Abstract
Intubated patients frequently become colonized by Pseudomonas aeruginosa, which is subsequently responsible for ventilator-associated pneumonia. This pathogen readily acquires resistance against available antimicrobials. Depending on the resistance mechanism selected for, resistance might either be lost or persist after removal of the selective pressure. We investigated the rapidity of selection, as well as the persistence, of antimicrobial resistance and determined the underlying mechanisms. We selected 109 prospectively collected P. aeruginosa tracheal isolates from two patients based on their prolonged intubation and colonization periods, during which they had received carbapenem, fluoroquinolone (FQ), or combined β-lactam-aminoglycoside therapies. We determined antimicrobial resistance phenotypes by susceptibility testing and used quantitative real-time PCR to measure the expression of resistance determinants. Within 10 days after the initiation of therapy, all treatment regimens selected resistant isolates. Resistance to β-lactam and FQ was correlated with ampC and mexC gene expression levels, respectively, whereas imipenem resistance was attributable to decreased oprD expression. Combined β-lactam-aminoglycoside resistance was associated with the appearance of small-colony variants. Imipenem and FQ resistance persisted for prolonged times once the selecting antimicrobial treatment had been discontinued. In contrast, resistance to β-lactams disappeared rapidly after removal of the selective pressure, to reappear promptly upon renewed exposure. Our results suggest that resistant P. aeruginosa is selected in less than 10 days independently of the antimicrobial class. Different resistance mechanisms lead to the loss or persistence of resistance after the removal of the selecting agent. Even if resistant isolates are not evident upon culture, they may persist in the lung and can be rapidly reselected.
Pseudomonas aeruginosa is one of the most important gram-negative opportunistic pathogens responsible for severe ventilator-associated pneumonia (VAP) (45). VAP is a major medical problem responsible for high mortality and elevated costs for intubated patients (4, 7, 23, 34). Prolonged endotracheal intubation, associated with exposure to antimicrobial therapies, frequently leads to colonization of the upper respiratory tract by P. aeruginosa (10). Once established, P. aeruginosa colonization is almost impossible to eradicate and leads to VAP in 10 to 15% of the patients (26). Therapeutic approaches are limited because P. aeruginosa readily acquires resistance against antimicrobials. Pneumonic lungs, which may carry P. aeruginosa populations of more than 10E8 CFU/ml of tracheal aspirate, are therefore likely to harbor resistant clones that can be selected under antimicrobial regimens. In order to prevent the selection of resistant isolates, shorter durations of antimicrobial therapies for VAP have been suggested recently (5, 6, 36).
P. aeruginosa displays an elevated intrinsic drug resistance that can be further increased through chromosomal mutations (38). The most frequently encountered mutations lead to derepression of the chromosomal AmpC β-lactamase (hydrolyzing most β-lactams except carbapenems), overexpression of multidrug efflux pumps (extruding fluoroquinolones [FQs], β-lactams [except imipenem], aminoglycosides, and tetracyclines), or reduced expression of porin pathways (OprD, limiting the permeation of most carbapenems), or they occur in antimicrobial targets such as, for instance, type II topoisomerases (reducing interaction with FQs) (29). Except for target mutations, all other acquired resistance mechanisms described above result from altered gene expression. Therefore, the presence of specific resistance genes does not per se account for their expression. To determine the expression of different antimicrobial resistance mechanisms in P. aeruginosa, we have recently validated the use of quantitative real-time PCR (qRT-PCR) (8). Knowledge of specific resistance mechanisms could be important, because they might influence the persistence of resistant clones after discontinuation of antimicrobial therapies, thereby compromising the success of subsequent treatments (1).
To date, studies analyzing the longitudinal follow-up of colonized patients over prolonged times have been performed only for cystic fibrosis (CF) patients with respect to β-lactam resistance (12). However, no study has examined the overall resistance phenotypes and their underlying mechanisms in intubated patients. We therefore decided to follow prospectively patients at the intensive care units of the University Hospital Geneva.
The aims of the study were (i) to follow precisely the kinetics of emergence and persistence of resistance phenotypes upon exposure to different classes of antimicrobials, (ii) to identify by gene expression analysis the resistance mechanisms acquired in response to particular antimicrobial treatments, and (iii) to establish a correlation between gene expression and levels of susceptibility to antimicrobials.
MATERIALS AND METHODS
Clinical setting.
From 1999 to 2001, we screened all intubated patients in both the surgical and the medical intensive care unit of the University Hospital Geneva for respiratory tract colonization by P. aeruginosa. We prospectively collected tracheal aspirates from a total of 40 intubated patients harboring P. aeruginosa in their tracheal aspirates. Day zero was defined as the first day a tracheal aspirate culture was found positive for P. aeruginosa; only one tracheal aspirate was collected on a given day. Collection was stopped at the time of death, extubation, or transfer of the patient to another unit. Among the 40 patients of our cohort, 2 patients were selected on the bases of (i) prolonged follow-up (more than 40 days), (ii) availability of clinical isolates from tracheal aspirates obtained at regular time intervals, and (iii) their exposure to various antimicrobial agents.
Antimicrobial regimens.
Antimicrobials were administered in standard regimens (37): imipenem (500 mg four times a day), piperacillin-tazobactam (4.5 g four times a day), tobramycin (1.7 mg/kg of body weight three times a day), cefepime (2 g twice a day), and ciprofloxacin (400 mg three times a day). The doses were adapted to the renal function during periods of significant reduction (<40 ml/min) of creatinine clearance.
Strain collection and susceptibility testing.
Upon collection, all tracheal aspirates were transferred to ice without delay and directly transported to the research facility. The tracheal aspirates were immediately homogenized with dithiothreitol, and aliquots were spread on Pseudomonas isolation agar (Pseudosel; Becton Dickinson, Cockeysville, MD). For each aspirate, one to seven colonies presenting various morphotypes were selected and frozen at −70°C as glycerol stocks. The schedule of tracheal aspirate collection for both patients is shown in Fig. 1. For patient A, the collection included 40 isolates from 18 individual tracheal aspirates collected over a total observation period of 92 days (see Table 1), whereas for patient B, we collected 69 isolates from 18 individual tracheal aspirates obtained over a 43-day study period (see Table 2). Susceptibilities to 13 antimicrobial agents were tested by the disk diffusion method according to the recommendations of the National Committee for Clinical Laboratory Standards (now CLSI) (35). Disks contained the following compounds: piperacillin, piperacillin-tazobactam, ceftazidime, cefepime, imipenem, meropenem, aztreonam, amikacin, gentamicin, netilmicin, tobramycin, norfloxacin, and ciprofloxacin (bioMérieux, France). The inhibition zones were measured with a video camera-equipped SIRscan 2000 instrument (i2a, Montpellier, France).
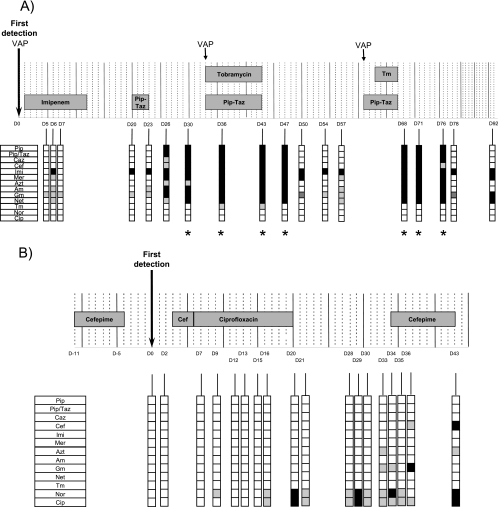
FIG. 1.
Schedule of antimicrobial treatments and evolution of resistance profiles of two intubated patients colonized by P. aeruginosa. Isolates of P. aeruginosa were cultured from tracheal aspirates during a period of 92 days for patient A (A) and 43 days for patient B (B). Day zero (D0) is the first day of detection of P. aeruginosa. Episodes of VAP and periods of antimicrobial treatments are indicated. For each patient, one tracheal aspirate was collected on each of the 18 days specified. The resistance profile of the most resistant isolate among those cultured from each tracheal aspirate is shown. Symbols designate susceptible (open squares), intermediate (gray squares), and resistant (black squares) isolates, according to CLSI breakpoints. Pip, piperacillin; Pip/Taz, piperacillin-tazobactam; Caz, ceftazidime; Cef, cefepime; Imi, imipenem; Mer, meropenem; Azt, aztreonam; Am, amikacin; Gm, gentamicin; Net, netilmicin; Tm, tobramycin; Nor, norfloxacin; Cip, ciprofloxacin. Asterisks indicate small-colony variants.
TABLE 1.
Resistance profiles of 40 isolates from patient A
| Isolatea | Genotypeb | Day | Susceptibilityc to the following antimicrobiald (zone inhibition diam range [mm]e)
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β-Lactams
|
Aminoglycosides
|
FQs
|
|||||||||||||
| Pip (17-18) | Pip/Taz (17-18) | Caz (14-18) | Cep (14-18) | Imi (13-16) | Mer (11-14) | Azt (15-22) | Am (14-17) | Gm (12-15) | Net (12-15) | Tm (12-15) | Nor (12-17) | Clp (15-21) | |||
| P21.21 | d | 5 | S | S | S | S | S | S | S | S | I | S | S | S | S |
| 65.18-1 | d | 6 | S | S | S | S | S | S | S | I | I | I | S | S | S |
| 65.18-2 | * | 6 | S | S | S | S | R | I | S | I | I | S | S | S | S |
| 65.18-3 | * | 6 | S | S | S | S | R | S | S | I | I | S | S | S | S |
| 65.18-4 | * | 6 | S | S | S | S | R | I | S | S | I | S | S | S | S |
| P21.23 | * | 7 | S | S | S | S | S | S | S | S | I | S | S | S | S |
| 1A5 | d | 20 | S | S | S | S | R | S | S | S | I | S | S | S | S |
| 1C7 | d | 23 | S | S | S | S | R | S | S | I | I | S | S | S | S |
| 1D7 | d | 23 | S | S | S | S | R | S | S | I | I | S | S | S | S |
| 65.33-1 | * | 26 | S | S | S | S | R | S | S | S | I | S | S | S | S |
| 65.33-2 | d | 26 | S | S | S | S | R | R | I | I | R | I | S | S | S |
| 65.33-3 | d | 26 | R | R | I | I | R | R | I | R | R | I | S | S | S |
| 65.33-4 | d | 26 | S | S | S | S | R | I | S | I | S | I | S | S | S |
| 65.36-1 | * | 30 | S | S | S | S | R | I | I | I | R | I | S | S | S |
| 65.36-2 | * | 30 | S | S | S | S | R | I | I | I | R | I | S | S | S |
| 65.36-3 | d | 30 | S | S | S | I | R | R | I | R | R | I | S | S | S |
| 65.36-4f | * | 30 | R | R | R | R | R | R | I | R | R | R | S | S | S |
| 65.38-1f | d | 36 | R | R | R | R | R | R | R | R | R | R | I | S | S |
| 65.38-2f | * | 36 | R | R | I | I | R | R | I | R | R | R | S | S | S |
| 65.38-3f | * | 36 | R | R | R | I | R | R | R | R | R | R | S | S | S |
| 65.38-4f | d | 36 | S | S | S | S | R | R | I | R | R | R | S | S | S |
| 1A9 | d | 43 | R | R | R | S | R | R | R | I | R | S | S | S | S |
| 1B9f | d | 43 | R | R | R | R | R | R | R | R | R | R | I | S | S |
| 1C9f | d | 43 | R | S | I | S | R | R | I | R | R | R | S | S | S |
| 2A1 | d | 47 | R | R | I | S | R | R | I | S | I | S | S | S | S |
| 2C1f | d | 47 | R | R | R | R | R | R | R | R | R | R | S | S | S |
| 2A2 | d | 50 | S | S | S | S | R | R | S | S | I | S | S | S | S |
| 2C2 | d | 50 | S | S | S | S | R | I | S | S | I | S | S | S | S |
| 2A3 | d | 54 | S | S | S | S | R | S | S | S | I | S | S | S | S |
| 2A5 | d | 57 | S | S | S | S | R | S | S | I | S | S | S | S | S |
| 2B5 | d | 57 | S | S | S | S | R | I | S | I | I | I | S | S | S |
| 2A6f | d | 68 | R | R | R | I | R | R | R | R | R | I | S | S | S |
| 2F6f | d | 68 | R | R | R | R | R | R | R | R | R | R | S | S | S |
| 2A7f | d | 71 | R | R | R | R | R | R | R | R | R | R | S | S | S |
| 2A9f | d | 76 | R | R | R | I | R | R | R | R | R | R | S | S | S |
| 3B1 | d | 78 | S | S | S | S | R | S | S | S | I | S | S | S | S |
| 65.68-1 | * | 92 | S | S | S | S | R | R | S | S | I | S | S | S | S |
| 65.68-2 | d | 92 | S | S | S | S | R | R | S | S | R | R | S | S | S |
| 65.68-3 | * | 92 | S | S | S | S | R | R | S | S | R | I | S | S | S |
| 65.68-4 | * | 92 | S | S | S | S | R | R | S | S | S | I | S | S | S |
Boldfaced isolates were used for qRT-PCR.
*, the isolate was not genotyped.
S, susceptible; I, intermediate; R, resistant.
Pip, piperacillin; Pip/Taz, piperacillin-tazobactam; Caz, ceftazidime; Cef, cefepime; Imi, imipenem; Mer, meropenem; Azt, aztreonam; Am, amikacin; Gm, gentamicin; Net, netilmicin; Tm, tobramycin; Nor, norfloxacin; Cip, ciprofloxacin.
Given as diameter for resistant isolates-diameter for susceptible isolates.
SCV.
TABLE 2.
Resistance profiles of 69 isolates from patient B
| Isolatea | Genotypeb | Day | Susceptibilityc to the following antimicrobiald (zone inhibition diam range [mm]e)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β-Lactams
|
Aminoglycosides
|
FQs
|
||||||||
| Pip/Taz (17-18) | Caz (14-18) | Cep (14-18) | Imi (13-16) | Am (14-17) | Gm (12-15) | Nor (12-17) | Clp (15-21) | |||
| 2A5 | a | 0 | S | S | S | S | S | S | S | S |
| 3D1 | * | 2 | S | S | S | S | S | S | S | S |
| 3D10 | a | 2 | S | S | S | S | S | S | S | S |
| 3E2 | b | 2 | S | S | S | S | S | S | S | S |
| 5F2 | b | 7 | S | S | S | S | S | S | S | S |
| 5F8 | * | 7 | S | S | S | S | S | S | S | S |
| 5G3 | * | 7 | S | S | S | S | S | S | S | S |
| 5H6 | * | 7 | S | S | S | S | S | S | S | S |
| 8A2f | a | 9 | S | S | S | S | S | S | S | S |
| 8A12 | a | 9 | S | S | S | S | S | S | I | S |
| 8C4 | * | 9 | S | S | S | S | S | S | S | S |
| 8B11 | * | 9 | S | S | S | S | S | S | S | S |
| 9A1 | * | 12 | S | S | S | S | S | S | S | S |
| 9A3 | c | 12 | S | S | S | S | S | S | S | S |
| 9A7 | * | 12 | S | S | S | S | S | S | S | S |
| 9A10 | * | 12 | S | S | S | S | S | S | S | S |
| 9B2 | * | 12 | S | S | S | S | S | S | S | S |
| 9B5 | c | 12 | S | S | S | S | S | S | S | S |
| 10A6 | * | 13 | S | S | S | S | S | S | S | S |
| 10A9 | * | 13 | S | S | S | S | S | S | S | S |
| 10B1 | * | 13 | S | S | S | S | S | S | S | S |
| 10B3 | * | 13 | S | S | S | S | S | S | S | S |
| 12A3 | * | 15 | S | S | S | S | S | S | S | S |
| 12A7 | * | 15 | S | S | S | S | S | S | S | S |
| 12B2 | * | 15 | S | S | S | S | S | S | S | S |
| 13D3f | a | 16 | S | S | S | S | S | S | I | I |
| 13D6 | * | 16 | S | S | S | S | S | S | I | I |
| 13D11 | * | 16 | S | S | S | S | S | S | S | S |
| 13E1 | * | 16 | S | S | S | S | S | S | S | S |
| 15G2f | a | 20 | S | S | S | S | S | S | R | R |
| 15G10f | a | 20 | S | S | S | S | S | S | I | I |
| 15H5 | * | 20 | S | S | S | S | S | S | S | S |
| 16D2f | a | 21 | S | S | S | S | S | S | I | I |
| 16D5 | a | 21 | S | S | S | S | S | S | S | S |
| 19B1f | a | 28 | S | S | S | S | S | S | I | I |
| 19B3 | * | 28 | S | S | S | S | S | S | S | S |
| 19B6 | * | 28 | S | S | S | S | S | S | S | S |
| 19C2f | a | 28 | S | S | I | S | S | S | I | I |
| 19C5 | * | 29 | S | S | S | S | S | S | S | S |
| 19C7 | * | 29 | S | S | S | S | S | S | S | S |
| 19C12 | a | 29 | S | S | S | S | S | S | I | I |
| 19D9f | a | 29 | S | S | S | S | S | S | R | R |
| 19D10 | a | 30 | S | S | S | S | S | S | I | I |
| 19E1 | a | 30 | S | S | S | S | S | S | I | I |
| 19E2 | a | 30 | S | S | S | S | S | S | S | S |
| 19E12 | * | 30 | S | S | S | S | S | S | I | I |
| 19E9f | a | 30 | S | S | S | S | S | S | S | S |
| 19F1f | a | 30 | S | S | S | S | S | S | I | I |
| 19F2 | * | 30 | S | S | S | S | S | S | I | I |
| 22D2 | * | 33 | S | S | S | S | S | I | S | S |
| 22D6 | * | 33 | S | S | S | S | S | I | S | S |
| 22D9f | a | 33 | S | S | S | S | S | S | S | S |
| 22E1f | a | 33 | S | S | S | S | S | S | S | S |
| 22E3 | * | 33 | S | S | S | S | S | S | S | S |
| 24C5 | * | 34 | S | S | S | S | S | S | S | S |
| 24D1 | * | 34 | S | S | S | S | S | I | S | S |
| 24D5f | a | 34 | S | S | I | S | S | S | R | I |
| 24E1 | a | 34 | S | S | S | S | S | S | I | I |
| 24E2 | a | 34 | S | S | S | S | S | S | I | I |
| 24E5 | * | 34 | S | S | S | S | S | I | S | S |
| 24E6f | a | 34 | S | S | S | S | S | S | I | I |
| 23C5f | a | 35 | S | S | S | S | S | S | I | I |
| 24F4 | a | 36 | S | S | I | S | S | I | S | S |
| 24G1f | a | 36 | S | S | S | S | S | S | S | I |
| 29F6 | * | 43 | S | S | S | S | S | S | S | S |
| 29G6 | a | 43 | S | S | S | S | S | S | S | S |
| 29G7 | a | 43 | S | S | S | S | S | S | S | S |
| 29G8 | * | 43 | S | S | S | S | S | S | S | S |
| 29H1f | a | 43 | S | S | R | S | S | S | R | R |
Boldfaced isolates were used for qRT-PCR.
*, the isolate was not genotyped.
S, susceptible; I, intermediate; R, resistant. All isolates were susceptible to piperacillin, aztreonam, meropenem, netilmicin, and tobramycin.
Pip/Taz, piperacillin-tazobactam; Caz, ceftazidime; Cef, cefepime; Imi, imipenem; Am, amikacin; Gm, gentamicin; Nor, norfloxacin; Cip, ciprofloxacin.
Given as diameter for resistant isolates-diameter for susceptible isolates.
Isolate with a small colony diameter.
RAPD analysis.
Strains presenting different antimicrobial resistance profiles were selected and analyzed by random amplification of polymorphic DNA (RAPD) using the Ready-to-Go RAPD kit (Pharmacia-Amersham). Primer 208 or 272 (32) was used in the PCR, with the following protocol: one cycle at 94°C for 5 min; 45 cycles at 94°C for 1 min, 36°C for 1 min, and 72°C for 2 min; and one cycle at 72°C for 10 min.
Real-time PCR analysis.
Isolation of total RNA, cDNA synthesis, and real-time PCR analysis were performed as previously described (8). P. aeruginosa strain PAO1 (17) was used as the reference.
Mutation analysis.
The genes encoding the quinolone resistance-determining regions (QRDRs) of DNA gyrase (gyrA and gyrB) and topoisomerase IV (parC and parE) were amplified by PCR and sequenced using the following primers: gyrAS (AGTCCTATCTCGACTACGCGAT), gyrAR (AGTCGACGGTTTCCTTTTCCAG), gyrBS (TGCGGTGGAACAGGAGATGGGCAAGTAC), gyrBR (CTGGCGGAAGAAGAAGGTCAACAGCAGGGT), parCS2 (CGAGCAGGCCTATCTGAACTAT), parCR2 (GAAGGACTTGGGATCGTCCGGA), parES (CGGCGTTCGTCTCGGGCGTGGTGAAGGA), and parER (TCGAGGGCGTAGTAGATGTCCTTGCCGA).
Analysis of results.
Results are presented as ratios of the expression of the target gene to that of the reference gene (rpsL), calculated as  (40), where E is the real-time PCR efficiency for a given gene and CT is the crossing point of the amplification curve with the threshold. An effect on gene transcription was considered significant when the corresponding ratio was ≥2.5 or ≤0.4. Correlations between gene expression and susceptibility data were analyzed with the linear fit program of the Origin software (Microcal, version 6).
(40), where E is the real-time PCR efficiency for a given gene and CT is the crossing point of the amplification curve with the threshold. An effect on gene transcription was considered significant when the corresponding ratio was ≥2.5 or ≤0.4. Correlations between gene expression and susceptibility data were analyzed with the linear fit program of the Origin software (Microcal, version 6).
RESULTS
Description of the two patients.
Patient A suffered from posttuberculosis restrictive pulmonary disease and severe mitral valve regurgitation. He was intubated and underwent surgical mitral valve replacement. Cefazolin was given for 3 days as postoperative prophylaxis. P. aeruginosa was detected for the first time in a bronchoalveolar lavage specimen on day zero (Fig. 1A). Patient A remained colonized by P. aeruginosa and suffered three episodes of acute P. aeruginosa VAP (defined as the combination of a compatible clinical picture, a new infiltrate on the chest X ray, and the presence of P. aeruginosa at >10E4 CFU/ml in a bronchoalveolar lavage specimen), at days 1, 33, and 61. The first VAP episode was treated by imipenem (days zero to 12), the two other episodes by a combination therapy with piperacillin-tazobactam and tobramycin (days 33 to 43 and days 61 to 67, respectively) (Fig. 1A). A 3-day piperacillin-tazobactam treatment was given starting on day 20 for severe P. aeruginosa purulent bronchitis. Extubation was impossible because of the underlying pulmonary disease, and a tracheotomy was performed after 25 days of mechanical ventilation (day 14). The patient was transferred to another hospital on day 92.
Patient B suffered from chronic respiratory insufficiency, with chronic hypoventilation and a sleep apnea syndrome, due to major obesity. Patient B was treated with cefepime for severe bronchitis without microbiological documentation of a specific bacterial pathogen (from day −11 to day −4 [Fig. 1B]). P. aeruginosa was first detected in a tracheal aspirate on day zero. The patient was treated with noninvasive mechanical ventilation until day 23, the date of intubation (Fig. 1B). In contrast to patient A, patient B did not experience P. aeruginosa VAP during the study period. Cefepime was administered from day 3 to day 6 for severe purulent bronchitis, followed by 14 days of ciprofloxacin (days 4 to 20) and cefepime (days 33 to 43). Because of the severity of respiratory insufficiency, extubation was impossible, and the patient underwent a tracheotomy before being transferred to another hospital. Despite repeated antimicrobial treatments, both patients remained colonized by P. aeruginosa throughout the entire study period.
Timing of antimicrobial therapies and evolution of phenotypic resistance in isolates from tracheal aspirations.
Eighteen tracheal aspirates were collected for each patient during a 3-month (patient A) or a 43-day (patient B) observation period (Fig. 1). To follow the sequential evolution of antimicrobial resistance for P. aeruginosa isolates, one to seven colonies were selected from each tracheal aspirate (see Materials and Methods). Tables 1 and 2 show the profiles of resistance against antimicrobial agents for a total of 40 isolates from patient A and 69 isolates from patient B, respectively.
Patient A received no antimicrobial agents during the 3 weeks preceding the study period. The first P. aeruginosa isolate from patient A, obtained from a bronchoalveolar lavage specimen during the first episode of P. aeruginosa VAP, was susceptible to all antimicrobials tested (except for being intermediate for gentamicin). Imipenem resistance appeared 6 days after the initiation of imipenem treatment (day 7 in Fig. 1A) and remained detectable in all of the 38 isolates thereafter (days 20 to 92), even in the absence of imipenem exposure. Piperacillin-tazobactam resistance emerged 6 days after the initiation of the first piperacillin-tazobactam treatment (day 26 in Fig. 1A). From that day until day 47, two to four P. aeruginosa clones with different resistance profiles were detected among isolates from a single tracheal aspirate. Surprisingly, one of the four isolates from day 26 was also resistant to amikacin and gentamicin (Fig. 1A and Table 1), though the patient had not received aminoglycosides at that point. Additional resistances to ceftazidime, cefepime, and netilmicin appeared on day 30, again without administration of any of these three antimicrobial agents. These resistance phenotypes therefore appeared 7 days after the discontinuation of the first piperacillin-tazobactam treatment. With the initiation of β-lactam-aminoglycoside combination therapy (day 33), isolates displaying a small-colony variant (SCV) morphology appeared (day 36 in Fig. 1A; Table 1). SCVs are autoaggregative cells that form colonies with unusually small diameters (0.5 to 1 mm after 24 h of incubation at 37°C) (14). To our knowledge, this is the first report of the isolation of SCVs from non-CF patients. The SCVs showed higher levels of resistance, in particular to gentamicin (no inhibition zone), than non-SCV aminoglycoside-resistant isolates (inhibition zone diameter, 11 to 12 mm). After this combination therapy, patient A remained without treatment for 2 weeks. During this period, isolates susceptible to piperacillin, but still resistant to imipenem, reappeared, suggesting that in this case the maintenance of a significant number of clones resistant to piperacillin, but not to imipenem, was dependent on continuing selective pressure. Markedly, 5 days after the initiation of the second combination therapy, multidrug-resistant isolates reemerged (day 68 in Fig. 1A). Again, SCVs were present among these isolates. All isolates remained susceptible to quinolone antimicrobials (Table 1), suggesting that efflux mechanisms were unlikely to be active in these isolates.
Patient B received cefepime for 8 days during the 2 weeks preceding the first detection of P. aeruginosa isolates in tracheal secretions. This therapy did not, however, select cefepime-resistant isolates (Fig. 1B). Three days after identification of P. aeruginosa in the tracheal aspirates, the patient was started again on cefepime for 2 days and then switched to ciprofloxacin (day 6, Fig. 1B). The first FQ-intermediate (FQ-I) and FQ-resistant (FQ-R) isolates were detected 7 and 10 days after the initiation of ciprofloxacin treatment, respectively. Surprisingly, among all the FQ-I and FQ-R isolates analyzed from days 16 to 43, 76% (16/21) displayed colony diameters 3- to 12-fold smaller than that of the FQ-susceptible reference strain PAO1 but did not share other phenotypes associated with SCVs (Fig. 1B; Table 2). FQ resistance persisted for at least 3 weeks in 40% (16/39) of the isolates after the interruption of ciprofloxacin treatment. A cefepime-intermediate and a cefepime-resistant isolate were detected, respectively, on day 36 and day 43 (10 days after initiation of the therapy), during the third cefepime treatment. Interestingly, these two isolates remained susceptible to the other six β-lactams tested, suggesting that resistance was not due to derepression of the chromosomal ampC β-lactamase; for one isolate (24F4), resistance resulted from overexpression of the MexXY efflux pump (data not shown) (16).
Together these observations suggest that for both patients, exposure to various classes of antimicrobial agents was followed by the selection of resistant clones in 6 to 10 days. Strikingly, clones resistant to certain β-lactams and aminoglycosides appeared even in the absence of exposure to these antimicrobials. The persistence of resistant clones after the discontinuation of antimicrobial therapy differed depending on the selecting agent. Moreover, SCVs were observed after exposure to aminoglycosides.
The appearance of new resistance phenotypes does not correlate with colonization by new P. aeruginosa isolates.
One explanation for the appearance of resistant clones in the absence of antimicrobial exposure could be cross-colonization with a new strain from another patient (41). We therefore determined whether resistant and susceptible clones were genotypically related by performing RAPD analysis on 27 isolates from patient A (Table 1) and 35 isolates from patient B (Table 2). The isolates were selected in such a manner that they covered the whole panel of resistance profiles. Isolates from patient A displayed identical banding patterns and therefore belonged to the same RAPD genotype (d) (Table 1). This result strongly suggests that all resistant isolates from this patient, including the multidrug-resistant SCV, were derived from the same susceptible clone isolated initially from the bronchoalveolar lavage specimen and not from the acquisition of a new P. aeruginosa strain (Fig. 1A).
For patient B, three different RAPD types (a, b, and c) were detected among isolates collected during the first 12 days of the observation period (Table 2). All isolates recovered thereafter belonged to RAPD type a, demonstrating that all FQ-I and FQ-R isolates derived from a susceptible type-a clone. Reanalysis of isolates from patient B with a second primer (primer 208) confirmed the classification obtained with primer 272 (data not shown).
Expression of specific resistance mechanisms correlates with resistance phenotypes.
Resistance to carbapenems is frequently related to decreased expression or loss of the carbapenem-specific porin OprD (47, 48). We therefore measured the expression of oprD by the isolates from patient A. With the exception of the initial imipenem-susceptible isolate, all the other, imipenem-resistant isolates showed decreased oprD expression compared to that of PAO1 (range, 0.4- to 0.01-fold). Resistance to meropenem was not linked to resistance to imipenem and appeared later during the study period. In contrast to imipenem, meropenem is a substrate of the MexAB-OprM efflux pump; however, meropenem susceptibilities did not correlate with mexA expression, suggesting the presence of other meropenem-specific resistance mechanisms. Strikingly, OprD mutants persisted for at least 2 months in this patient despite the discontinuation of imipenem therapy.
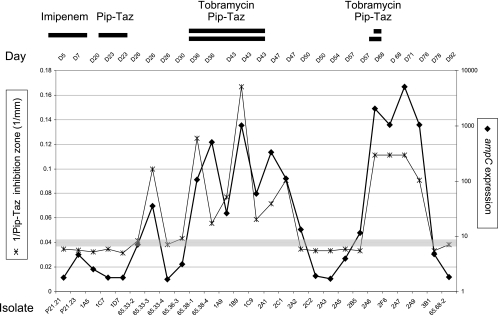
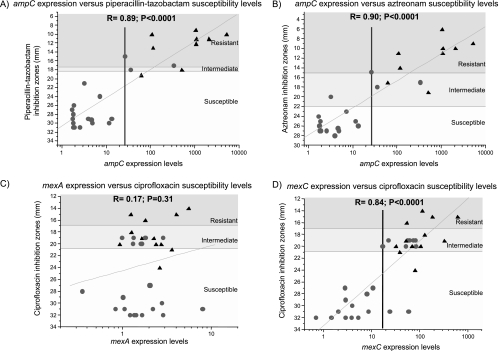
Resistance to penicillins and cephalosporins is usually mediated by the inducible ampC β-lactamase present on the P. aeruginosa chromosome. We therefore analyzed the expression of this gene in 27 isolates (Table 1) from patient A by qRT-PCR and compared it to expression by the susceptible reference strain PAO1. We observed a parallel evolution between phenotypic β-lactam resistance (expressed as 1 divided by the inhibition zone diameter) and the levels of ampC expression by the different isolates collected during the 3-month study period (Fig. 2). Indeed, for all isolates displaying phenotypic resistance toward piperacillin-tazobactam, the expression of ampC was increased (20- to 5,000-fold). Plotting ampC expression against piperacillin-tazobactam inhibition zone diameters revealed the presence of two populations, the more resistant of which was composed mainly of SCVs (Fig. 3A). A good correlation was found between piperacillin-tazobactam (Fig. 3A), aztreonam (Fig. 3B), and ceftazidime resistance levels and ampC expression (Table 3), implicating AmpC as the major resistance determinant for these isolates. A threshold level 20- to 25-fold higher than ampC expression by the reference strain PAO1 allowed discrimination between cephalosporin/penicillin-susceptible and -resistant isolates (Fig. 3A and 3B).
FIG. 2.
Temporal evolution of ampC expression and β-lactam resistance for 27 P. aeruginosa isolates from patient A. For each isolate, the expression of ampC was measured by qRT-PCR (right scale) and the inhibition diameter for piperacillin-tazobactam was determined (expressed as 1/inhibition diameter) (left scale). For each tracheal aspirate, one to three isolates were tested as indicated. Days of collection, names of isolates, and periods of antimicrobial treatments are indicated. Gray horizontal bar, zone of intermediate susceptibility for piperacillin-tazobactam.
FIG. 3.
Correlations between the expression of different resistance determinant genes and antibiotic susceptibility levels. ampC expression levels for 27 isolates from patient A were determined by qRT-PCR and plotted against their piperacillin-tazobactam (A) and aztreonam (B) inhibition diameters. mexA (C) and mexC (D) expression levels were determined for 35 isolates of patient B and plotted against their ciprofloxacin inhibition diameters. Zones of resistance as defined by inhibition diameters are indicated by shading: white, susceptible; light gray, intermediate; dark gray, resistant. •, isolates with colony diameters comparable to that of PAO1; ▴, small-colony variants. Correlations were calculated with the linear fit program of the Origin software (version 6).
TABLE 3.
Correlations between gene expression and levels of resistance to β-lactams for isolates from patient A
| Gene | Linear correlation coefficient (R)a for expression of the indicated gene and the following β-lactamb:
|
|||
|---|---|---|---|---|
| Pip-Taz | Caz | Imi | Azt | |
| ampC | 0.89 | 0.90 | 0.45 | 0.90 |
| oprD | NA | NA | 0.50 | NA |
| mexY | 0.07 | 0.03 | 0.26 | NA |
| mexA | 0.32 | 0.28 | 0.06 | 0.34 |
Boldfaced values, P < 0.001; NA, not analyzed.
Pip-Taz, piperacillin-tazobactam; Caz, ceftazidime; Imi, imipenem; Azt, aztreonam.
Resistance to aminoglycosides in P. aeruginosa can result from their enzymatic inactivation or from their extrusion by the MexXY efflux pump. Although we observed >20-fold overexpression by 2 of the 11 aminoglycoside-resistant isolates from patient A, there was no general correlation between resistance and expression of this efflux pump (data not shown). This finding supports previous reports on MexXY efflux pump expression by P. aeruginosa CF isolates (43, 46), suggesting that yet unrecognized resistance determinants may contribute to aminoglycoside resistance.
FQs are known to be substrates for all characterized efflux pumps in P. aeruginosa. Expression of at least one gene from each of the four main efflux pumps, MexAB-OprM, MexCD-OprJ, MexEF-OprN, and MexXY, was therefore analyzed by qRT-PCR for 35 isolates from patient B (Table 2). No correlation was found between expression of mexA (Fig. 3C; Table 4), mexE, or mexY and FQ resistance, although mexA was overexpressed more than threefold by two FQ-R isolates. An excellent correlation (R ≥ 0.8; P < 0.001) was found, however, between FQ resistance levels and mexC expression: all FQ-I and FQ-R isolates overexpressed this gene (Fig. 3D; Table 4). The expression levels of oprJ and the regulator gene nfxB also correlated with FQ resistance levels (Table 4) and mexC expression levels. A threshold level of approximately 20-fold for mexC expression separated FQ-susceptible from FQ-I and FQ-R isolates (Fig. 3D). Resistance to FQ antimicrobials can also result from mutations in the gyrase and topoisomerase IV target genes (39). Sequencing of the QRDRs encoded by gyrA and parC for 10 out of 23 isolates displaying FQ-I or FQ-R phenotypes revealed no alterations. Furthermore, no alterations were found in the QRDRs encoded by gyrB and parE for the four FQ-R isolates. Together these results suggest that overexpression of the MexCD-OprJ efflux system was solely responsible for decreased FQ susceptibilities in the isolates analyzed from patient B.
TABLE 4.
Correlations between gene expression and levels of resistance to FQs for isolates from patient B
| FQ | Linear correlation coefficient (R)a for the indicated FQ and expression of the following gene:
|
|||||
|---|---|---|---|---|---|---|
| mexC | oprJ | nfxB | mexA | mexE | mexY | |
| Norfloxacin | 0.79 | 0.80 | 0.83 | 0.18 | 0.03 | 0.31 |
| Ciprofloxacin | 0.84 | 0.87 | 0.82 | 0.17 | 0.11 | 0.30 |
Boldfaced values, P < 0.001.
DISCUSSION
Our study provides evidence for the emergence of resistance in P. aeruginosa isolates collected from two patients, both colonized for prolonged periods, who had received different antimicrobial treatments. Irrespective of the antimicrobial agent administered, resistant clones appeared after 6 to 10 days of treatment. Resistance phenotypes correlated with the expression of specific resistance genes.
Isolates resistant to imipenem and piperacillin emerged, at the latest, 6 days after the initiation of therapy. All imipenem-resistant isolates showed decreased oprD expression. However, the n-fold decrease in oprD expression did not correlate well with imipenem susceptibility levels, suggesting that additional factors, such as posttranscriptional regulation of oprD (9) or interplay with AmpC expression, might play a role, as suggested previously (28).
Resistance to penicillins and cephalosporins in P. aeruginosa can be mediated by the chromosomal AmpC β-lactamase or the MexAB-OprM efflux pump (33). For patient A, piperacillin-tazobactam therapy selected resistant isolates characterized by derepressed ampC expression rather than MexAB-OprM overproduction. These isolates were also resistant to ceftazidime and aztreonam. Indeed, the susceptibilities to these β-lactams correlated with ampC expression levels, which might be explained by the fact that both compounds, though weak inducers of ampC, are nevertheless hydrolyzed by this enzyme when expressed at high levels (2, 27). Since ampC expression was determined in the absence of any inducer, it is expected that all of the isolates were partially or totally derepressed for ampC (11). Sequencing of the ampR and ampD regulator genes from two highly β-lactam-resistant isolates revealed no modification, suggesting the involvement of other previously suspected regulatory loci (2, 24).
Surprisingly, piperacillin-tazobactam resistance coincided with the appearance of aminoglycoside resistance in an isolate from patient A, although this patient had not received aminoglycosides at this point. However, the original isolate already showed intermediate susceptibility to gentamicin. With one exception, the β-lactam-aminoglycoside-panresistant phenotype was always associated with the SCV morphotype. Compared to the corresponding wild-type isolate, SCVs are characterized by a smaller colony size (13). These morphotypes show increased fitness under stationary-growth conditions in comparison with clonal wild types (14). SCVs are hyperpiliated and exhibit increased twitching motility and an increased capacity to form biofilms (14). To date, such SCVs have been isolated only from CF patients, and our study shows for the first time that SCVs can also be isolated from a non-CF environment. As described previously, SCVs emerge under aminoglycoside treatment, either in vitro with gentamicin (25) or in vivo with tobramycin, as in our case (13). In the lung environment, SCVs seemed to be outcompeted by the wild-type morphotype in the absence of selective pressure. However, they remained present in the lung reservoir, since they were reselected rapidly during the subsequent combination therapy. SCVs present a serious risk for the treatment of P. aeruginosa infections, since they might escape diagnosis in the clinical laboratory due to their atypical colony morphology.
FQs are prominent in selecting efflux-mediated mechanisms in vitro (22) and in vivo (20, 21) and are considered to be risk factors for the development of VAP (44). In our study, ciprofloxacin treatment resulted in the emergence of FQ resistance, due to MexCD-OprJ overexpression. This pump, together with MexEF-OprN, was the predominant mechanism of FQ resistance in P. aeruginosa isolates from CF patients (19, 39). While in most reports, target mutations in the gyrA and parC genes were associated with efflux-mediated resistance (15, 20, 39), isolates from patient B seemed to have acquired FQ resistance solely through MexCD-OprJ overexpression. The identification of these different resistance mechanisms should contribute to the optimization of therapeutic treatments, particularly in view of the potential therapeutic use of efflux pump inhibitors (31, 42), which are also expected to reduce the risk of the emergence of resistance (30).
Our study further shows that acquisition of resistance determinants independent of the antimicrobial treatment administered was due not to cross-colonization with new P. aeruginosa strains but to mutations occurring in an originally susceptible strain colonizing the patient for prolonged periods (more than 90 days for patient A). Relapse of P. aeruginosa infections with single strains persisting for prolonged periods has been documented (41), and these results underline the importance of developing methods to reduce persistent colonization.
All mechanisms identified in these isolates were chromosomally encoded, underlining the intrinsic capacities of this organism to develop resistance. Interesting differences were observed between the type of resistance mechanism and the persistence of the corresponding isolate in the absence of antimicrobial agents. Whereas both imipenem- and FQ-resistant isolates were detected in tracheal aspirates for prolonged periods after treatment discontinuation, β-lactam-resistant isolates rapidly became undetectable but reappeared upon reexposure to β-lactam antimicrobials within a short time. This suggests that ampC overexpression has a more pronounced impact on P. aeruginosa fitness than OprD loss or MexCD-OprJ overexpression. As a result, persistence of imipenem- and FQ-resistant clones might be visible to the clinician, while β-lactam-resistant isolates with derepressed ampC might escape surveillance. This is of major importance when therapies are given for VAP, since inappropriate antimicrobial therapies are associated with increased mortality (3, 18). Together these data strongly support our previous observations (1) that clinicians should be extremely cautious in the choice of antimicrobial agents when a P. aeruginosa infection is suspected and should avoid using classes of antimicrobials to which their patients have been exposed during the previous months (41).
Recently, the standard duration of treatment of VAP—14 to 21 days—has been challenged (5, 36). It has been suggested that reducing the treatment duration to 8 days might be able to prevent the selection of resistant isolates without negatively affecting the clinical outcome (6). Our results support this hypothesis; indeed, resistant P. aeruginosa isolates were cultured from tracheal aspirates independently of the antimicrobial used after treatment durations of 6 to 10 days. Shorter exposure to the selective pressure of antimicrobials might potentially reduce the risk of selection of resistant isolates. However, whether shorter treatment durations can be used for P. aeruginosa VAP without increasing the risk for recurrent infections is not clear and deserves further studies (6).
General conclusions.
Our study was limited to the analyses of only two patients; therefore, it is difficult to draw general conclusions from our observations. However, we believe that the detailed analysis of the longitudinal collection of tracheal isolates from these two patients clearly illustrates the impressive capacity of P. aeruginosa to adapt to the selective pressure of various classes of antimicrobial agents and adds to our understanding of the dynamics of resistance selection and persistence of this pathogen. Whether imipenem, FQ, or β-lactam-aminoglycoside combination therapy was administered, resistant clones appeared within 6 to 10 days. None of the different therapies, including combination therapies, were able to eradicate P. aeruginosa in the two patients. Clinicians should be aware that resistant P. aeruginosa isolates are selected after treatment periods exceeding 6 days and may rapidly reemerge when the same treatment is administered a second time. The potential prevention of resistance selection during shorter treatment durations of VAP needs to be further investigated.
Acknowledgments
We thank Colette Rouiller for performing the antibiotic susceptibility testing and Rachel Comte for skillful technical assistance. We further thank the physicians and nursing staff of the medical and surgical intensive care units of the University Hospital of Geneva for collection of samples.
This work was supported by a NRP49 grant to T.K. and C.V.D. (4049-063239) and a grant to C.V.D. (No. 32-51940.97) from the Swiss National Science Foundation.
Footnotes
Published ahead of print on 29 January 2007.
REFERENCES
- 1.Boffi El Amari, E., E. Chamot, R. Auckenthaler, J. C. Pechère, and C. Van Delden. 2001. Influence of previous exposure to antibiotic therapy on the susceptibility pattern of Pseudomonas aeruginosa bacteremic isolates. Clin. Infect. Dis. 33:1859-1864. [DOI] [PubMed] [Google Scholar]
- 2.Campbell, J. I., O. Ciofu, and N. Hoiby. 1997. Pseudomonas aeruginosa isolates from patients with cystic fibrosis have different β-lactamase expression phenotypes but are homogeneous in the ampC-ampR genetic region. Antimicrob. Agents Chemother. 41:1380-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chamot, E., E. Boffi El Amari, P. Rohner, and C. Van Delden. 2003. Effectiveness of combination antimicrobial therapy for Pseudomonas aeruginosa bacteremia. Antimicrob. Agents Chemother. 47:2756-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chastre, J., and J. Y. Fagon. 2002. Ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 165:867-903. [DOI] [PubMed] [Google Scholar]
- 5.Chastre, J., C. E. Luyt, A. Combes, and J. L. Trouillet. 2006. Use of quantitative cultures and reduced duration of antibiotic regimens for patients with ventilator-associated pneumonia to decrease resistance in the intensive care unit. Clin. Infect. Dis. 43(Suppl. 2):S75-S81. [DOI] [PubMed] [Google Scholar]
- 6.Chastre, J., M. Wolff, J. Y. Fagon, S. Chevret, F. Thomas, D. Wermert, E. Clementi, J. Gonzalez, D. Jusserand, P. Asfar, D. Perrin, F. Fieux, and S. Aubas. 2003. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA 290:2588-2598. [DOI] [PubMed] [Google Scholar]
- 7.Crouch Brewer, S., R. G. Wunderink, C. B. Jones, and K. V. Leeper, Jr. 1996. Ventilator-associated pneumonia due to Pseudomonas aeruginosa. Chest 109:1019-1029. [DOI] [PubMed] [Google Scholar]
- 8.Dumas, J. L., C. Van Delden, K. Perron, and T. Köhler. 2006. Analysis of antibiotic resistance gene expression in Pseudomonas aeruginosa by quantitative real-time-PCR. FEMS Microbiol. Lett. 254:217-225. [DOI] [PubMed] [Google Scholar]
- 9.Epp, S. F., T. Köhler, P. Plésiat, M. Michea-Hamzehpour, J. Frey, and J. C. Pechère. 2001. C-terminal region of Pseudomonas aeruginosa outer membrane porin OprD modulates susceptibility to meropenem. Antimicrob. Agents Chemother. 45:1780-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.George, D. L., P. S. Falk, R. G. Wunderink, K. V. Leeper, Jr., G. U. Meduri, E. L. Steere, C. E. Corbett, and C. G. Mayhall. 1998. Epidemiology of ventilator-acquired pneumonia based on protected bronchoscopic sampling. Am. J. Respir. Crit. Care Med. 158:1839-1847. [DOI] [PubMed] [Google Scholar]
- 11.Giwercman, B., P. A. Lambert, V. T. Rosdahl, G. H. Shand, and N. Hoiby. 1990. Rapid emergence of resistance in Pseudomonas aeruginosa in cystic fibrosis patients due to in-vivo selection of stable partially derepressed β-lactamase producing strains. J. Antimicrob. Chemother. 26:247-259. [DOI] [PubMed] [Google Scholar]
- 12.Giwercman, B., C. Meyer, P. A. Lambert, C. Reinert, and N. Hoiby. 1992. High-level β-lactamase activity in sputum samples from cystic fibrosis patients during antipseudomonal treatment. Antimicrob. Agents Chemother. 36:71-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Häussler, S., B. Tümmler, H. Weissbrodt, M. Rohde, and I. Steinmetz. 1999. Small-colony variants of Pseudomonas aeruginosa in cystic fibrosis. Clin. Infect. Dis. 29:621-625. [DOI] [PubMed] [Google Scholar]
- 14.Häussler, S., I. Ziegler, A. Lottel, F. von Gotz, M. Rohde, D. Wehmhohner, S. Saravanamuthu, B. Tümmler, and I. Steinmetz. 2003. Highly adherent small-colony variants of Pseudomonas aeruginosa in cystic fibrosis lung infection. J. Med. Microbiol. 52:295-301. [DOI] [PubMed] [Google Scholar]
- 15.Higgins, P. G., A. C. Fluit, D. Milatovic, J. Verhoef, and F. J. Schmitz. 2003. Mutations in GyrA, ParC, MexR and NfxB in clinical isolates of Pseudomonas aeruginosa. Int. J. Antimicrob. Agents 21:409-413. [DOI] [PubMed] [Google Scholar]
- 16.Hocquet, D., P. Nordmann, F. El Garch, L. Cabanne, and P. Plésiat. 2006. Involvement of the MexXY-OprM efflux system in emergence of cefepime resistance in clinical strains of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 50:1347-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holloway, B. W., V. Krishnapillai, and A. F. Morgan. 1979. Chromosomal genetics of Pseudomonas. Microbiol. Rev. 43:73-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibrahim, E. H., G. Sherman, S. Ward, V. J. Fraser, and M. H. Kollef. 2000. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 118:146-155. [DOI] [PubMed] [Google Scholar]
- 19.Jalal, S., O. Ciofu, N. Hoiby, N. Gotoh, and B. Wretlind. 2000. Molecular mechanisms of fluoroquinolone resistance in Pseudomonas aeruginosa isolates from cystic fibrosis patients. Antimicrob. Agents Chemother. 44:710-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jalal, S., and B. Wretlind. 1998. Mechanisms of quinolone resistance in clinical strains of Pseudomonas aeruginosa. Microb. Drug Resist. 4:257-261. [DOI] [PubMed] [Google Scholar]
- 21.Join-Lambert, O. F., M. Michea-Hamzehpour, T. Köhler, F. Chau, F. Faurisson, S. Dautrey, C. Vissuzaine, C. Carbon, and J. Pechère. 2001. Differential selection of multidrug efflux mutants by trovafloxacin and ciprofloxacin in an experimental model of Pseudomonas aeruginosa acute pneumonia in rats. Antimicrob. Agents Chemother. 45:571-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Köhler, T., M. Michea-Hamzehpour, P. Plésiat, A. L. Kahr, and J. C. Pechère. 1997. Differential selection of multidrug efflux systems by quinolones in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 41:2540-2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kollef, M. H. 1999. Avoidance of tracheal intubation as a strategy to prevent ventilator-associated pneumonia. Intensive Care Med. 25:553-555. [DOI] [PubMed] [Google Scholar]
- 24.Langaee, T. Y., L. Gagnon, and A. Huletsky. 2000. Inactivation of the ampD gene in Pseudomonas aeruginosa leads to moderate-basal-level and hyperinducible AmpC β-lactamase expression. Antimicrob. Agents Chemother. 44:583-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langford, P. R., H. Anwar, I. Gonda, and M. R. Brown. 1989. Outer membrane proteins of gentamicin induced small colony variants of Pseudomonas aeruginosa. FEMS Microbiol. Lett. 52:33-36. [DOI] [PubMed] [Google Scholar]
- 26.Levine, S. A., and M. S. Niederman. 1991. The impact of tracheal intubation on host defenses and risks for nosocomial pneumonia. Clin. Chest Med. 12:523-543. [PubMed] [Google Scholar]
- 27.Livermore, D. M. 1997. β-Lactamases: quantity and resistance. Clin. Microbiol. Infect. 3(Suppl. 4):S10-S19. [PubMed] [Google Scholar]
- 28.Livermore, D. M. 1992. Interplay of impermeability and chromosomal β-lactamase activity in imipenem-resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 36:2046-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livermore, D. M. 2002. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin. Infect. Dis. 34:634-640. [DOI] [PubMed] [Google Scholar]
- 30.Lomovskaya, O., A. Lee, K. Hoshino, H. Ishida, A. Mistry, M. S. Warren, E. Boyer, S. Chamberland, and V. J. Lee. 1999. Use of a genetic approach to evaluate the consequences of inhibition of efflux pumps in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 43:1340-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lomovskaya, O., M. S. Warren, A. Lee, J. Galazzo, R. Fronko, M. Lee, J. Blais, D. Cho, S. Chamberland, T. Renau, R. Leger, S. Hecker, W. Watkins, K. Hoshino, H. Ishida, and V. J. Lee. 2001. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob. Agents Chemother. 45:105-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahenthiralingam, E., M. E. Campbell, J. Foster, J. S. Lam, and D. P. Speert. 1996. Random amplified polymorphic DNA typing of Pseudomonas aeruginosa isolates recovered from patients with cystic fibrosis. J. Clin. Microbiol. 34:1129-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masuda, N., E. Sakagawa, S. Ohya, N. Gotoh, H. Tsujimoto, and T. Nishino. 2000. Substrate specificities of MexAB-OprM, MexCD-OprJ, and MexXY-OprM efflux pumps in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44:3322-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehta, R. M., and M. S. Niederman. 2003. Nosocomial pneumonia in the intensive care unit: controversies and dilemmas. J. Intensive Care Med. 18:175-188. [DOI] [PubMed] [Google Scholar]
- 35.National Committee for Clinical Laboratory Standards. 2004. Performance standards for antimicrobial susceptibility testing, vol. 24, no. 1. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 36.Niederman, M. S. 2006. Use of broad-spectrum antimicrobials for the treatment of pneumonia in seriously ill patients: maximizing clinical outcomes and minimizing selection of resistant organisms. Clin. Infect. Dis. 42(Suppl. 2):S72-S81. [DOI] [PubMed] [Google Scholar]
- 37.Niederman, M. S., D. E. Craven, M. Bonten, J. Chastre, W. A. Craig, J. Y. Fagon, J. Hall, G. A. Jacoby, M. H. Kollef, C. M. Luna, L. A. Mandell, A. Torres, and R. G. Wunderink. 2005. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 171:388-416. [DOI] [PubMed] [Google Scholar]
- 38.Normark, B. H., and S. Normark. 2002. Evolution and spread of antibiotic resistance. J. Intern. Med. 252:91-106. [DOI] [PubMed] [Google Scholar]
- 39.Oh, H., J. Stenhoff, S. Jalal, and B. Wretlind. 2003. Role of efflux pumps and mutations in genes for topoisomerases II and IV in fluoroquinolone-resistant Pseudomonas aeruginosa strains. Microb. Drug Resist. 9:323-328. [DOI] [PubMed] [Google Scholar]
- 40.Pfaffl, M. W., G. W. Horgan, and L. Dempfle. 2002. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rello, J., D. Mariscal, F. March, P. Jubert, F. Sanchez, J. Valles, and P. Coll. 1998. Recurrent Pseudomonas aeruginosa pneumonia in ventilated patients. Relapse or reinfection? Am. J. Respir. Crit. Care Med. 157:912-916. [DOI] [PubMed] [Google Scholar]
- 42.Renau, T. E., R. Leger, E. M. Flamme, J. Sangalang, M. W. She, R. Yen, C. L. Gannon, D. Griffith, S. Chamberland, O. Lomovskaya, S. J. Hecker, V. J. Lee, T. Ohta, and K. Nakayama. 1999. Inhibitors of efflux pumps in Pseudomonas aeruginosa potentiate the activity of the fluoroquinolone antibacterial levofloxacin. J. Med. Chem. 42:4928-4931. [DOI] [PubMed] [Google Scholar]
- 43.Sobel, M. L., G. A. McKay, and K. Poole. 2003. Contribution of the MexXY multidrug transporter to aminoglycoside resistance in Pseudomonas aeruginosa clinical isolates. Antimicrob. Agents Chemother. 47:3202-3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trouillet, J. L., A. Vuagnat, A. Combes, N. Kassis, J. Chastre, and C. Gibert. 2002. Pseudomonas aeruginosa ventilator-associated pneumonia: comparison of episodes due to piperacillin-resistant versus piperacillin-susceptible organisms. Clin. Infect. Dis. 34:1047-1054. [DOI] [PubMed] [Google Scholar]
- 45.Vincent, J. L., D. J. Bihari, P. M. Suter, H. A. Bruining, J. White, M. H. Nicolas-Chanoin, M. Wolff, R. C. Spencer, and M. Hemmer. 1995. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) study. JAMA 274:639-644. [PubMed] [Google Scholar]
- 46.Vogne, C., J. R. Aires, C. Bailly, D. Hocquet, and P. Plésiat. 2004. Role of the multidrug efflux system MexXY in the emergence of moderate resistance to aminoglycosides among Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Antimicrob. Agents Chemother. 48:1676-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolter, D. J., N. D. Hanson, and P. D. Lister. 2004. Insertional inactivation of oprD in clinical isolates of Pseudomonas aeruginosa leading to carbapenem resistance. FEMS Microbiol. Lett. 236:137-143. [DOI] [PubMed] [Google Scholar]
- 48.Yoneyama, H., and T. Nakae. 1993. Mechanism of efficient elimination of protein D2 in outer membrane of imipenem-resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 37:2385-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]