneri serotypes in Israel during a period of three years: 2000-2002. Epidemiol. Infect. 132:1049-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Versalovic, J., T. Koeuth, and J. R. Lupski. 1991. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 19:6823-6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White, P. A., C. J. McIver, and W. D. Rawlinson. 2001. Integrons and gene cassettes in the Enterobacteriaceae. Antimicrob. Agents Chemother. 45:2658-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams, J. G., A. R. Kubelik, K. J. Livak, J. A. Rafalski, and S. V. Tingey. 1990. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 18:6531-6535. [DOI] [PMC free article] [PubMed] [Google Scholar]