Abstract
The antiviral activity of a new series of thymidine analogs was determined against vaccinia virus (VV), cowpox virus (CV), herpes simplex virus, and varicella-zoster virus. Several compounds were identified that had good activity against each of the viruses, including a set of novel 5-substituted deoxyuridine analogs. To investigate the possibility that these drugs might be phosphorylated preferentially by the viral thymidine kinase (TK) homologs, the antiviral activities of these compounds were also assessed using TK-deficient strains of some of these viruses. Some of these compounds were shown to be much less effective in the absence of a functional TK gene in CV, which was unexpected given the high degree of amino acid identity between this enzyme and its cellular homolog. This unanticipated result suggested that the CV TK was important in the mechanism of action of these compounds and also that it might phosphorylate a wider variety of substrates than other type II enzymes. To confirm these data, we expressed the VV TK and human TK1 in bacteria and isolated the purified enzymes. Enzymatic assays demonstrated that the viral TK could efficiently phosphorylate many of these compounds, whereas most of the compounds were very poor substrates for the cellular kinase, TK1. Thus, the specific phosphorylation of these compounds by the viral kinase may be sufficient to explain the TK dependence. This unexpected result suggests that selective phosphorylation by the viral kinase may be a promising new approach in the discovery of highly selective inhibitors of orthopoxvirus replication.
Effective therapies for orthopoxvirus infections are required to combat potential infections of variola virus or monkeypox virus and also to treat adverse events associated with vaccination with vaccinia virus (VV) (7, 8, 26). Cidofovir (CDV) exhibits good antiviral activity against a wide spectrum of orthopoxviruses, including VV, cowpox virus (CV), variola virus, ectromelia virus, and monkeypox virus (3, 18, 35, 43, 51). There is also a small body of clinical experience using CDV to treat molluscum contagiosum and orf virus infections (15, 27). Thus, CDV is a potentially useful drug for the treatment of orthopoxvirus infections and there is an Investigational New Drug Approval for the emergency treatment of smallpox and complications from vaccination. Unfortunately, the utility of this compound is limited by the lack of oral bioavailability and inherent toxicity reduces its usefulness in the clinic (13).
Recent advances in the development of therapeutics for these infections have identified a number of highly active compounds (52). Among these, inhibitors of the VV I7L proteinase have been identified that block virion maturation (9). Inhibitors of the p37 major envelope protein (F13L) are also good inhibitors of viral replication both in vitro and in vivo (54). Ether lipid analogs of CDV have also been shown to be orally bioavailable and highly effective inhibitors of orthopoxvirus infection both in vitro and in vivo (35, 36, 38). The thymidine analog, (N)-methanocarbathymidine, is also active against orthopoxviruses both in vitro and in vivo, and its activity appears to be dependent on a functional orthopoxvirus TK gene (45). This mechanism of action has historically proven to be extraordinarily effective in the therapy of herpesvirus infections, so we sought to identify other potential nucleoside analogs that could be selectively phosphorylated by this viral enzyme.
While herpesviruses and orthopoxviruses both express proteins with TK activity (39, 40), the enzymes are distinct in several fundamental respects, including molecular weight, quaternary structure, and substrate specificity. Herpesvirus enzymes belong to the type I family of TKs, whereas the VV TK is a type II enzyme (5). The type I enzyme encoded by the herpes simplex virus (HSV) UL23 gene (42) is active as a homodimer and is unaffected by allosteric effectors (34). This enzyme, like other members of this family, can phosphorylate a broad range of substrates, including thymidine, 2′-deoxycytidine, and many synthetic nucleoside analogs (19, 25, 34). The prototypic type II TK is encoded by the J2R gene in VV and is closely related to the human cytosolic TK1, which is also a member of this family (32). This group of enzymes is active as homotetramers (31) and is allosterically controlled by both dTTP and dTDP (6, 30). Members of this family are also characterized by a very narrow substrate specificity limited to thymidine and a few closely related analogs.
Early studies by Prusoff and coworkers identified a number of 5-substituted 2′-deoxyuridine analogs, such as idoxuridine (IDU) and trifluoridine (TFT), which exhibited antiviral activity (28). Although some of these compounds were associated with significant toxicity, they could selectively inhibit the replication of both HSV (1, 11, 12) and VV (33, 44). Early studies with VV demonstrated that IDU competed with thymidine as a substrate for the DNA polymerase and was incorporated in viral DNA (48). Interestingly, a functional TK was apparently involved in the mechanism of action of the drug, since recombinant viruses that did not express this enzyme were comparatively resistant to its activity (10). HSV was also sensitive to this compound and similarly required a functional virus TK for activity (25). Subsequent studies identified related compounds, such as brivudine, that were remarkably active against HSV yet did not exhibit the toxicity of earlier compounds (16, 17). Like IDU, these compounds derive their remarkable specificity through selective phosphorylation by herpesvirus TK homologs and remained unactivated in uninfected cells, since they are not substrates for cellular nucleoside kinases (55). However, these compounds were inactive against the orthopoxviruses, since they were not phosphorylated by the viral type II TK homologs and were not converted to active metabolite.
Recently, a new series of deoxyuridine analogs with large substituents at the 5 position were described that retained activity against both VV and CV (21-24). Here, we report that the compounds exhibit an unexpected TK dependence in orthopoxviruses. Enzymatic assays demonstrated that these novel compounds were good substrates for the VV TK, whereas they were poor substrates for the human homolog, TK1. These results suggest that although these enzymes are closely related, selective activation of antiviral drugs by the VV TK is a viable approach in the discovery of highly specific drugs to treat orthopoxvirus infections. Studies presented here were designed to describe the unique substrate specificity of the VV TK in an effort to develop better antiviral drugs for the treatment of orthopoxvirus infections.
MATERIALS AND METHODS
Cells, viruses, and drugs.
Methods for obtaining and passaging human foreskin fibroblast (HFF) cells were described previously (49). Culture medium for all cell lines was minimal essential medium (MEM) containing 10% fetal bovine serum (FBS) and standard concentrations of l-glutamine, penicillin, and gentamicin. VV strains WR, Copenhagen, and IHD were obtained from the American Type Culture Collection (ATCC, Manassas, VA). Working stocks of these viruses were propagated in Vero cells obtained from the ATCC. CV strain Brighton was kindly provided by John W. Huggins (Department of Viral Therapeutics, Virology Division, U.S. Army Medical Research Institute of Infectious Disease, Frederick, MD). CV strains delta crmA (TK+) and TK:GFP lacZ (TK−) were obtained from Pete Turner (University of Florida, Gainesville, FL) and were described previously (2). The wild-type (WT) HSV-1 strain F and the TK-deficient DM2.1, as well as the WT HSV-2 strain MS and the TK-altered strain AG-3, were described and propagated as reported previously (29). CDV (Vistide) was a gift from Mick Hitchcock (Gilead Sciences Inc., Foster City, CA), and other compounds were either purchased (Sigma Chemical company, St. Louis, MO) or provided through the NIAID, NIH, Bethesda, MD. Substituted 2′-deoxyuridine derivatives were synthesized in the lab of Paul Torrence and are as follows: (1) 5-(2,2-dicyanovinyl)-2′-deoxyuridine; (2) 5-(2-carboxyethyl-2-cyanovinyl)-2′-deoxyuridine; (3) 5-(2-amino-3-cyano-5-oxo-5,6,7,8-tetrahydro-4H-chromen-4-yl)-1-(2-deoxypento-furanosyl)-pyrimidine-2,4(1H,3H)-dione; (4) 1-(2-deoxypentofuranosyl)-5-(3-methyl-5-oxo-1-phenyl-4,5-dihydro-4H-pyrazol-4-ylidene)pyrimidine-2,4(1H,3H)-dione; (5) 5-bis(3-methyl-5-oxo-1-phenyl-4,5-dihydro-4H-pyrazol-4-yl)methyl-1-(2-deoxypentofuranosyl)pyrimidine-2,4 (1H,3H)-dione; (6) 5-(2-amino-3-cyano-5-oxo-6,6-dimethyl-5,6,7,8-tetrahydro-4H-chromen-4-yl)-1-(2-deoxypento-furanosyl)-pyrimidine-2,4(1H,3H)-dione.
VV, CV, and HSV plaque reduction assays.
For assays against VV and CV, HFF cells were added to six-well plates and incubated for two days at 37°C with 5% CO2 and 90% humidity. On the day of the assay, the drug at two times the final desired concentration was diluted serially 1:5 in 2× MEM with 10% FBS to provide six concentrations. Aspiration of culture medium from triplicate wells for each drug concentration was followed by the addition of 0.2 ml per well of diluted virus which would give 20 to 30 plaques per well in MEM containing 10% FBS or 0.2 ml medium for drug toxicity wells. The plates were incubated for 1 h with shaking every 15 min. An equal amount of 1% agarose was added to an equal volume of each drug dilution; this mixture was added to each well in 2-ml volumes, and the plates were incubated for three days. The cells were stained with a solution of 0.01% neutral red in phosphate-buffered saline (PBS) and incubated for 5 to 6 h. The stain was aspirated, and plaques were counted using a stereomicroscope at ×10 magnification and 50% effective concentration (EC50) values were calculated by standard methods. The HSV plaque reduction assays were essentially the same as for VV and CV with the following changes. The drug solutions were prepared at the desired concentration in MEM with 2% FBS, and a liquid overlay with pooled human serum containing antibodies to HSV instead of agarose was used. At 72 h following infection, the media containing the drug was aspirated and the monolayers were stained with 1 ml of a solution of 0.01% crystal violet in 60% methanol for 10 min. Residual stain was then washed from the wells with 1 ml PBS, and plaques were counted. Varicella-zoster virus (VZV) assays were performed in the same manner, except the plaques were stained at 10 days following infection.
CV β-galactosidase assay.
Monolayers of HFF cells in 96-well plates were incubated at 37°C for 24 h in a humidified incubator. Drugs were then diluted in the plates, and either TK+ or TK− strains of CV were added at a multiplicity of infection of 0.05 PFU/cell. At 48 h postinfection, the medium was removed and the β-galactosidase substrate, chlorophenol red-β-galactopyranoside, was added at a final concentration of 50 μg/ml in PBS. The conversion of the colorimetric substrate was determined by measuring the absorbance at 570 nm, and EC50 values were calculated by standard methods (46). The EC50 ratio for TK− and TK+ viruses, respectively, was calculated and used as a measure of TK dependence.
Cytotoxicity determination.
HFF cells were added to 96-well, black-walled plates at a concentration of 2.5 × 104 cells per well. After 24 h, the media was aspirated and 125 μl of each drug concentration in MEM with 2% FBS was added to the first row of wells in triplicate. Serial 1:5 dilutions were performed using a Beckman BioMek liquid handling system. After compound addition, the plates were incubated for 7 days in a 5% CO2 incubator at 37°C. To each well 35 μl of CellTiter-Glo (Promega, Madison, WI) reagent was added, and luminescence was measured with a luminometer. Standard methods were used to determine the drug concentration which inhibited cell proliferation by 50% (IC50).
Enzyme preparation.
The full-length open reading frame for the TK encoded by the WR strain of VV and a cDNA encoding human TK1 were amplified and cloned into pET15b (Novagen, Madison, WI) and pET151 d vector (Invitrogen, Carlsbad, CA), respectively, to amino-terminal His tags. Primers for amplifying J2R were 5′-CAC CAT GAA CGG CGG ACA TAT TC-3′ and 5′-TGA GTC GAT GTA ACA CTT TCT TAA-3′, and primers for amplifying TK1 were 5′-CAC CAT GAG CTG CAT TAA CCT GCC CAC T-3′ and 5′-CTA GTT GGC AGG GCT GCA TT-3′. These plasmids were transformed into Escherichia coli strain BL21(DE3) (Invitrogen), grown to exponential phase (optical density at 600 nm of 0.4 to 0.6), and induced with 0.5 mM IPTG (isopropyl-β-d-thiogalactopyranoside; Sigma) at 37°C for 4 h. Cells were collected by centrifugation, and pellets were stored at −80°C. Pellets were thawed on ice and resuspended in enzyme lysis buffer consisting of 50 mM Tris, pH 8.0, 500 mM NaCl, 2 mM MgCl2, 2 mM imidazole, 0.05% Tween 20, 10% sucrose, 1 mg/ml lysozyme, and protease inhibitor cocktail (Sigma Chemical Company, St. Louis, MO). Samples were incubated on ice for 30 min and sonicated (10 six-second bursts), and 0.5 mg/ml DNase I from bovine pancreas (Sigma Chemical Company) was added to the lysate, which was incubated an additional 15 min on ice. The lysate was clarified by centrifugation for 30 min at 25,000 rpm. Ni-nitrilotriacetic agarose beads (QIAGEN, Germantown, MD) were equilibrated in lysis buffer and added to the supernatant. The agarose bead suspension was agitated for 2 h at 4°C and then loaded onto a column for subsequent purification steps. The column was washed with 10 bed volumes of wash buffer (20 mM Tris, 500 mM NaCl, 2 mM MgCl2, 20 mM imidazole, 0.5% Tween 20). Proteins were eluted with 5 bed volumes of elution buffer (20 mM Tris, pH 8.0, 500 mM NaCl, 2 mM MgCl2, 250 mM imidazole, 0.05% Tween 20), aliquoted, and stored at −80°C. The proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis to assess purity. Higher-molecular-weight species were observed in the human TK1 preparation, so mass spectrometry was used to identify these proteins. This analysis identified these products as human TK1, consistent with multimeric forms of the enzyme. This analysis also identified one contaminating bacterial protein (b2255, gi16130190) that is of unknown function but is not homologous to any known kinases. It also does not exhibit detectable ATPase activity, since the concentrated enzyme did not significantly degrade ATP in 1 h of incubation.
Enzyme activity assays.
Kinetic parameters for substrates of TK1 and VV TK were determined by measuring ATP utilization with a luciferase-based assay. Briefly, substrates were diluted in black clear-bottom, 96-well plates in buffer containing 20 mM HEPES, pH 7.4, 250 mM NaCl, 2 mM dithiothreitol, 1 mM MgCl2, and 50 μM ATP. Reactions were initiated by the addition of the purified enzyme, and the mixtures were incubated at room temperature for 1 h, after which the Kinase-Glo luciferase reagent (Promega) was added. The resulting luminescence was used to measure the quantity of residual ATP in the reaction. Reactions were linear over 90 min and were dependent on the addition of dThd or other suitable substrates.
RESULTS
Antiviral activity and TK dependence in orthopoxviruses.
Recently described 5-substituted deoxyuridine analogs that were shown to possess antiviral activity are related in that they have rather bulky substituents in the 5 position of the pyrimidine ring (Fig. 1). These compounds proved to be very active against VV and CV in plaque assays and were relatively nontoxic (Table 1). We hypothesized that these compounds might be selective inhibitors of orthopoxvirus replication because they were preferentially phosphorylated by the viral TK homologs. A recently reported TK dependence assay was used to see if the presence of the CV TK homolog affected the activity of the compounds (47). In this assay, the CDV negative control was equally effective against the TK+ and TK− strains of the virus, since it is a monophosphate analog and does not require the first phosphorylation step (Table 1). By contrast, the IDU positive control requires an initial phosphorylation by the viral kinase and consequently was much less active in a TK− strain, as reported previously (10). In this series of experiments, each of the analogs was more than 20-fold less active in the TK− strain, which suggested that they required the viral TK for optimal activity. This unexpected result was intriguing, since it suggested that the orthopoxvirus TK activity expressed in infected cells might be able to confer sensitivity to antiviral drugs in the same manner as the herpesvirus TK homologs impart sensitivity to acyclovir.
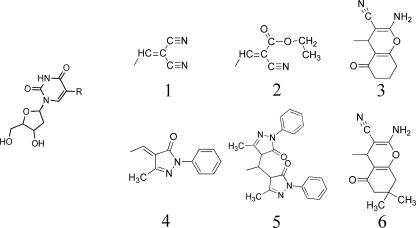
FIG. 1.
Structures of 5-substituted deoxyuridine analogs. Compounds 1 to 6 are analogs of deoxyuridine and contain the substitutions shown.
TABLE 1.
Antiviral activity against orthopoxviruses
| Compound | EC50 (μM)a
|
TK dependenceb (μM)
|
Toxicityc (μM) | |||
|---|---|---|---|---|---|---|
| VV | CV | TK− | TK+ | TK−/TK+ ratio | ||
| 1 | 17 ± 6.4 | 21 ± 12 | 46 ± 15 | 1.9 ± 0.6 | 23 ± 1.2 | 180 ± 73 |
| 2 | 18 ± 14 | 11 ± 9.2 | 71 ± 25 | 1.9 ± 0.5 | 35 ± 9.3 | >300 ± 0 |
| 3 | 4.6 ± 2.0 | 2.0 ± 0.3 | 67 ± 6 | 1.9 ± 0.2 | 36 ± 4.6 | 200 ± 11 |
| 4 | 6.9 ± 0.9 | 5.6 ± 5.2 | 73 ± 1.2 | 0.9 ± 0.2 | 84 ± 14 | 159 ± 13 |
| 5 | 11 ± 1.0 | 9.0 ± 7.0 | 51 ± 0 | 2.7 ± 1.7 | 23 ± 12 | >234 ± 58 |
| 6 | 7.9 ± 2.5 | 8.4 ± 3.6 | 67 ± 0 | 3.1 ± 2.4 | 38 ± 32 | >233 ± 96 |
| IDU | 5.6 ± 0.2 | 1.8 ± 0.2 | 76 ± 8.4 | 1.1 ± 0.3 | 75 ± 15 | >293 ± 0 |
| CDV | 11 ± 3.8 | 32 ± 7.5 | 16 ± 12 | 10 ± 3.5 | 1.9 ± 1.9 | >317 ± 0 |
Effective concentrations that reduced plaque formation by 50% (EC50). Some values were reported previously (22).
EC50 values were determined in β-galactosidase assays in TK− and TK+ strains of CV as described previously (47).
Cytotoxicity was determined by CellTiter-Glo assays (Promega) with standard deviation values shown.
Antiviral activity against herpesviruses.
The TK dependence observed in the first set of experiments prompted an examination of the efficacy of these compounds against herpesviruses, which also express enzymes with TK activity. This series of drugs was evaluated against HSV-1, HSV-2, and VZV using standard plaque assays. Each of these compounds had good antiviral activity against WT strains of HSV-1, HSV-2, and VZV (Table 2). These compounds were also tested in TK-deficient strains of HSV-1 and HSV-2 to see if the drugs also exhibited TK dependence in these viruses. Only compound 5 was substantially less effective in the TK-negative strains of these viruses. This contrasts with the results obtained with CV and suggests that other viral targets may also be involved in the mechanism of action of these compounds in herpesviruses.
TABLE 2.
Antiviral activity against HSV and VZV
| Compound | EC50 (μM)a
|
||||
|---|---|---|---|---|---|
| HSV-1 TK+ (E-377) | HSV-1 TK− (DM2.1) | HSV-2 TK+ (MS) | HSV-2 TK− (AG-3) | VZV (Ellen) | |
| 1 | 11 ± 0.3 | 14 ± 4.6 | 11 ± 2.6 | 12 ± 4.6 | 15 ± 1.5 |
| 2 | 7.6 ± 0.8 | 16 ± 5.7 | 8.3 ± 2.6 | 11 ± 1.1 | 48 ± 11 |
| 3 | 8.6 ± 4.8 | 13 ± 1.3 | 12 ± 3.5 | 12 ± 4.4 | 16 ± 11 |
| 4 | 7.9 ± 5.5 | 9.6 ± 2.9 | 4.2 ± 2.6 | 7.6 ± 0.9 | 15 ± 4.5 |
| 5 | 7.9 ± 0.3 | 25 ± 6.0 | 10 ± 0.7 | 39 ± 12 | 10 ± 1.8 |
| 6 | 7.3 ± 1.9 | 17 ± 0.6 | 13 ± 5.3 | 14 ± 2.9 | 9.2 ± 3.8 |
| ACVb | 1.3 ± 0 | >444 ± 0 | 3.1 ± 0 | >444 ± 0 | 8.5 ± 6.3 |
EC50, effective concentration that reduced plaque formation by 50%. Virus strains used were described previously (47).
ACV, acyclovir.
Purification of VV TK and characterization of its enzymatic activity.
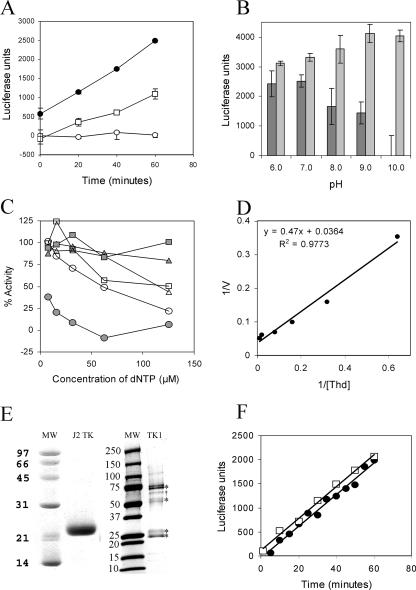
The TK dependence observed in CV suggested that this enzyme was important in the mechanism of action of these new drugs. We hypothesized that the viral TK might be activating the drugs directly. To test this hypothesis, we wanted to determine the relative phosphorylation efficiency of these substrates by human TK1 and J2 in VV. The VV enzyme was selected rather than the CV kinase, since it is more closely related to the enzyme expressed by variola virus. And since the CV and VV kinases are 98% identical at the amino level, they might be expected to exhibit similar activities. Both VV TK and human TK1 were expressed in bacteria, and the histidine-tagged enzymes were purified to see if the viral enzyme selectively phosphorylated these compounds. Enzymatic activity was determined in an ATP utilization assay using luciferase as a reporter. For VV TK, we demonstrated that the utilization of ATP was dependent on thymidine or IDU as phosphate acceptors, and reactions were linear over the 60-min assay period (Fig. 2A). Assays were also optimized with respect to pH (Fig. 2B), salt concentration, and Mg2+ requirements (data not shown). The enzyme required Mg2+, and concentrations of salt over 100 mM reduced its activity, while the pH optimum was approximately 9. A previous report identified dTTP and dTDP as allosteric effectors of the enzyme (30). We confirmed the allosteric inhibition with dTTP with the new assay and also observed >50% inhibition of enzymatic activity for dUTP concentrations greater than 60 μM (Fig. 2C). The assay was reproducible, and Lineweaver-Burk plots for thymidine also yielded Km values between 13 and 49 μM (Fig. 2D; Tables 3 and 4). Km values were confirmed in a standard spectrophotometric assay (50) and an isothermal calorimetry assay that directly measures the heat of binding of the substrate to the enzyme and generated values of 27 and 24 μM, respectively (P. F. Torrence and R. F. Smith, unpublished data). These values agree well with the reported value of 15 μM for the human TK1 homolog purified from E. coli, which occurs predominantly as a dimer (4). These data suggested that the assay conditions were suitable for assessing the kinetic parameters of other potential substrates. Human TK1 was also purified by similar methods (Fig. 2E). Higher-molecular-weight species were observed and were confirmed by mass spectrometry to be TK1, consistent with multimers of this enzyme. Kinase assays with this enzyme yielded results similar to those described for the viral enzyme, including thymidine-dependent ATP utilization, reactions that were linear over the assay period, and Km values that were similar to those reported in the literature (Fig. 2F) (4).
FIG. 2.
Characteristics of the enzymatic activity of J2 TK and human TK1. (A) ATP utilization by VV TK was dependent on either 100 μM thymidine (filled circles) or 100 μM IDU (open squares), while no ATP was consumed in the absence of a nucleoside substrate (open circles). (B) Thymidine-dependent utilization of ATP by VV TK was determined at pH 6, 7, 8, 9, and 10. Luciferase activity was measured following a kinase reaction in the presence (dark gray bars) and absence (light gray bars) of 50 μM thymidine. (C) VV TK enzymatic activity was determined in the presence of the potential allosteric effector molecules shown. Shaded circles and open circles represent dTTP and dUTP, respectively, while shaded squares represent the control without added inhibitors. Open triangles, shaded triangles, and open squares represent dGTP, dCTP, and dATP, respectively. (D) A Lineweaver-Burk plot for thymidine as a substrate of VV TK. (E) Coomassie-stained gels of purified J2 TK and human TK1. Higher-molecular-weight species indicated by asterisks in the human TK1 lane were identified as TK1 by mass spectrometry and are consistent with multimers. One contaminating bacterial protein was also identified as indicated by the dagger (†) symbol and is a bacterial protein of unknown function (b2255, gi16130190). (F) Enzymatic assays for both J2 (filled circles) and TK1 (open squares) are linear with time, and both require thymidine or a related substrate for ATP utilization.
TABLE 3.
Kinetic parameters for substrates of VV TK and human TK1 using known antiviral agents
| Substrate | VV TK
|
Human TK1
|
||||
|---|---|---|---|---|---|---|
| Km (μM)a | Vmax (μM min−1 mg−1) | Vmax/Kmb | Km (μM)a | Vmax (μM min−1 mg−1) | Vmax/Kmb | |
| dThd | 49 ± 7.6 | 289 ± 137 | 5.9 | 60 ± 13 | 619 ± 70 | 10 |
| BrdU | 30 ± 6.2 | 281 ± 59 | 9.4 | 46 ± 0 | 573 ± 62 | 12 |
| IDU | 31 ± 4.7 | 222 ± 58 | 7.2 | 38 ± 11 | 577 ± 26 | 15 |
| FDU | 113 ± 85 | 108 ± 63 | 0.96 | 96 ± 11 | 472 ± 22 | 5 |
| TFT | 14 ± 4.3 | 333 ± 44 | 24 | 47 ± 2.8 | 545 ± 68 | 12 |
| FIAU | 4.3 ± 2.5 | 99 ± 41 | 22.7 | 66 ± 3.5 | 233 ± 6 | 3.5 |
| FIAC | >135 | <0.14 | <0.001 | >135 | <25 | <0.19 |
| CDV | >158 | <0.025 | <0.0002 | >159 | <25 | <0.16 |
Average of three or more determinations with standard deviations shown.
Calculated efficiency using 0.5 μg of purified VV TK and TK1 in each assay.
TABLE 4.
Kinetic parameters for substrates of VV TK and human TK1 using new experimental compounds
| Substrate | VV TK
|
Human TK1
|
||||
|---|---|---|---|---|---|---|
| Km (μM)a | Vmax (μM min−1 mg−1)a | Vmax/Kmb | Km (μM)a | Vmax (μM min−1 mg−1)a | Vmax/Kmb | |
| 1 | 9.6 ± 5.5 | 120 ± 46 | 12 | 113 ± 20 | 130 ± 65 | 1.2 |
| 2 | 13 ± 5.2 | 119 ± 6 | 9 | 118 ± 14 | 98 ± 21 | 0.8 |
| 3 | 103 ± 2.9 | 91 ± 33.4 | 0.9 | >200 | <55 | <0.28 |
| 4 | 11 ± 7.9 | 106 ± 7 | 9.6 | >200 | <55 | <0.28 |
| 5 | 21 ± 1.1 | 91 ± 3 | 4.3 | >200 | <55 | <0.28 |
| 6 | 97 ± 26 | 78 ± 7 | 0.8 | >200 | <55 | <0.28 |
| dThd | 21 ± 12 | 302 ± 7 | 15 | 23 ± 13 | 334 ± 192 | 14.5 |
| CDV | >200 | <55 | <0.28 | >200 | <55 | <0.28 |
Average of three or more determinations with standard deviations shown.
Calculated efficiency using 0.5 μg of purified VV TK and TK1 in each assay.
A series of known pyrimidine analogs was assayed with purified VV TK and human TK1 to further characterize this system. The viral enzyme appeared to phosphorylate dThd, IDU, and bromodeoxyuridine (BrdU) with similar efficiencies, while the phosphorylation of fluorodeoxyuridine (FDU) appeared to be somewhat less efficient (Table 3). TFT and fialuridine (FIAU) appeared to be superior substrates for the VV TK, whereas the cytidine analog, fiacitabine (FIAC), was not a substrate for the viral enzyme. Human TK1 also appeared to phosphorylate most of the substrates with comparable efficiencies, with the single exception of FIAU. This drug had a Km with the viral TK that was significantly lower than with TK1, suggesting that it was a better substrate for the viral enzyme (Table 1). The efficiency with which the viral enzyme phosphorylates this compound was also more than sixfold higher than that observed with TK1. This selective phosphorylation by the viral enzyme is also consistent with the previously reported activity of this compound against VV and CV (37). The potential phosphorylation of several other compounds was also evaluated in this system, but they did not appear to be suitable substrates, including CDV, acyclovir, penciclovir, ganciclovir, brivudine, and sorivudine (data not shown). These results demonstrate that, in many respects, the substrate specificities of these enzymes are related but also that there are significant differences that might be exploited in the discovery of new antiviral agents.
5-Substituted deoxyuridine derivatives as substrates for VV TK and TK1.
The TK dependence exhibited by the compounds in Table 1 suggested that the CV TK was important for their antiviral activity. To test the hypothesis that this might be mediated through selective phosphorylation by the viral TK, we determined their kinetic parameters using purified VV TK and human TK1 (Table 4). This analysis confirmed that all six analogs were substrates for the viral TK, and many of them had Km values that were similar to thymidine, the natural substrate for this enzyme. Compounds 1, 2, and 4 were phosphorylated with the highest efficiency. These results contrast with those obtained with the cellular enzyme, where only compounds 1 and 2 were substrates, and these had Km values that exceeded 100 μM and were not phosphorylated efficiently. Of interest, four analogs were phosphorylated by the viral TK with efficiencies that were 10-fold greater than those measured for TK1. Compound 4 appeared to be one of the best substrates for the viral kinase, and its phosphorylation efficiency was 34-fold higher for the viral enzyme. This result is consistent with the TK dependence data, where this compound also exhibited the greatest TK dependence (Table 1). Since the viral enzyme preferentially phosphorylates this series of deoxyuridine analogs as well as FIAU, we conclude that the substrate specificity of the viral enzyme is broader than had been suspected and is distinct from that of TK1. These data, taken together with the TK dependence data, suggest that selective phosphorylation is important in the mechanism of action of these compounds and that orthopoxvirus TK homologs can confer specificity to this series of compounds.
Predicted structure of J2.
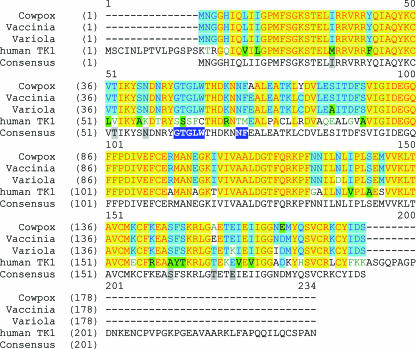
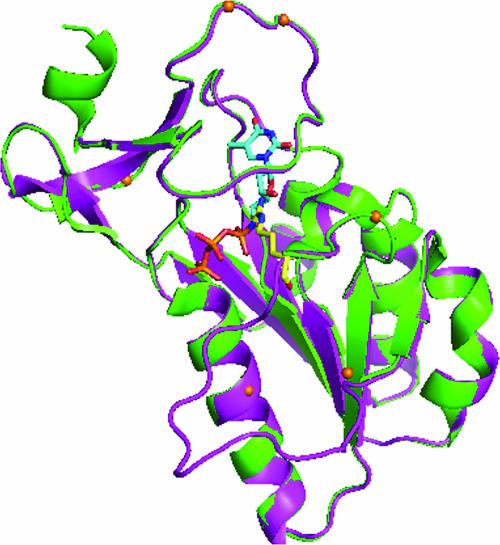
The distinct substrate specificity of the J2R kinase was interesting, since this enzyme is so closely related to the human homolog, TK1. Although the conserved domains in these proteins share 70% identity at the amino acid level, there are regions where they diverge (Fig. 3). To see if these regions might be near the substrate recognition site, we modeled the structure of this enzyme based on an amino acid alignment with four crystal structures of other TKs, including human (Protein Database [PDB] IDs 1XBT and 1W4R), Clostridium acetobutylicum (PDB ID 1XX6), and Ureaplasma parvum (PDB ID 2B8T). Each of these structures is a type II enzyme and would be predicted to be structurally related. The identity was 70, 37, and 35% between the conserved domains of the viral enzyme and the human, C. acetobutylicum, and U. parvum TKs, respectively. The coordinates of human TK 1XBT were used as the template to model VV TK, using the program Modeler (41). Since the identity between the conserved domains of the two proteins is 70% (Fig. 3), the reliability of the model for VV TK is high. The active site residues are rather well conserved, suggesting the enzyme specificity of VV TK is similar to that of human TK. This is consistent with results obtained for most compounds in this report; however, significant differences were also observed and may be related to the subtle differences between the two enzymes. One potentially important difference is a substitution of a slightly smaller serine residue for the threonine 163 in TK1 that is in close contact with the 5 methyl group in thymidine. Another difference is the conformation of the side chain of residue Arg45 (Fig. 4). In the crystal structure of 1XBT, the human TK is in complex with the allosteric inhibitor dTTP (53), where the side chain of its active site arginine (Arg60) coordinates with the triphosphate of the inhibitor. The loop next to this arginine (residues 61 to 74) is disordered in this structure. The homologous Arg45 in VV TK was modeled without the bound inhibitor, and this amino acid is located within the space to be occupied by dTTP if present. The conformation of the loop next to Arg45 (residues 46 to 59) was modeled in VV TK (Fig. 4). This loop contains seven residues with significant differences from those in the human TK (Fig. 3). The difference in this loop may offer an opportunity for exploitation of VV TK-specific substrates, especially the five residues (47 to 51) next to Arg45. Moieties representing modifications of the triphosphate of dTTP may result in selective binding to VV TK. Residues within 12 Å of the active site (Fig. 3) may also contribute to the selectivity of a potential inhibitor of VV TK. In the human TK1 structure, the binding pocket is rather open around the 3′ and 4′ carbons of the deoxyribose portion of the molecule (53). Thus by extension, it may be possible to modify this region of the molecule to increase its selectivity for the viral enzyme, which appears to be borne out by the selective phosphorylation of FIAU, which has a fluorine in the 2′ position of an arabinose sugar. After the manuscript was submitted, we determined the crystal structure of the VV TK, and it was also reported by another laboratory (20). The model presented here is very close to the actual structure for the enzyme, and continued efforts with compounds crystallized in the active site will help define the molecular basis of the observed differences in substrate specificity.
FIG. 3.
Alignment of amino acid sequences for selected TK homologs. Amino acid sequences of TK homologs are shown for cowpox virus (NP_619890), vaccinia virus (YP_232976), variola virus (NP_042123), and human TK1 (AAH07986) with the consensus sequence shown below. In the consensus line, text in dark blue boxes represents the significant differences in the loop next to the active site residue Arg45. Text highlighted in gray denotes amino acid differences within 12 Å of the bound inhibitor dTTP.
FIG. 4.
Model of VV TK structure derived from the crystal structure of 1XBT by blastp (www.ncbi.nlm.nih.gov). Modeled J2R TK structure is shown in magenta overlaid on the reported structure of human TK1 1XBT (green). The modeled side chain of Arg45 (yellow) was shown to overlap with the bound dTTP in 1XBT. Orange dots represent the amino acid differences near the active site. The drawing was created with the program PyMol (Delano Scientific, San Carlos, CA).
DISCUSSION
Experiments summarized here described the antiviral activities of a new series of 5-substituted pyrimidine analogs against selected orthopoxviruses and herpesviruses. These data led us to conduct additional experiments examining the mechanisms of action of these compounds. Results from these studies suggested that (i) VV TK can selectively phosphorylate deoxyuridine nucleosides with rather large substituents at the 5 position, (ii) the phosphorylation of these drugs by orthopoxvirus TK homologs is important in the mechanisms of action of these compounds, and (iii) the strategy of selective phosphorylation can be used in the discovery of new inhibitors against orthopoxviruses. These results were unexpected, inasmuch as TK1 shares a high degree of amino acid identity with the VV homolog and was thought to share a similarly narrow substrate specificity (53). They are also important because selective phosphorylation has proven to be one of the most successful strategies for the treatment of herpesvirus infections. Indeed, the treatments of choice for HSV, VZV, Epstein-Barr virus, and cytomegalovirus infections each rely on a viral-encoded enzyme to selectively phosphorylate the drugs, and this activation is required for the compounds to be metabolized to the active form that inhibits the viral DNA polymerase. Unfortunately, these compounds are not active in orthopoxvirus infections, primarily because the initial phosphorylation step is not catalyzed by the TK homologs in these viruses (47). This view is supported by a previous report in which VV became fully sensitive to acyclovir when the HSV TK homolog was supplied in trans to activate the drug (14). Thus, a strategy of improving the selective activation of nucleosides could be used to improve the efficacy of drug candidates. Results presented here also support this idea and demonstrate that selective phosphorylation is a feasible strategy for orthopoxviruses.
The activation of the compounds by the VV TK suggests that the substrate specificities of the VV TK and TK1 are sufficiently different for such a strategy to be effective. The modeled structure of this enzyme suggests that there are a few amino acid differences near the active sites of these two enzymes that may contribute to the broader substrate specificity observed with the viral enzyme. In this regard, solving the three-dimensional structure of this enzyme should facilitate the design of new inhibitors that are better substrates for the viral kinase, and this project is well under way. This information will represent an important step in the development of even more selective and effective pyrimidine analogs for use in treating orthopoxviruses that rely on the selective activation by the viral kinase, resulting in a highly effective and nontoxic drug. In this endeavor it will also be important to consider differences in the J2R homologs in other orthopoxviruses, particularly variola and monkeypox, which each have 5 amino acid substitutions compared to VV (http://www.poxvirus.org/data.asp). Of particular interest are the E153T and E156K polymorphisms in variola, compared to VV, since each of these amino acids is predicted to lie within 12 Å of the active site of the bound dTTP inhibitor. Also of interest will be the coordinates of substrates cocrystallized in the active sites of the enzymes. These and other similar experiments remain on the critical path of development of these and related compounds.
The TK dependence observed with this series of compounds in CV taken together with the preferential phosphorylation by the VV TK are consistent and suggest a mechanism of action for this series of compounds. We propose that the viral TK activates these compounds by catalyzing the addition of the alpha phosphate on these compounds. Subsequent phosphorylation events by viral or cellular enzymes further phosphorylate the compound to the level of the triphosphate, which in turn inhibits the viral DNA polymerase. Studies presented here do not directly demonstrate the addition of a phosphate to the 5′ position of these compounds, and it remains possible that some other metabolite is the active form and that viral DNA synthesis is inhibited indirectly through the inhibition of thymidylate synthetase or other cellular targets. It is also possible that these compounds are also substrates for other kinases, including the mitochondrial thymidine kinase, TK2, or even the thymidylate kinase encoded by VV, and that other phosphorylation steps are also important in the mechanism of action of these compounds. Nevertheless, the TK dependence data in CV suggests that the viral TK exerts a major influence on the activities of the compounds regardless of the final targets of the inhibitors. Data in favor of our proposed mechanism of action including direct phosphorylation of the drugs are (i) the compounds are analogs of known substrates that are phosphorylated by the VV TK, (ii) the luminescent TK assays presented require a phosphate acceptor for the hydrolysis of ATP and each of the compounds exhibit this property, (iii) the isothermal calorimetry study demonstrated direct binding of the compounds to the enzyme, (iv) the compounds require the viral TK to be active in vitro, and (v) the potent inhibition of DNA synthesis by each of the analogs (EC50 values of less than 2 μM) is consistent with direct inhibition of the DNA polymerase by triphosphate metabolites. Further experiments are required to identify the active metabolites and the final targets of these compounds.
Perhaps the most significant aspect of these studies is how they affect the perception of the potential of the orthopoxvirus TK homologs to play a role in the therapy of these infections. The data presented here suggest that the orthopoxvirus and herpesviruses TK homologs can each be used in a common approach towards the development of specific antiviral therapies. While neither enzyme is required for viral replication, both can be exploited by drugs used to inhibit the replication of these viruses. To be sure, the range of substrates that the VV TK will phosphorylate is more restricted than those phosphorylated by the herpesvirus TK homologs, and the TK dependence in herpesviruses is more robust than that seen in the orthopoxviruses, but opportunities remain. Additional experiments may identify better substrates for the kinase that have the potential to be highly selective therapies for the treatment of orthopoxvirus infections.
Acknowledgments
We thank Geraldine Jefferson, Shalisa Sanders, and Robin Conley for their expert technical assistance.
These studies were supported by Public Health Service contract NO1-AI-30049 and grant 1-U54-AI-057157 from the NIAID, NIH. We also acknowledge contract USAMRIID DAMD 17-03-C-0081 (P.F.T.) from the U.S. Army Medical Research Materiel Command and the State of Arizona Proposition 301 Funds (P.F.T.) for financial support and Robert Smith for excellent technical assistance.
Footnotes
Published ahead of print on 26 February 2007.
REFERENCES
- 1.Albert, D. M., M. Lahav, P. N. Bhatt, T. W. Reid, R. E. Ward, R. C. Cykiert, T. S. Lin, D. C. Ward, and W. H. Prusoff. 1976. Successful therapy of herpes hominis keratitis in rabbits by 5-iodo-5′-amino-2′5′-dideoxyuridine (AIU): a novel analog of thymidine. Investig. Ophthalmol. 15470-478. [PubMed] [Google Scholar]
- 2.Ali, A. N., P. C. Turner, M. A. Brooks, and R. W. Moyer. 1994. The SPI-1 gene of rabbitpox virus determines host range and is required for hemorrhagic pock formation. Virology 202305-314. [DOI] [PubMed] [Google Scholar]
- 3.Baker, R. O., M. Bray, and J. W. Huggins. 2003. Potential antiviral therapeutics for smallpox, monkeypox and other orthopoxvirus infections. Antivir. Res. 5713-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berenstein, D., J. F. Christensen, T. Kristensen, R. Hofbauer, and B. Munch-Petersen. 2000. Valine, not methionine, is amino acid 106 in human cytosolic thymidine kinase (TK1). Impact on oligomerization, stability, and kinetic properties. J. Biol. Chem. 27532187-32192. [DOI] [PubMed] [Google Scholar]
- 5.Black, M. E., and D. E. Hruby. 1992. A single amino acid substitution abolishes feedback inhibition of vaccinia virus thymidine kinase. J. Biol. Chem. 2679743-9748. [PubMed] [Google Scholar]
- 6.Black, M. E., and D. E. Hruby. 1992. Site-directed mutagenesis of a conserved domain in vaccinia virus thymidine kinase. Evidence for a potential role in magnesium binding. J. Biol. Chem. 2676801-6806. [PubMed] [Google Scholar]
- 7.Bray, M. 2003. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antivir. Res. 58101-114. [DOI] [PubMed] [Google Scholar]
- 8.Breman, J. G., and D. A. Henderson. 2002. Diagnosis and management of smallpox. N. Engl. J. Med. 3461300-1308. [DOI] [PubMed] [Google Scholar]
- 9.Byrd, C. M., T. C. Bolken, A. M. Mjalli, M. N. Arimilli, R. C. Andrews, R. Rothlein, T. Andrea, M. Rao, K. L. Owens, and D. E. Hruby. 2004. New class of orthopoxvirus antiviral drugs that block viral maturation. J. Virol. 7812147-12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrd, C. M., and D. E. Hruby. 2004. Construction of recombinant vaccinia virus: cloning into the thymidine kinase locus. Methods Mol. Biol 26931-40. [DOI] [PubMed] [Google Scholar]
- 11.Cheng, Y. C., B. Goz, and W. H. Prusoff. 1975. Deoxyribonucleotide metabolism in herpes simplex virus infected HeLa cells. Biochim. Biophys. Acta 390253-263. [DOI] [PubMed] [Google Scholar]
- 12.Cheng, Y. C., J. P. Neenan, B. Goz, D. C. Ward, and W. H. Prusoff. 1975. Synthesis and biological activity of some novel analogs of thymidine. Ann. N. Y. Acad. Sci. 255332-341. [DOI] [PubMed] [Google Scholar]
- 13.Cundy, K. C., Z. H. Li, M. J. Hitchcock, and W. A. Lee. 1996. Pharmacokinetics of cidofovir in monkeys. Evidence for a prolonged elimination phase representing phosphorylated drug. Drug Metab. Dispos. 24738-744. [PubMed] [Google Scholar]
- 14.Darby, G., B. A. Larder, K. F. Bastow, and H. J. Field. 1980. Sensitivity of viruses to phosphorylated 9-(2-hydroxyethoxymethyl)guanine revealed in TK-transformed cells. J. Gen. Virol. 48451-454. [DOI] [PubMed] [Google Scholar]
- 15.Davies, E. G., A. Thrasher, K. Lacey, and J. Harper. 1999. Topical cidofovir for severe molluscum contagiosum. Lancet 3532042. [DOI] [PubMed] [Google Scholar]
- 16.De Clercq, E. 1993. Antiviral agents: characteristic activity spectrum depending on the molecular target with which they interact. Adv. Virus Res. 421-55. [DOI] [PubMed] [Google Scholar]
- 17.De Clercq, E. 1980. Antiviral and antitumor activities of 5-substituted 2′-deoxyuridines. Methods Find. Exp. Clin. Pharmacol. 2253-267. [PubMed] [Google Scholar]
- 18.De Clercq, E., A. Holy, I. Rosenberg, T. Sakuma, J. Balzarini, and P. C. Maudgal. 1986. A novel selective broad-spectrum anti-DNA virus agent. Nature 323464-467. [DOI] [PubMed] [Google Scholar]
- 19.Elion, G. B. 1983. The biochemistry and mechanism of action of acyclovir. J. Antimicrob. Chemother. 12(Suppl. B)9-17. [DOI] [PubMed] [Google Scholar]
- 20.El Omari, K., N. Solaroli, A. Karlsson, J. Balzarini, and D. K. Stammers. 2006. Structure of vaccinia virus thymidine kinase in complex with dTTP: insights for drug design. BMC Struct. Biol. 622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan, X., X. Zhang, L. Zhou, K. A. Keith, E. R. Kern, and P. F. Torrence. 2006. 5-(Dimethoxymethyl)-2′-deoxyuridine: a novel gem diether nucleoside with anti-orthopoxvirus activity. J. Med. Chem. 493377-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan, X., X. Zhang, L. Zhou, K. A. Keith, E. R. Kern, and P. F. Torrence. 2006. Assembling a smallpox biodefense by interrogating 5-substituted pyrimidine nucleoside chemical space. Antivir. Res. 71201-205. [DOI] [PubMed] [Google Scholar]
- 23.Fan, X., X. Zhang, L. Zhou, K. A. Keith, E. R. Kern, and P. F. Torrence. 2006. A pyrimidine-pyrazolone nucleoside chimera with potent in vitro anti-orthopoxvirus activity. Bioorg. Med. Chem. Lett. 163224-3228. [DOI] [PubMed] [Google Scholar]
- 24.Fan, X., X. Zhang, L. Zhou, K. A. Keith, M. N. Prichard, E. R. Kern, and P. F. Torrence. 2006. Toward orthopoxvirus countermeasures: a novel heteromorphic nucleoside of unusual structure. J. Med. Chem. 494052-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Field, H., A. McMillan, and G. Darby. 1981. The sensitivity of acyclovir-resistant mutants of herpes simplex virus to other antiviral drugs. J. Infect. Dis. 143281-285. [DOI] [PubMed] [Google Scholar]
- 26.Fulginiti, V. A., A. Papier, J. M. Lane, J. M. Neff, and D. A. Henderson. 2003. Smallpox vaccination: a review, part II. Adverse events. Clin. Infect. Dis. 37251-271. [DOI] [PubMed] [Google Scholar]
- 27.Geerinck, K., G. Lukito, R. Snoeck, R. De Vos, E. De Clercq, Y. Vanrenterghem, H. Degreef, and B. Maes. 2001. A case of human orf in an immunocompromised patient treated successfully with cidofovir cream. J. Med. Virol. 64543-549. [DOI] [PubMed] [Google Scholar]
- 28.Goz, B., and W. H. Prusoff. 1970. Pharmacology of viruses. Annu. Rev. Pharmacol. 10143-170. [DOI] [PubMed] [Google Scholar]
- 29.Hartline, C. B., E. A. Harden, S. L. Williams-Aziz, N. L. Kushner, R. J. Brideau, and E. R. Kern. 2005. Inhibition of herpesvirus replication by a series of 4-oxo-dihydroquinolines with viral polymerase activity. Antivir. Res. 6597-105. [DOI] [PubMed] [Google Scholar]
- 30.Hruby, D. E. 1985. Inhibition of vaccinia virus thymidine kinase by the distal products of its own metabolic pathway. Virus Res. 2151-156. [DOI] [PubMed] [Google Scholar]
- 31.Hruby, D. E., and L. A. Ball. 1982. Mapping and identification of the vaccinia virus thymidine kinase gene. J. Virol. 43403-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hruby, D. E., R. A. Maki, D. B. Miller, and L. A. Ball. 1983. Fine structure analysis and nucleotide sequence of the vaccinia virus thymidine kinase gene. Proc. Natl. Acad. Sci. USA 803411-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jack, M. K., and R. W. Sorenson. 1963. Vaccinial keratitis treated with IDU. Arch. Ophthalmol. 69730-732. [DOI] [PubMed] [Google Scholar]
- 34.Jamieson, A. T., and J. H. Subak-Sharpe. 1974. Biochemical studies on the herpes simplex virus-specified deoxypyrimidine kinase activity. J. Gen. Virol. 24481-492. [DOI] [PubMed] [Google Scholar]
- 35.Keith, K. A., M. J. Hitchcock, W. A. Lee, A. Holy, and E. R. Kern. 2003. Evaluation of nucleoside phosphonates and their analogs and prodrugs for inhibition of orthopoxvirus replication. Antimicrob. Agents Chemother. 472193-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keith, K. A., W. B. Wan, S. L. Ciesla, J. R. Beadle, K. Y. Hostetler, and E. R. Kern. 2004. Inhibitory activity of alkoxyalkyl and alkyl esters of cidofovir and cyclic cidofovir against orthopoxvirus replication in vitro. Antimicrob. Agents Chemother. 481869-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kern, E. R. 2003. In vitro activity of potential anti-poxvirus agents. Antivir. Res. 5735-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kern, E. R., C. Hartline, E. Harden, K. Keith, N. Rodriguez, J. R. Beadle, and K. Y. Hostetler. 2002. Enhanced inhibition of orthopoxvirus replication in vitro by alkoxyalkyl esters of cidofovir and cyclic cidofovir. Antimicrob. Agents Chemother. 46991-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kit, S., D. R. Dubbs, and M. Anken. 1967. Altered properties of thymidine kinase after infection of mouse fibroblast cells with herpes simplex virus. J. Virol. 1238-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kit, S., L. J. Piekarski, and D. R. Dubbs. 1963. Induction of thymidine kinase by vaccinia-infected mouse fibroblasts. J. Mol. Biol. 622-33. [DOI] [PubMed] [Google Scholar]
- 41.Marti-Renom, M. A., A. C. Stuart, A. Fiser, R. Sanchez, F. Melo, and A. Sali. 2000. Comparative protein structure modeling of genes and genomes. Annu. Rev. Biophys. Biomol. Struct. 29291-325. [DOI] [PubMed] [Google Scholar]
- 42.McGeoch, D. J., M. A. Dalrymple, A. J. Davison, A. Dolan, M. C. Frame, D. McNab, L. J. Perry, J. E. Scott, and P. Taylor. 1988. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J. Gen. Virol. 69(Pt. 7)1531-1574. [DOI] [PubMed] [Google Scholar]
- 43.Neyts, J., H. Sobis, R. Snoeck, M. Vandeputte, and E. De Clercq. 1993. Efficacy of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)-cytosine and 9-(1,3-dihydroxy-2-propoxymethyl)-guanine in the treatment of intracerebral murine cytomegalovirus infections in immunocompetent and immunodeficient mice. Eur. J. Clin. Microbiol. Infect. Dis. 12269-279. [DOI] [PubMed] [Google Scholar]
- 44.Neyts, J., E. Verbeken, and E. De Clercq. 2002. Effect of 5-iodo-2′-deoxyuridine on vaccinia virus (orthopoxvirus) infections in mice. Antimicrob Agents Chemother. 462842-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prichard, M. N., K. A. Keith, D. C. Quenelle, and E. R. Kern. 2006. Activity and mechanism of action of N-methanocarbathymidine against herpesvirus and orthopoxvirus infections. Antimicrob Agents Chemother. 501336-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prichard, M. N., and C. Shipman, Jr. 1990. A three-dimensional model to analyze drug-drug interactions. Antivir. Res. 14181-205. [DOI] [PubMed] [Google Scholar]
- 47.Prichard, M. N., A. D. Williams, K. A. Keith, E. A. Harden, and E. R. Kern. 2006. Distinct thymidine kinases encoded by cowpox virus and herpes simplex virus contribute significantly to the differential antiviral activity of nucleoside analogs. Antivir. Res. 711-6. [DOI] [PubMed] [Google Scholar]
- 48.Prusoff, W. H., Y. S. Bakhle, and J. F. McCrea. 1963. Incorporation of 5-iodo-2′-deoxyuridine into the deoxyribonucleic acid of vaccinia virus. Nature 1991310-1311. [DOI] [PubMed] [Google Scholar]
- 49.Rybak, R. J., C. B. Hartline, Y. L. Qiu, J. Zemlicka, E. Harden, G. Marshall, J. P. Sommadossi, and E. R. Kern. 2000. In vitro activities of methylenecyclopropane analogues of nucleosides and their phosphoralaninate prodrugs against cytomegalovirus and other herpesvirus infections. Antimicrob. Agents Chemother. 441506-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schelling, P., G. Folkers, and L. Scapozza. 2001. A spectrophotometric assay for quantitative determination of kcat of herpes simplex virus type 1 thymidine kinase substrates. Anal. Biochem. 29582-87. [DOI] [PubMed] [Google Scholar]
- 51.Stittelaar, K. J., J. Neyts, L. Naesens, G. van Amerongen, R. F. van Lavieren, A. Holy, E. De Clercq, H. G. Niesters, E. Fries, C. Maas, P. G. Mulder, B. A. van der Zeijst, and A. D. Osterhaus. 2006. Antiviral treatment is more effective than smallpox vaccination upon lethal monkeypox virus infection. Nature 439745-748. [DOI] [PubMed] [Google Scholar]
- 52.Tseng, C. K. 2005. Overview of antiviral drug discovery and development, p. 31-82. In P. F. Torrence (ed.), Antiviral drug discovery for emerging diseases and bioterrorism threats. Wiley-Interscience, Hoboken, NJ.
- 53.Welin, M., U. Kosinska, N. E. Mikkelsen, C. Carnrot, C. Zhu, L. Wang, S. Eriksson, B. Munch-Petersen, and H. Eklund. 2004. Structures of thymidine kinase 1 of human and mycoplasmic origin. Proc. Natl. Acad. Sci. USA 10117970-17975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang, G., D. C. Pevear, M. H. Davies, M. S. Collett, T. Bailey, S. Rippen, L. Barone, C. Burns, G. Rhodes, S. Tohan, J. W. Huggins, R. O. Baker, R. L. Buller, E. Touchette, K. Waller, J. Schriewer, J. Neyts, E. DeClercq, K. Jones, D. Hruby, and R. Jordan. 2005. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus challenge. J. Virol. 7913139-13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zou, F. C., G. E. Dutschman, E. De Clercq, and Y. C. Cheng. 1984. Differential binding affinities of sugar-modified derivatives of (E)-5-(2-bromovinyl)-2′-deoxyuridine for herpes simplex virus-induced and human cellular deoxythymidine kinases. Biochem. Pharmacol. 331797-1800. [DOI] [PubMed] [Google Scholar]