Abstract
The rise in the rates of glycopeptide resistance among Staphylococcus aureus isolates is concerning and underscores the need for the development of novel potent compounds. Ceragenins CSA-8 and CSA-13, cationic steroid molecules that mimic endogenous antimicrobial peptides, have previously been demonstrated to possess broad-spectrum activities against multidrug-resistant bacteria. We examined the activities of CSA-8 and CSA-13 against clinical isolates of vancomycin-intermediate S. aureus (VISA), heterogeneous vancomycin-intermediate S. aureus (hVISA), as well as vancomycin-resistant S. aureus (VRSA) and compared them to those of daptomycin, linezolid, and vancomycin by susceptibility testing and killing curve analysis. We also examined CSA-13 for its concentration-dependent activity, inoculum effect, postantibiotic effect (PAE), and synergy in combination with various antimicrobials. Overall, the MICs and minimal bactericidal concentrations of CSA-13 were fourfold lower than those of CSA-8. Time-kill curve analysis of the VRSA, VISA, and hVISA clinical isolates demonstrated concentration-dependent bactericidal killing. An inoculum effect was also observed when a higher starting bacterial density was used, with the time required to achieve 99.9% killing reaching 1 h with a 6-log10-CFU/ml starting inoculum, whereas it was ≥24 h with a 8- to 9-log10-CFU/ml starting inoculum with 10× the MIC (P ≤ 0.001). A concentration-dependent PAE was demonstrated with CSA-13, nearly doubling from 2× to 4× the MIC (P = 0.03). With respect to the CSA-13 antimicrobial combinations, time-kill curve analysis showed no difference in the log10 CFU/ml at 24 h for the majority of the organisms tested. However, early synergy at 4 to 8 h was detected against the VRSA Pennsylvania strain (2002) when CSA-13 was tested in combination with gentamicin, while early additivity was demonstrated against all of the other organisms.
Staphylococcus aureus is a common, virulent pathogen associated with a variety of infections, ranging from moderate to severe, in the community as well as in the nosocomial setting. The current National Nosocomial Infection Surveillance System report describes an increase in the proportion of hospital intensive care units in which Staphylococcus aureus is resistant to methicillin from 35.9% in 1992 to 64.4% in 2003 (13, 19). Infections with methicillin-resistant S. aureus (MRSA) have led to a significant increase in the use of vancomycin, with the consequential development of intermediate and resistant pathogens, such as vancomycin-resistant enterococci, vancomycin-intermediate S. aureus (VISA), heterogeneous vancomycin-intermediate S. aureus (hVISA), and vancomycin-resistant S. aureus (VRSA).
The rise of multidrug resistance has prompted renewed interest in the development of novel antimicrobial agents. It is not surprising that pathogenic species have adopted survival mechanisms to counteract both old and new antimicrobials (2). The drive to produce newer agents targeting novel sites that may circumvent resistance is critical to the long-term control of bacterial infection. One frequently studied target is the bacterial membrane. This is an appealing target, given that most structural elements are conserved and resistance to membrane-targeting antibiotics would require major changes in the membrane structure, which may influence the permeability barrier (20). Many agents that target the bacterial membrane are cationic, facially amphiphilic molecules, including endogenous antimicrobial peptides. Unlike typical amphiphilic compounds, facially amphiphilic molecules display separate hydrophilic and hydrophobic faces. Examples of facially amphiphilic antimicrobial peptides include the magainins and the human antimicrobial peptide LL37. Most antimicrobial peptides display broad-spectrum antibacterial activities and target the bacterial membrane. However, many antimicrobial peptides are difficult to synthesize and purify due to their complexity and size (23). In addition, antimicrobial peptides can be substrates for proteases, which limit their in vivo half-lives.
Recently, facially amphiphilic compounds designed to mimic the activities of antimicrobial peptides have been generated from a steroid scaffolding. Li and colleagues prepared multiple series of cholic acid derivatives with amine groups appended on one face of the steroid (for example, see Fig. 1), and this non-peptide-based approach has yielded a class of compounds termed ceragenins with antibacterial activities comparable to those of antimicrobial peptides (10, 15, 17). Given that the amphiphilic properties of ceragenins and antimicrobial peptides are similar, it has been postulated that these agents share a mechanism of action that includes the depolarization of bacterial membranes (9). As a consequence of the bacterial membrane activities of ceragenins, they effectively permeabilize the outer membranes of gram-negative bacteria, thus making these organisms susceptible to hydrophobic antibiotics (12, 16).
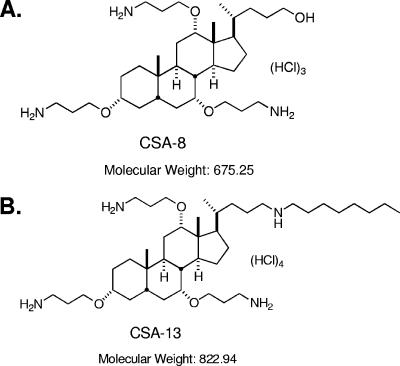
FIG. 1.
(A) Chemical structure of CSA-8 (molecular weight, 675.25); (B) chemical structure of CSA-13 (molecular weight, 822.94) (12).
Many of the ceragenins reported in the literature and at recent scientific meetings display broad-spectrum antibacterial activities. Some of these agents, such as CSA-13, are bactericidal at low concentrations, with similar MICs and minimal bactericidal concentrations (MBCs). Savage et al. found that the covalent attachment of CSA-13 to a polymer backbone to generate thin films inhibited bacterial growth and may be helpful in controlling bacterial infections associated with medical devices (P. B. Savage, D. Michaelis, T. Orsak, J. Nielson, J. Ostler, S. Salt, and C. Genbert, Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-1235, 2005). MIC testing with common gram-negative aerobic and facultative bacilli, strict anaerobes such as Propionibacterium acnes and Clostridium perfringens, as well as multidrug resistant S. aureus demonstrated that CSA-13 possess a broad spectrum of activity (P. B. Savage, H. F. Chambers, C. Genberg, R. N. Jones, J. Nielson, T. Orsak, J. Ostler, and T. R. Fritshe, Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-1231, 2005; P. B. Savage, H. F. Chambers, C. Genberg, R. N. Jones, J. Nielson, T. Orsak, J. Ostler, and T. R. Fritshe, 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-1232, 2005). Bogdanovich et al. found that CSA-13 was bactericidal against select strains of VRSA, VISA, and vancomycin-intermediate coagulase-negative staphylococci at 4× the MIC at 24 h in time-kill studies (T. Bogdanovich, P. B. Savage, C. Genberg, and P. C. Appelbaum, Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-1233, 2005).
To better understand the antibacterial activities of ceragenins, we compared the activities of two representative compounds (CSA-8 and CSA-13) to those of daptomycin, linezolid, and vancomycin against clinical isolates of VISA, hVISA, as well as VRSA. We also examined CSA-13 for concentration-dependent activity, inoculum effect, postantibiotic effect (PAE), and synergy in combination with various antimicrobials.
MATERIALS AND METHODS
Bacterial strains.
Fifty clinical isolates of hVISA and VISA as well as 4 clinical isolates of VRSA from NARSA and the clinical collections of Keiichi Hiramatsu, Tokyo, Japan, and the Anti-Infective Research Laboratory, Detroit, MI, were used for susceptibility testing (Mu50; MI-14379; NJ-992; Mu3; hVISA SA-118; VISA SA-179; ATCC 25923 control; SA-494 control; and four clinical strains of VRSA, including the Pennsylvania, Michigan, and New York strains) (4-6, 18). All hVISA strains were identified by subpopulation analysis. Four clinical isolates were selected for time-kill analysis as well as for evaluation of the postantibiotic effects. They include the prototypical strain VISA Mu50, hVISA Mu3, and VRSA strains from Pennsylvania and Michigan (VRSA-PA [2002]) and VRSA-MI [2002], respectively).
Antimicrobial agents.
CSA-8 and CSA-13 were synthesized and provided by one of the authors (Paul B. Savage). Linezolid (analytical grade) was provided by Pfizer, Inc. Vancomycin, doxycycline, and gentamicin (analytical grade) were obtained from Sigma-Aldrich. Tigecycline and daptomycin (analytical-grade powders) were provided by Wyeth and Cubist Pharmaceuticals, respectively.
Medium.
Mueller-Hinton broth (Difco Laboratories, Detroit, MI) supplemented with magnesium (12.5 mg/liter) and calcium (25 mg/liter) was used for all microdilution susceptibility testing and time-kill analyses. In addition, Mueller-Hinton broth adjusted with 50 mg/liter calcium and 12.5 mg/liter magnesium was used for the daptomycin studies (11, 14). Trypticase soy agar (Difco Laboratories) was used for growth and quantification of organisms.
Susceptibility testing.
MICs as well as MBCs were determined by standardized broth microdilution techniques with a starting inoculum of 5 × 105 CFU/ml, according to Clinical and Laboratory Standards Institute guidelines, and incubation for 24 h at 35°C. MICs were determined in duplicate, with broth microdilution testing being performed up to an MIC of 32 μg/liter for both CSA-8 and CSA-13.
Time-kill analysis.
Time-kill experiments were performed with CSA-13 and four clinical isolates (isolates Mu3, Mu50, VRSA-PA, and VRSA-MI) in triplicate. A starting inoculum of 5 × 105 CFU/ml as well as a high starting inoculum of 1 × 108 to 1 × 109 CFU/ml was used to determine whether there was an inoculum effect on CSA-13. CSA-13 was tested at 0.5×, 1×, 4×, and 10× the MIC for both starting inocula of 106 and 108 CFU/ml. Tests with CSA-13 alone and in combination with gentamicin, doxycycline, linezolid, vancomycin, tigecycline, and daptomycin at 0.5× the MIC with a starting inoculum of 1 × 106 CFU/ml were also performed for combination time-kill testing. Aliquots (0.1 ml) were removed from the cultures at 0, 1, 4, 8, and 24 h and were serially diluted in cold 0.9% sodium chloride. Synergy, additivity, antagonism, and indifference were defined as follows: >2-log killing, <2-log but >1-log killing, >1-log growth, and ±1-log killing, respectively. Bacterial counts were determined by spiral plating if appropriate dilutions by use of an automatic spiral plater (WASP; DW Scientific, West Yorkshire, England) and by counting the colonies by use of a protocol colony counter (Synoptics Limited, Frederick, MD). The lower limit of detection for the colony counts was 2 log10 CFU/ml. Time-kill curves were constructed by plotting the mean colony counts (log10 CFU/ml) versus time. To eliminate antibiotic carryover, all samples were diluted sufficiently prior to plating, or if the concentration was close to the MIC postdilution, they were subjected to vacuum filtration with 0.9% sodium chloride.
PAE.
The PAE was determined by the equation T − C, where T refers to the time required for the CFU count to increase 1 log10 above the count observed immediately after drug removal, and C refers to the time required for the CFU count in an untreated control culture to increase 1 log10 above the count observed immediately after completion of the same procedure with the test culture used for drug removal. PAE was determined by using the method of rapid drug removal by repeated washing, dilution, filtration, or drug inactivation. For a concentration-dependent effect with PAE, the four selected clinical strains were exposed to CSA-13 for 0.5 h at 1×, 2×, and 4× the MIC.
Statistical analysis.
Statistical analysis was performed with SPSS statistical software (release 14.0; SPSS, Inc., Chicago, IL). Colony counts at 8 h and 24 h and the time to 99.9% killing (T99.9) were compared between groups by using one-way analysis of variance, followed by Tukey's post-hoc test for multiple comparisons. A P value of ≤0.05 indicated statistical significance.
RESULTS
Susceptibility.
Susceptibility testing of the clinical isolates demonstrated that the MIC50, MIC90, and MBC of CSA-8 were 4, 8, and 8 mg/liter, respectively, and that those of CSA-13 were 1, 1, and 1 mg/liter, respectively (n = 50) (Table 1). Overall, the MICs and MBCs of CSA-13 were fourfold lower than those of CSA-8. The MIC50 and MIC90 of CSA-13 were lower than those of vancomycin, and the MIC90 of CSA-13 was lower than that of linezolid. In addition, the susceptibility data for CSA-8 and CSA-13 were also the same for the four VRSA strains tested (Table 1). Both the MICs and MBCs of CSA-13 were lower than those of linezolid, vancomycin, and CSA-8 (by fourfold) for most of the VRSA isolates.
TABLE 1.
Activities of CSA-13, CSA-8, and comparator antimicrobial agents of 4 clinical strains of VRSA and 50 clinical isolates of hGISA and GISA
| Antimicrobial agent | MIC (μg/ml)/MBC (μg/ml) for hGISA/GISAa strains (n = 50)
|
MIC (μg/ml)/MBC (μg/ml)
|
|||||
|---|---|---|---|---|---|---|---|
| Range | 50% | 90% | VRSA-NY (2004) | VRSA-MI (2002) | VRSA-PA (2002) | VRSA-MI (2005) | |
| CSA-8 | 4-8/4-16 | 4/8 | 8/8 | 4/4 | 4/4 | 4/4 | 4/4 |
| CSA-13 | 1-2/1-2 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 |
| Daptomycin | 0.125-1/0.125-1 | 0.5/0.5 | 1/1 | 0.125/0.125 | 0.125/0.125 | 0.5/1 | 0.125/0.125 |
| Linezolid | 0.25-2/0.5-64 | 1/8 | 2/64 | 1/16 | 2/4 | 2/8 | 0.25/0.5 |
| Vancomycin | 1-8/1-16 | 4/8 | 8/8 | 1024/>2,048 | 32/64 | 256/>256 | 128/>256 |
hGISA/GISA, glycopeptide-intermediate S. aureus; heterogeneous glycopeptide-intermediate S. aureus.
Concentration-dependent bactericidal killing.
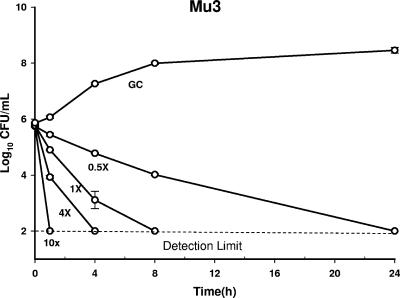
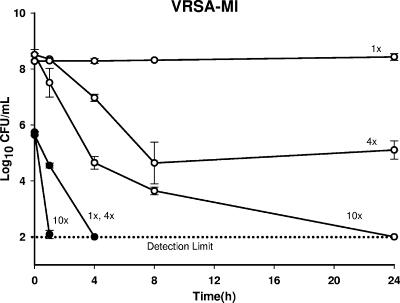
Time-kill curve analysis of the VRSA, VISA, and hVISA clinical isolates demonstrated concentration-dependent bactericidal killing, with 10× the MIC of CSA-13 achieving the earliest killing (1 h) for all isolates tested. Bactericidal killing (detection limit, 99.9%) was distinct and statistically different at 24 h and 1 h when CSA-13 was tested at 0.5× and 10× the MIC (P < 0.002) against all four strains. Bactericidal killing was observed at 24, 8, 4, and 1 h at 0.5×, 1×, 4×, and 10× the MIC, respectively, for Mu3 (Fig. 2). At 1× and 4× the MIC, the times to bactericidal kill (T99.9%) were 8 h and 4 h, respectively, for all of the isolates except VRSA-MI. With VRSA-MI, bactericidal killing was similar with 1× and 4× the MIC at 4 h (Fig. 3). However, time-kill curve analysis of all four strains demonstrated bacterial killing from 1.72 to 3.92 log10 CFU/ml by 8 h, with a mean killing of −3.4 ± 0.65 log10 CFU/ml (median, −3.69 log10 CFU/ml) at 8 h and a mean killing of −3.75 ± 0.07 log10 CFU/ml (median, −3.74 log10 CFU/ml) at 24 h when the organism was tested at all four concentrations.
FIG. 2.
Time-kill curve of CSA-13 activity against Mu3 at 0.5×, 1×, 4×, and 10× the MIC over a 24-h period. GC, growth control of the organism. The limit of detection is 2 log10 CFU/ml.
FIG. 3.
Time-kill curve of CSA-13 activity with starting densities of both 6 log10 CFU/ml (closed circles) and 8 to 9 log10 CFU/ml (open circles) at 1×, 4×, and 10× the MIC. The limit of detection is 2 log10 CFU/ml.
An inoculum effect was also observed for CSA-13 at a starting bacterial density of 8 to 9 log10 CFU/ml compared to that achieved with a starting bacterial density of 6 log10 CFU/ml. At 1×, 4×, and 10× the MICs for Mu3, Mu50, and VRSA-PA, the T99.9% were 8, 4, and 1 h, respectively, at an inoculum of 6 log10 CFU/ml, whereas the T99.9% were ≥24 h at an inoculum of 8 to 9 log10 CFU/ml. For VRSA-MI at 1×, 4×, and 10× the MIC, the T99.9% were 4 h, 4 h, and 1 h, respectively, at 6 log10 CFU/ml, whereas they were ≥24 h at 8 to 9 log10 CFU/ml (P ≤ 0.001) (Fig. 3). In fact, the time to bactericidal killing extended beyond 24 h at 1×, 4×, and 10× the MIC with the higher inoculum. In addition, there was a difference in bacterial killing between the higher and the lower inoculum densities for all the isolates tested with CSA-13 at 0.5×, 1×, and 10× the MIC at both 8 h and 24 h (P ≤ 0.05). A difference in bacterial killing between the two inocula was also detected for Mu3 at 24 h when it was tested with CSA-13 at 4× the MIC (P ≤ 0.001). A concentration-dependent effect of CSA-13 was also demonstrated in the PAE. The PAE nearly doubled at 2.28 ± 0.5 h (average ± standard deviation) when the VRSA strains and Mu3 were exposed to CSA-13 at 4× the MIC compared to that at 2× the MIC (P = 0.03) (Fig. 4). PAE also increased accordingly by 1.13 ± 0.51 h (average ± standard deviation) upon exposure to CSA-13 from 1× to 2× the MIC for all four strains and by 1.58 ± 0.92 h upon exposure to CSA-13 from 2× to 4× the MIC for Mu50.
FIG. 4.
Concentration-dependent PAE of CSA-13 for VRSA-PA (A) and VRSA-MI (B) measured at 1×, 2×, and 4× the MIC. GC, growth control of the organism.
Combination testing.
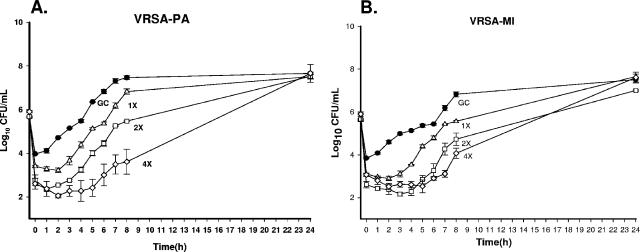
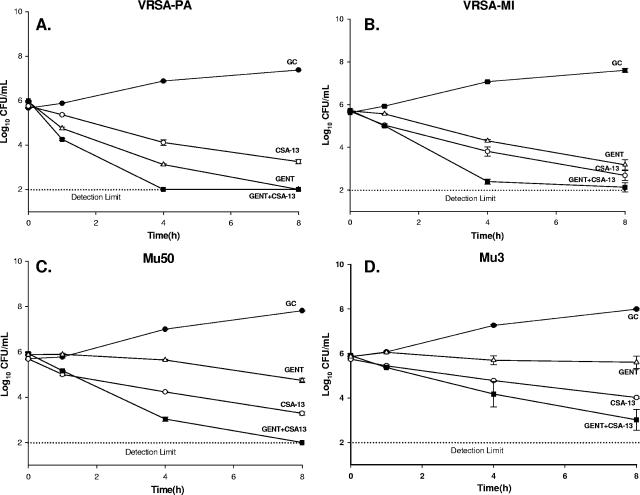
Overall, the addition of other antimicrobial agents to CSA-13 did not improve the activity of CSA-13 since the agent was so potent when it was used alone. Time-kill curve analysis of the clinical isolates showed no difference in the log killing at 24 h for the majority of the organisms tested with CSA-13 in combination with gentamicin, doxycycline, linezolid, vancomycin, tigecycline, and daptomycin. Specifically, the combinations with doxycycline and tigecycline demonstrated indifference at all time points (1, 4, 8, and 24 h). However, early synergy at 4 h was detected against VRSA-PA when it was tested with CSA-13 plus gentamicin, whereas early additivity was demonstrated at 4, 4, and 8 h with VRSA-MI, Mu50, and Mu3, respectively (Fig. 5). In addition, early additivity at 4 to 8 h was detected against Mu3, Mu50, and VRSA-PA when they were tested with CSA-13 plus daptomycin, CSA-13 plus linezolid, and CSA-13 plus vancomycin, respectively.
FIG. 5.
Time-kill curves of CSA-13 in combination with gentamicin (GENT + CSA-13) against VRSA-PA (A), VRSA-MI (B), Mu50 (C), and Mu3 (D). GC, growth control of the organism.
DISCUSSION
Ceragenins are unique, low-molecular-mass compounds that have potent bactericidal activities against both gram-negative and gram-positive bacteria, particularly clinically relevant pathogens like MRSA. Susceptibility data from our study demonstrated that CSA-13 has an MIC50/MBC50 ratio of 1, suggesting bactericidal activity. This was confirmed by time-kill analysis, which demonstrated that CSA-13 has potent bactericidal activity even at a concentration of 0.5× the MIC. Indeed, various CSA-13 concentrations, i.e., those at, below, and above the MIC, demonstrated concentration-dependent antimicrobial activities. This phenomenon was also demonstrated with the postantibiotic effect.
In addition, varying the initial inoculum size in the time-kill studies showed an inoculum effect with CSA-13. Further increasing the inoculum to 108 CFU/ml substantially prolonged the time to 99.9% killing, i.e., from 1 h to 24 h at 10× the MIC, by testing with VRSA-MI. This inoculum effect is similar to that described in previous reports on beta-lactams and glycopeptides and may be a reflection of CSA-13's mechanism of action, given that the literature has suggested that antimicrobials targeting the cell wall have been associated with an inoculum effect (3, 21). Combination time-kill studies demonstrated that CSA-13 is a potent agent when it is used by itself. Early synergy and additivity were noted with the combination of gentamicin and CSA-13 at 4 to 8 h; but they were not noted at 24 h, as CSA-13 killed the isolates to the detection limit on its own. This result is similar to those of other studies of the use of gentamicin in combination, which found early synergy with bactericidal agents, such as daptomycin, an antimicrobial agent that targets the bacterial membrane (8, 22). Tsuji and Rybak found that the addition of a single high dose of gentamicin (5 mg/kg of body weight) to daptomycin resulted in early bactericidal killing and synergy at 4 h, whereas bactericidal killing was achieved at 32 h when daptomycin was used alone at 6 mg/kg in a 96-h simulated in vitro pharmacodynamic/pharmacokinetic model with simulated endocardial vegetations (22). Dawis et al. also found synergy in vitro in 20% of MRSA isolates and 60% of Pseudomonas aeruginosa isolates tested with gatifloxacin alone or in combination with gentamicin (7). Another gentamicin combination study by Anadiotis et al. found synergy in vitro against 38.5% and 84.7% of the S. pneumoniae isolates tested with the beta-lactam penicillin (which targets the penicillin binding proteins in the bacterial membrane) and gentamicin at concentrations of 1 mg/liter and 2 mg/liter, respectively (1).
Our findings of CSA-13's potent bactericidal activity against clinically relevant gram-positive pathogens are similar to those of previous studies performed by Savage et al. (45th ICAAC, abstr. F-1231 and F-1232) and Bogdanovich et al. (45th ICAAC). Similar to Bogdanovich et al. (45th ICAAC), we found bactericidal activity by 24 h in all the isolates tested with 1× and 4× the MIC as well as demonstrated killing against the VRSA strains, Savage et al. (45th ICAAC, abstr. F-1231) reported an MIC50 and MIC90 of 0.5 mg/liter for 32 clinical isolates of MRSA. These susceptibility data were similar to the MIC50 and MIC90 of 1 mg/liter that we have reported here for the 50 clinical isolates of S. aureus (VISA and hVISA strains) with raised vancomycin MICs. Both CSA-8 and CSA-13 are promising antimicrobials and may have important therapeutic implications for infections caused by hVISA and VISA strains. Given that these small compounds are experimental antimicrobial agents, future safety, efficacy, and pharmacokinetic studies in vivo should be performed to correlate these promising in vitro data.
Footnotes
Published ahead of print on 8 January 2007.
REFERENCES
- 1.Anadiotis, L., J. P. Maskell, and A. M. Sefton. 2002. Comparative in-vitro activity of penicillin alone and combined with gentamicin against clinical isolates of Streptococcus pneumoniae with decreased susceptibility to penicillin. Int. J. Antimicrob. Agents 19:173-178. [DOI] [PubMed] [Google Scholar]
- 2.Boman, H. G. 1995. Peptide antibiotics and their role in innate immunity. Annu. Rev. Immunol. 13:61-92. [DOI] [PubMed] [Google Scholar]
- 3.Casellas, J. M., G. Tomé, C. Bantar, P. Bertolini, N. Blázquez, N. Borda, E. Couto, N. Cudmani, J. Guerrera, M. J. Juárez, T. López, A. Littvik, E. Méndez, R. Notario, G. Ponce, M. Quinteros, F. Salamone, M. Sparo, E. Sutich, S. Vaylet, and L. Wolff. 2003. Argentinean collaborative multicenter study on the in vitro comparative activity of piperacillin-tazobactam against selected bacterial isolates recovered from hospitalized patients. Diagn. Microbiol. Infect. Dis. 47:527-537. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2002. Staphylococcus aureus resistant to vancomycin—United States, 2002. Morb. Mortal. Wkly. Rep. 51:565-567. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2002. Staphylococcus aureus resistant to vancomycin—United States, 2002. Morb. Mortal. Wkly. Rep. 51:902. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 2004. Staphylococcus aureus resistant to vancomycin—United States, 2004. Morb. Mortal. Wkly. Rep. 53:322-323. [Google Scholar]
- 7.Dawis, M. A., H. D. Isenberg, K. A. France, and S. G. Jenkins. 2003. In vitro activity of gatifloxacin alone and in combination with cefepime, meropenem, piperacillin and gentamicin against multidrug-resistant organisms. J. Antimicrob. Chemother. 51:1203-1211. [DOI] [PubMed] [Google Scholar]
- 8.Deshpande, L. M., and R. N. Jones. 2003. Bactericidal activity and synergy studies of BAL9141, a novel pyrrolidinone-3-ylidenemethyl cephem, tested against streptococci, enterococci and methicillin-resistant staphylococci. Clin. Microbiol. Infect. 9:1120-1124. [DOI] [PubMed] [Google Scholar]
- 9.Ding, B., Q. Guan, J. P. Walsh, J. S. Boswell, T. W. Winter, E. S. Winter, S. S. Boyd, C. Li, and P. B. Savage. 2002. Correlation of the antibacterial activities of cationic peptide antibiotics and cationic steroid antibiotics. J. Med. Chem. 45:663-669. [DOI] [PubMed] [Google Scholar]
- 10.Guan, Q., E. J. Schmidt, S. R. Boswell, C. Li, G. W. Allman, and P. B. Savage. 2000. Preparation and characterization of cholic acid-derived antimicrobial agents with controlled stabilities. Org. Lett. 2:2837-2840. [DOI] [PubMed] [Google Scholar]
- 11.Hanberger, H., L. E. Nilsson, R. Maller, and B. Isaksson. 1991. Pharmacodynamics of daptomycin and vancomycin on Enterococcus faecalis and Staphylococcus aureus demonstrated by studies of initial killing and postantibiotic effect and influence of Ca2+ and albumin on these drugs. Antimicrob. Agents Chemother. 35:1710-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kikuchi, K., E. M. Bernard, A. Sadownik, S. L. Regen, and D. Armstrong. 1997. Antimicrobial activities of squalamine mimics. Antimicrob. Agents Chemother. 41:1433-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klevens, R. M., J. R. Edwards, F. C. Tenover, L. C. McDonald, T. Horan, R Gaynes, and National Nosocomial Infections Surveillance System. 2006. Changes in the epidemiology of methicillin-resistant Staphylococcus aureus in intensive care units in US hospitals, 1992-2003. Clin. Infect. Dis. 42:389-391. [DOI] [PubMed] [Google Scholar]
- 14.Lamp, K. C., M. J. Rybak, E. M. Bailey, and G. W. Kaatz. 1992. In vitro pharmacodynamic effects of concentration, pH, and growth phase on serum bactericidal activities of daptomycin and vancomycin. Antimicrob. Agents Chemother. 36:2709-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li, C., L. P. Budge, C. D. Driscoll, B. M. Willardson, G. W. Allman, and P. B. Savage. 1999. Incremental conversion of outer-membrane permeabilizers into potent antibiotics for gram-negative bacteria. J. Am. Chem. Soc. 121:931-940. [Google Scholar]
- 16.Li, C., M. R. Lewis, A. B. Gilbert, M. D. Noel, D. H. Scoville, G. W. Allman, and P. B. Savage. 1999. Antimicrobial activities of amine- and guanidine-functionalized cholic acid derivatives. Antimicrob. Agents Chemother. 43:1347-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li, C., A. S. Peters, E. L. Meredith, G. W. Allman, and P. B. Savage. 1998. Design and synthesis of potent sensitizers of gram-negative bacteria based on a cholic acid scaffolding. J. Am. Chem. Soc. 120:2961-2962. [Google Scholar]
- 18.Michigan Department of Community Health. 3 March 2005. MDCH has confirmed its second case of vancomycin-resistant Staphylococcus aureus (VRSA). Michigan Department of Community Health, Lansing.
- 19.National Nosocomial Infections Surveillance System. 2004. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1992 through June 2004: a report from the NNIS system. Am. J. Infect. Control 32:470-485. [DOI] [PubMed] [Google Scholar]
- 20.Savage, P. B., C. Li, U. Taotafa, B. Ding, and Q. Guan. 2002. Antibacterial properties of cationic steroid antibiotics: a minireview. FEMS Microbiol. Lett. 217:1-7. [DOI] [PubMed] [Google Scholar]
- 21.Soriano, F., P. Coronel, M. Gimeno, M. Jimenez, P. Garcia-Corbeira, and R. Fernandez-Roblas. 1996. Inoculum effect and bactericidal activity of cefditoren and other antibiotics against Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis. Eur. J. Clin. Microbiol. Infect. Dis. 15:761-763. [DOI] [PubMed] [Google Scholar]
- 22.Tsuji, B. T., and M. J. Rybak. 2005. Short-course gentamicin in combination with daptomycin or vancomycin against Staphylococcus aureus in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 49:2735-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van't Hof, W., E. C. Veerman, E. J. Helmerhorst, and A. V. Amerongen. 2001. Antimicrobial peptides: properties and applicability. Biol. Chem. 382:597-619. [DOI] [PubMed] [Google Scholar]