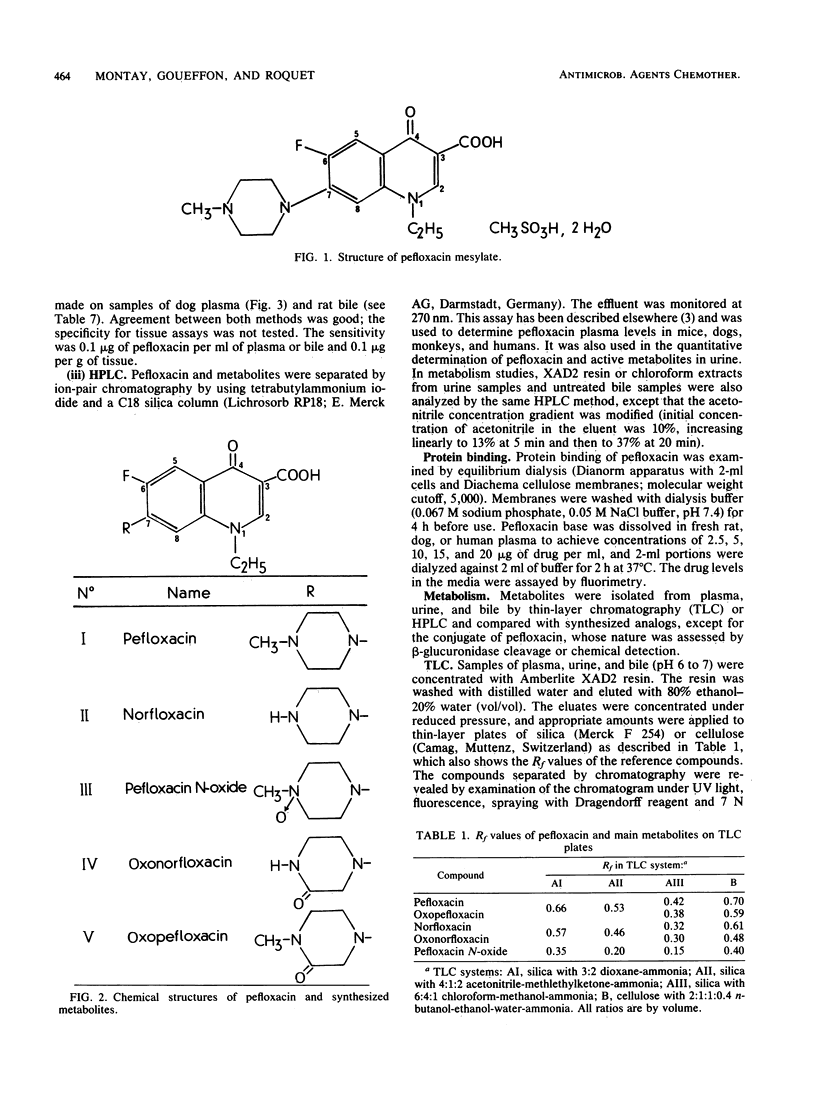
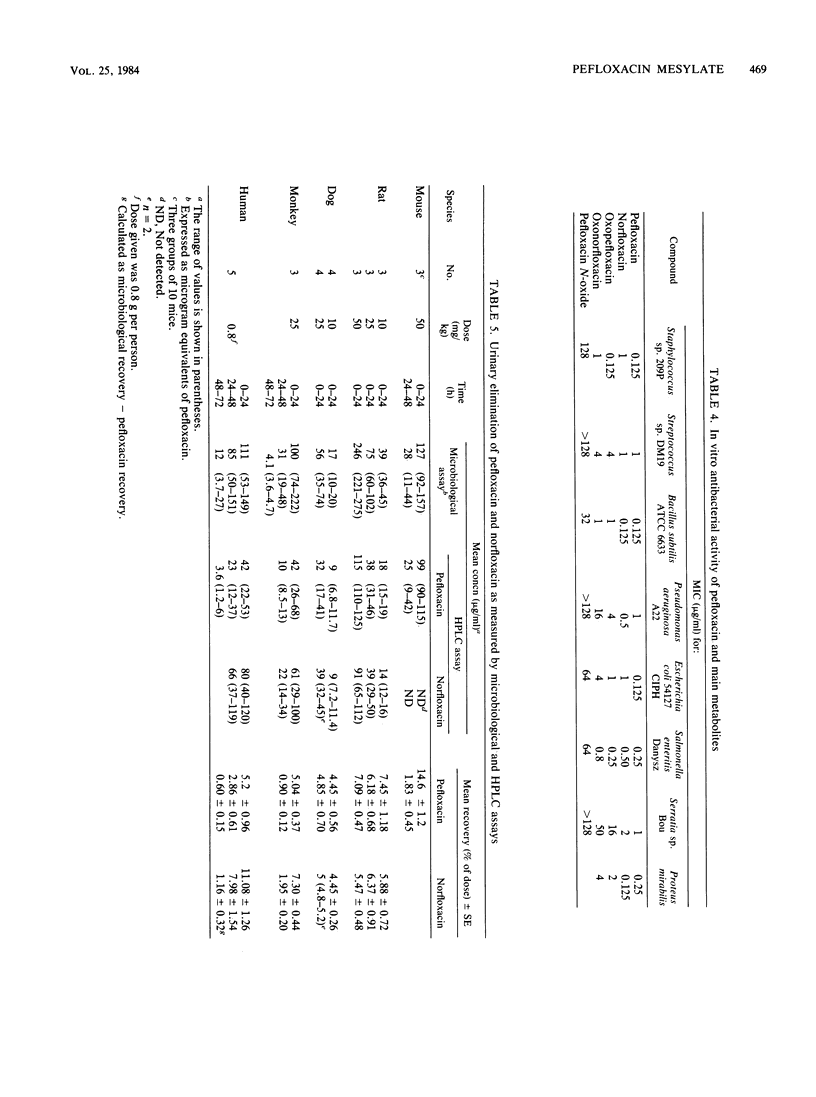
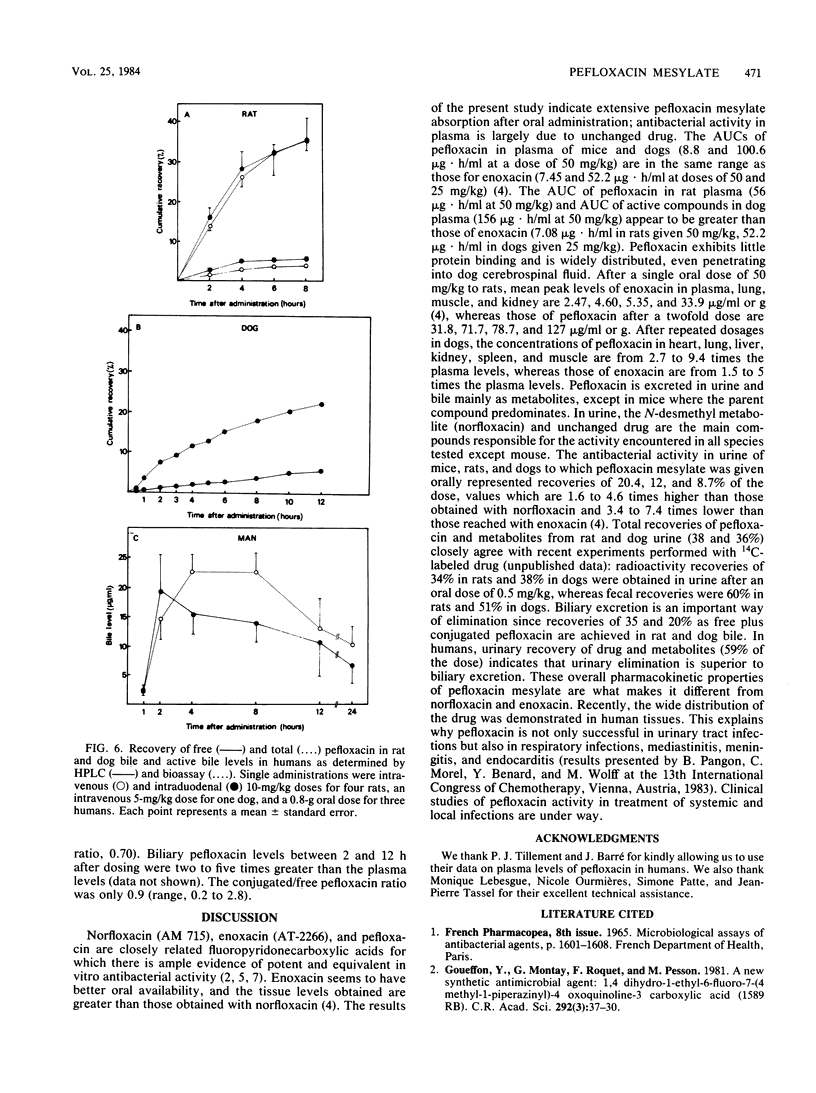
Abstract
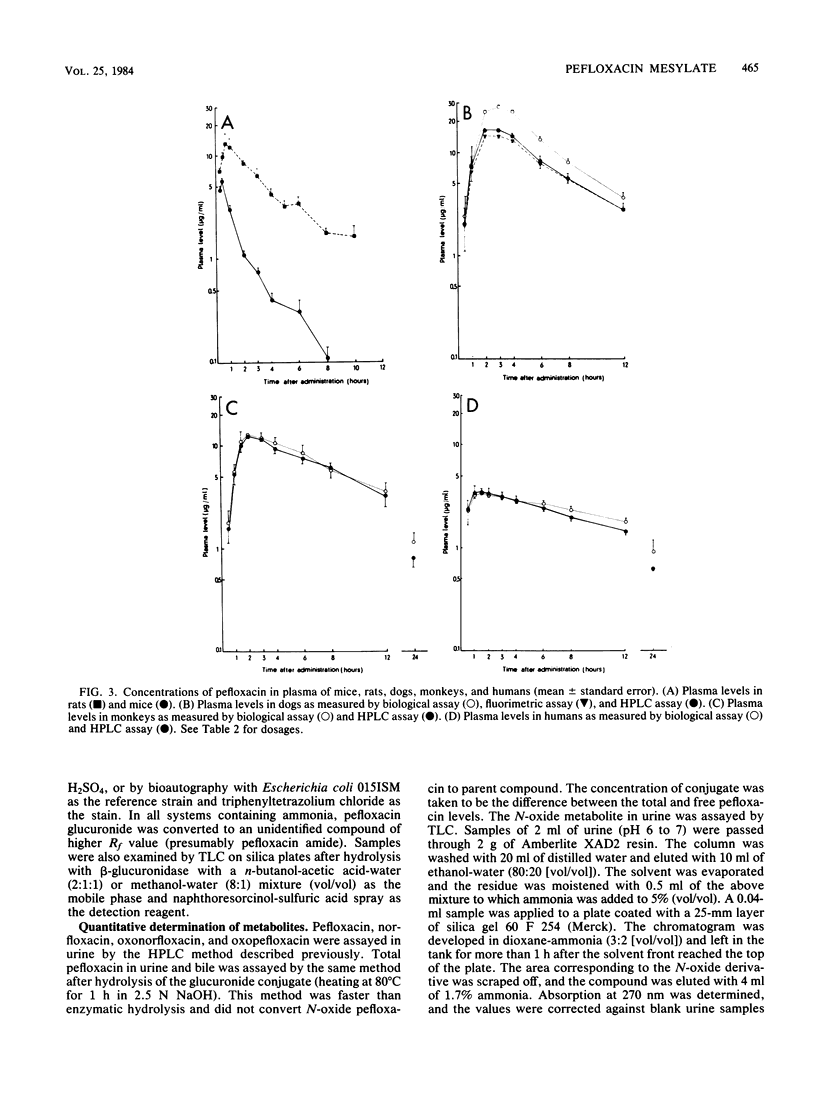
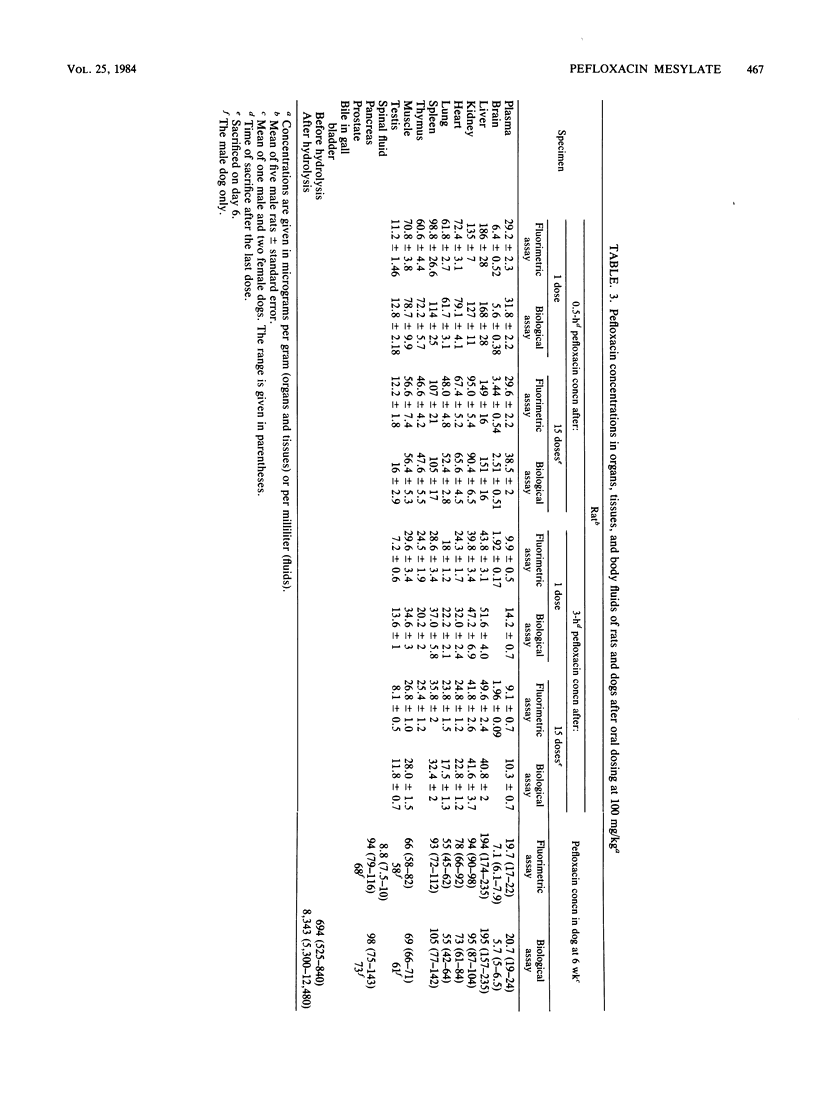
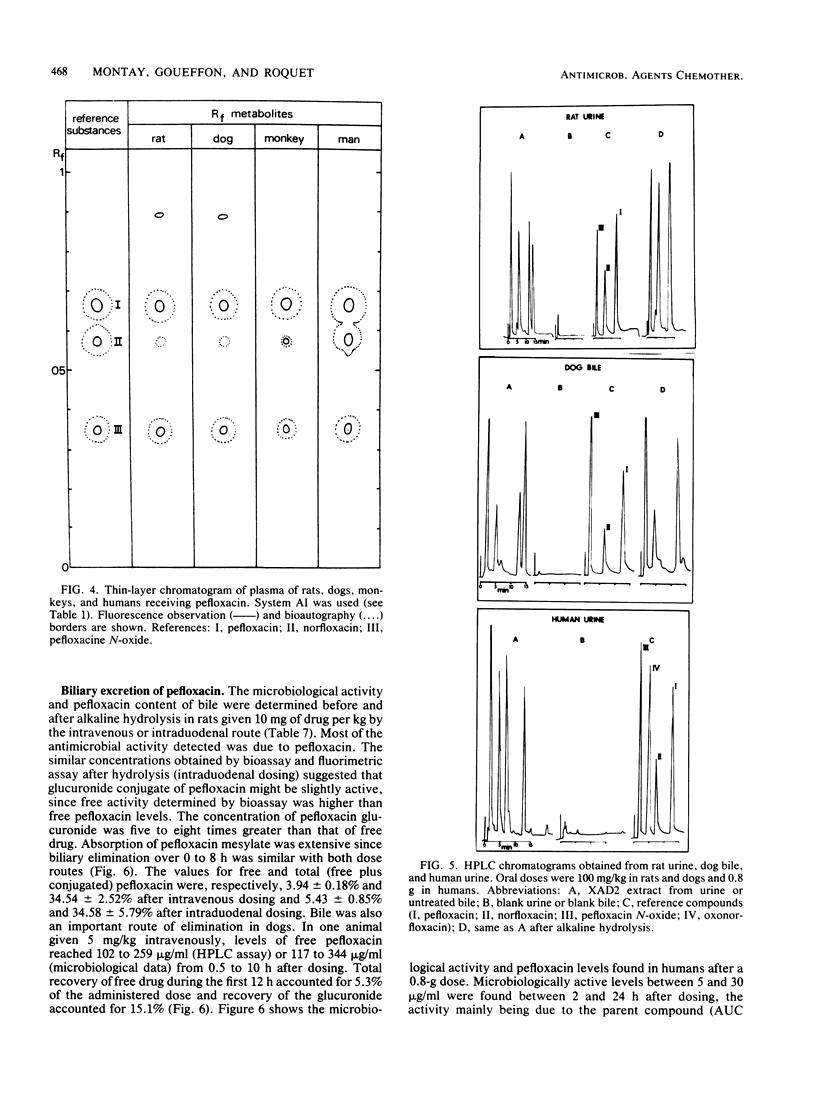
Pefloxacin mesylate is well absorbed by the oral route. The antimicrobial activity in dog, cynomolgus monkey, and human plasma was essentially due to unchanged drug which respectively accounted for 64, 94, and 84% of the total activity (ratios derived from relative area under the curve [AUC] values). Half-lives ranged from 1.9 h in mice to 8.6 h in humans. Protein binding was weak, about 20% in plasma. Except in brain, concentrations in most of the organs and tissues tested in rats and dogs were higher than the plasma levels. Microbiological activity in urine was mainly due to pefloxacin and norfloxacin, the N-desmethyl metabolite. The norfloxacin/pefloxacin ratios were 0 in mice, ca. 1 in rats and dogs, 1.6 in cynomolgus monkeys, and 2.3 in humans. The principal urinary compounds were unchanged drug in mice, pefloxacin glucuronide and pefloxacin N-oxide in rats and dogs, norfloxacin and pefloxacin in monkeys, and pefloxacin N-oxide and norfloxacin in humans. The urinary recovery of identified metabolites was 29.5% of the dose in mice, 37.8% in rats, 36.3% in dogs, 26.5% in monkeys, and 58.9% in humans. Biliary excretion occurred and was extensive in rats and dogs, mainly as a glucuronide conjugate of the drug. In rat and human bile, the main active compound was unchanged pefloxacin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Goueffon Y., Montay G., Roquet F., Pesson M. Sur un nouvel antibactérien de synthèse : l'acide éthyl-1 fluoro-6 (méthyl-4 pipérazinyl-1)-7 oxo-4 dihydro-1.4 quinoléine-3 carboxylique (1589 R.B.). C R Seances Acad Sci III. 1981 Jan 5;292(1):37–40. [PubMed] [Google Scholar]
- Montay G., Blain Y., Roquet F., Le Hir A. High-performance liquid chromatography of pefloxacin and its main active metabolites in biological fluids. J Chromatogr. 1983 Feb 11;272(2):359–365. doi: 10.1016/s0378-4347(00)86139-3. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Kurobe N., Kashimoto S., Ohue T., Takase Y., Shimizu M. Pharmacokinetics of AT-2266 administered orally to mice, rats, dogs, and monkeys. Antimicrob Agents Chemother. 1983 Jul;24(1):54–60. doi: 10.1128/aac.24.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Minami A., Katae H., Inoue S., Yamagishi J., Takase Y., Shimizu M. In vitro antibacterial properties of AT-2266, a new pyridonecarboxylic acid. Antimicrob Agents Chemother. 1983 May;23(5):641–648. doi: 10.1128/aac.23.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thabaut A., Durosoir J. L. Activité antibactérienne comparée in vitro de la péfloxacine (1589 RB), de l'acide nalidixique, de l'acide pipémidique et de la fluméquine. Pathol Biol (Paris) 1982 Jun;30(6):394–397. [PubMed] [Google Scholar]
- Thibault M., Koumaré B., Soussy C. J., Duval J. Relations structure-activité dans le groupe des quinolones: étude de l'activité antibactérienne de deux nouveaux composés. Ann Microbiol (Paris) 1981 May-Jun;132(3):267–281. [PubMed] [Google Scholar]



