Abstract
The susceptibility trends for the species of the Bacteroides fragilis group against various antibiotics from 1997 to 2004 were determined by using data for 5,225 isolates referred by 10 medical centers. The antibiotic test panel included ertapenem, imipenem, meropenem, ampicillin-sulbactam, piperacillin-tazobactam, cefoxitin, clindamycin, moxifloxacin, tigecycline, chloramphenicol, and metronidazole. From 1997 to 2004 there were decreases in the geometric mean (GM) MICs of imipenem, meropenem, piperacillin-tazobactam, and cefoxitin for many of the species within the group. B. distasonis showed the highest rates of resistance to most of the β-lactams. B. fragilis, B. ovatus, and B. thetaiotaomicron showed significantly higher GM MICs and rates of resistance to clindamycin over time. The rate of resistance to moxifloxacin of B. vulgatus was very high (MIC range for the 8-year study period, 38% to 66%). B. fragilis, B. ovatus, and B. distasonis and other Bacteroides spp. exhibited significant increases in the rates of resistance to moxifloxacin over the 8 years. Resistance rates and GM MICs for tigecycline were low and stable during the 5-year period over which this agent was studied. All isolates were susceptible to chloramphenicol (MICs < 16 μg/ml). In 2002, one isolate resistant to metronidazole (MIC = 64 μg/ml) was noted. These data indicate changes in susceptibility over time; surprisingly, some antimicrobial agents are more active now than they were 5 years ago.
Pathogens of the Bacteroides fragilis group are the anaerobic pathogens that are the most frequently isolated from blood and abscesses. They are also among the most antibiotic-resistant isolates in anaerobic and mixed infections (21). Susceptibility to antibiotics varies considerably among the species of the group, yet most clinical laboratories do not routinely determine the species of the organism or test the susceptibilities of any anaerobic isolates, including those in the B. fragilis group, due to technical difficulties surrounding Bacteroides susceptibility testing (21). Consequently, the treatment of anaerobic infections is selected empirically, based on published reports on patterns of susceptibility (14, 15, 19, 20). Therefore, the importance for reference laboratories to provide information on the patterns of susceptibility of the species within the group is important clinically. For over 20 years we have conducted a national survey on the susceptibility patterns of these important pathogens and our laboratory at Tufts New England Medical Center served as a reference center for the storage and testing of Bacteroides clinical isolates. We undertook this analysis to determine the susceptibility trends of the various species, using data from 1997 to 2004 for 5,225 isolates referred by 10 geographically diverse medical centers distributed throughout the United States.
(This study was presented in part at the 22nd European Congress on Clinical Microbiology and Infectious Diseases, Nice, France, April 2006.)
MATERIALS AND METHODS
Medical centers.
The isolates were referred from medical centers representing various geographical areas of the United States: Albany Medical Center, Albany, NY; Carolinas Medical Center, Charlotte, NC; Duke University Medical Center, Durham, NC; Loyola University Medical Center, Maywood, IL; New England Medical Center, Boston, MA; Mt. Sinai Medical Center, New York, NY; Pittsburgh Veterans Administration Center, Pittsburgh, PA; R. M. Alden Research Laboratories Santa Monica, CA; University of Michigan Medical Center, Ann Arbor, MI; and Wadsworth Veterans Administration Hospital, Los Angeles, CA.
Antimicrobial agents.
Standard powders of the following antibiotics were obtained from the indicated manufacturers: cefoxitin, ertapenem, and imipenem, Merck & Co., West Point, PA; ampicillin and sulbactam, Pfizer, Inc., New York, NY; piperacillin, tazobactam, and tigecycline, Wyeth Ayerst Research, Pearl River, NY; meropenem, Astra-Zeneca Pharmaceuticals, Wilmington, DE; moxifloxacin, Bayer Pharmaceuticals, West Haven, CT; clindamycin, Pharmacia Upjohn, Kalamazoo, MI, and United States Phamacopeia (USP), Rockville, MD; and metronidazole and chloramphenicol, Sigma Chemical, St. Louis, MO.
Bacterial isolates.
Nonduplicate clinical isolates of the B. fragilis group were referred for susceptibility testing to the Special Studies Laboratory at New England Medical Center by the medical centers participating in the survey. The isolates were shipped on prereduced agar slants and were stored until the time of testing. A total of 5,225 isolates were analyzed. The identification of the isolates was confirmed by using the API RapidANA II system and/or the standard methodology (11, 23).
Susceptibility testing.
MICs were determined by the agar dilution method following the recommendations of Clinical Laboratory Standards Institute (CLSI; formerly the NCCLS) (13). The plates were prepared on the day of the test by using enriched brucella agar (brucella agar supplemented with 5% lysed defibrinated sheep red blood cells and 1 μg/ml vitamin K). For the preparation of the inocula, the organisms were grown to logarithmic phase, and the turbidity was adjusted to that of a 0.5 McFarland standard (∼108 CFU/ml). The inocula were delivered to the surface of the agar plate with a Steers replicator, resulting in an organism concentration of 105 CFU/spot. The inoculated plates were incubated at 37°C in an anaerobic chamber for 48 h. In all tests, B. fragilis ATCC 25285 and B. thetaiotaomicron ATCC 29741 were used as controls. Tests were repeated when the MICs of the control organisms were outside of the CLSI-specified range (13). For tigecycline, the range used was that determined in a standardized study involving eight laboratories (D. Hecht, data presented at CLSI meeting, San Diego, CA, June 2006).
Data analysis.
Data were stored in Microsoft Excel spreadsheets. Statistical analysis was performed by using the SAS system for Windows, version 8.01. Trends for increased or decreased resistance over the 8 years were tested by using the Cochran-Armitage test of trend (1). The breakpoints for resistance used for data analysis were those established by regulatory agencies and were as follows: for the carbapenems, β-lactam-β-lactamase inhibitor combinations, cefoxitin, and clindamycin, the breakpoints used were those recommended by CLSI for anaerobic bacteria (13); for moxifloxacin and tigecycline, FDA-established breakpoints for resistance were used, since CLSI does not have any currently published susceptibility criteria recommendations for these two agents (Tygacil package insert [Wyeth Pharmaceuticals] and Avelox package insert [Bayer Pharmaceuticals]). Trends for increased or decreased MICs over time were evaluated by using linear regression analysis of the log10 MIC results. P values from the linear regression analysis are presented together with the geometric mean (GM) MIC, calculated as the antilog of the arithmetic average of the observed log10 MICs. An alpha level of 0.05 was used to determine statistical significance.
RESULTS
Table 1 shows the distribution by species of the 5,225 isolates included in the study. As previously reported by us and other investigators, B. fragilis continues to be the most common species within the group (52.1%), followed by B. thetaiotaomicron and B. ovatus (18.7% and 10.4%, respectively) (3, 5, 21). Among the 202 isolates grouped under Bacteroides “other,” B. caccae was the most frequent isolate: 150 (74%) isolates over the 8-year study period. In addition, there were 34 B. eggerthii isolates, 2 B. merdae isolates, 15 B. stercoris isolates, and 1 Bacteroides “other” isolate not identified. Because of their small numbers (compared to the numbers of the other species), the data for these isolates was compiled into one group.
TABLE 1.
Distribution of the species within the Bacteroides fragilis group
| Species/group | No. (%) of isolates
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1997 | 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | All yr | |
| B. distasonis | 41 (6.4) | 52 (6.2) | 35 (5.8) | 36 (6.1) | 17 (3.1) | 32 (5.7) | 28 (3.9) | 33 (4.5) | 274 (5.2) |
| B. fragilis | 336 (52.5) | 434 (51.5) | 314 (52.2) | 288 (48.9) | 286 (51.7) | 277 (49.6) | 378 (53.2) | 409 (56.0) | 2,722 (52.1) |
| B. ovatus | 67 (10.5) | 120 (14.3) | 57 (9.5) | 61 (10.4) | 73 (13.2) | 45 (8.1) | 71 (10.0) | 51 (7.0) | 545 (10.4) |
| B. thetaiotaomicron | 102 (15.9) | 128 (15.2) | 118 (19.6) | 136 (23.1) | 98 (17.7) | 123 (22.0) | 152 (21.4) | 122 (16.6) | 979 (18.7) |
| B. uniformis | 44 (6.9) | 28 (3.3) | 9 (1.5) | 11 (1.9) | 16 (2.9) | 24 (4.3) | 23 (3.2) | 42 (5.8) | 197 (3.8) |
| B. vulgatus | 32 (5.0) | 45 (5.3) | 45 (7.5) | 35 (5.9) | 40 (7.2) | 31 (5.6) | 29 (4.1) | 49 (6.7) | 306 (5.9) |
| Other species within the B. fragilis groupa | 18 (2.8) | 35 (4.2) | 24 (4.0) | 22 (3.7) | 23 (4.2) | 26 (4.7) | 29 (4.1) | 25 (3.4) | 202 (3.9) |
| All the species in the B. fragilis group | 640 (12.3) | 842 (16.1) | 602 (11.5) | 589 (11.3) | 553 (10.6) | 558 (10.7) | 710 (13.6) | 729 (14.0) | 5,225 (100.1) |
| Non-B. fragilis species | 304 (47.5) | 408 (48.5) | 288 (47.8) | 301 (51.1) | 267 (48.3) | 281 (50.4) | 332 (46.8) | 322 (44.0) | 2,503 (47.9) |
Includes 150 B. caccae isolates, 34 B. eggerthii isolates, 2 B. merdae isolates, 15 B. stercoris isolates, and 1 nonidentified isolate.
Table 2 is a summary of the susceptibilities of the isolates for the 8 years of the study period expressed as MIC range, GM MIC, MIC90, and percent resistant. The percent resistance was calculated by using the CLSI- or FDA-recommended breakpoints for the each antibiotic (13; Tygacil package insert [Wyeth Pharmaceuticals] and Avelox package insert [Bayer Pharmaceuticals]). In general, of the species tested, B. fragilis was the most susceptible to most agents. B. fragilis showed the lowest rates of resistance to the carbapenems and β-lactam-β-lactamase inhibitor combinations, and the rates of resistance to cefoxitin and tigecycline were approximately 5% each, while the rates of resistance to clindamycin and moxifloxacin were 19% and 27%, respectively.
TABLE 2.
Susceptibilities of the isolates by species: all isolates, 8 years of data
| Species (no. of isolates) | Ertapenem (>16 μg/ml)b
|
Imipenem (>16 μg/ml)
|
Meropenem (>16 μg/ml)
|
Ampicillin-sulbactam (>32 μg/ml)
|
Piperacillin-tazobactam (>128 μg/ml)
|
Cefoxitin (>64 μg/ml)
|
Clindamycin (>8 μg/ml)
|
Moxifloxacin (>8 μg/ml)
|
Tigecycline (>16 μg/ml)
|
|||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC range (μg/ml) | GM MIC (μg/ml) | MIC90 (μg/ml) | % Resistant | MIC range (μg/ml) | GM MIC (μg/ml) | MIC90 (μg/ml) | % Resistant | MIC range (μg/ml) | GM MIC (μg/ml) | MIC90 (μg/ml) | % Resistant | MIC range (μg/ml) | GM MIC (μg/ml) | MIC90 (μg/ml) | % Resistant | MIC range (μg/ml) | GM MIC (μg/ml) | MIC90 (μg/ml) | % Resistant | MIC range (μg/ml) | GM MIC (μg/ml) | MIC90 (μg/ml) | % Resistant | MIC range (μg/ml) | GM MIC (μg/ml) | MIC90 (μg/ml) | % Resistant | MIC range (μg/ml) | GM MIC (μg/ml) | MIC90 (μg/ml) | % Resistant | MIC range (μg/ml) | GM MIC (μg/ml) | MIC90 (μg/ml) | % Resistant | |
| B. fragilis (2,722) | 0.125-64 | 0.79 | 2 | 1.4 | 0.125-64 | 0.31 | 1 | 0.5 | 0.125-64 | 0.38 | 1 | 1.0 | 0.25-256 | 3.36 | 16 | 1.7 | 0.25-512 | 1.14 | 4 | 0.4 | 2-256 | 15.42 | 32 | 5.2 | 0.5-256 | 1.89 | 256 | 19.3 | 0.06-128 | 1.83 | 8 | 27.3 | 0.06-32 | 1.59 | 8 | 5.1 |
| B. distasonis (274) | 0.125-32 | 1.77 | 4 | 1.5 | 0.125-16 | 0.95 | 2 | 0.4 | 0.125-16 | 0.58 | 2 | 1.1 | 0.5-128 | 10.73 | 32 | 16.8 | 0.5-512 | 9.17 | 16 | 1.1 | 2-256 | 34.18 | 128 | 29.9 | 0.5-256 | 4.51 | 256 | 29.6 | 0.125-64 | 2.32 | 16 | 29.2 | 0.25-32 | 2.09 | 8 | 2.1 |
| B. ovatus (545) | 0.125-16 | 1.58 | 4 | 0.6 | 0.125-16 | 0.39 | 1 | 0.2 | 0.25-64 | 3.64 | 16 | 2.2 | 0.25-512 | 4.22 | 16 | 0.4 | 2-256 | 27.16 | 64 | 17.2 | 0.5-256 | 5.89 | 256 | 33.4 | 0.5-128 | 5.49 | 32 | 38.3 | 0.125-16 | 1.52 | 8 | 3.3 | ||||
| B. thetaiotaomicron (979) | 0.125-32 | 1.69 | 4 | 0.4 | 0.125-32 | 0.45 | 1 | 0.2 | 0.125-32 | 0.47 | 1 | 0.1 | 0.5-256 | 3.75 | 16 | 2.1 | 0.25-512 | 8.00 | 16 | 0.4 | 2-256 | 29.50 | 64 | 16.8 | 0.5-256 | 6.62 | 256 | 33.3 | 0.125-128 | 3.71 | 32 | 26.3 | 0.25-32 | 1.57 | 8 | 3.6 |
| B. uniformis (197) | 0.125-32 | 0.88 | 2 | 0.5 | 0.125-32 | 0.43 | 1 | 0.5 | 0.125-32 | 0.41 | 1 | 0.5 | 0.25-32 | 3.54 | 16 | 1.5 | 0.25-32 | 2.33 | 8 | 0.0 | 2-256 | 12.91 | 32 | 4.6 | 0.5-256 | 4.22 | 256 | 28.9 | 0.06-64 | 5.57 | 32 | 38.6 | 0.06-16 | 0.77 | 4 | 3.4 |
| B. vulgatus (306) | 0.125-16 | 0.82 | 2 | 0.3 | 0.125-16 | 0.50 | 1 | 0.3 | 0.125-8 | 0.47 | 1 | 0.0 | 0.5-128 | 5.46 | 16 | 2.0 | 0.25-512 | 5.05 | 16 | 0.7 | 2-256 | 10.50 | 32 | 7.5 | 0.5-256 | 4.31 | 256 | 35.3 | 0.5-128 | 7.92 | 64 | 54.7 | 0.25-16 | 1.05 | 4 | 1.60 |
| Other speciesa (202) | 0.125-64 | 1.03 | 4 | 0.5 | 0.125-4 | 0.35 | 1 | 0.0 | 0.125-64 | 0.40 | 1 | 0.5 | 0.25-64 | 3.46 | 16 | 1.0 | 0.25-128 | 3.23 | 16 | 0.5 | 2-256 | 17.61 | 64 | 10.9 | 0.5-256 | 3.63 | 256 | 28.2 | 0.25-128 | 3.85 | 32 | 36.4 | 0.125-64 | 1.51 | 8 | 7.20 |
| Non-B. fragilis spp. (2,503) | 0.125-64 | 1.4 | 4 | 0.6 | 0.125-32 | 0.47 | 1 | 0.2 | 0.125-64 | 0.48 | 1 | 0.3 | 0.25-256 | 4.33 | 16 | 3.6 | 0.25-512 | 5.63 | 16 | 0.5 | 2-256 | 23.32 | 64 | 15.7 | 0.5-256 | 5.4 | 256 | 32.4 | 0.06-128 | 4.36 | 32 | 34.5 | 0.06-64 | 1.44 | 8 | 3.5 |
| B. fragilis group (5,225) | 0.125-64 | 1.04 | 4 | 1.0 | 0.125-64 | 0.38 | 1 | 0.4 | 0.125-64 | 0.42 | 1 | 0.6 | 0.25-256 | 3.79 | 16 | 2.6 | 0.25-512 | 2.46 | 16 | 0.5 | 2-256 | 18.80 | 64 | 10.3 | 0.5-256 | 3.13 | 256 | 25.6 | 0.06-128 | 4.36 | 32 | 34.5 | 0.06-64 | 1.52 | 8 | 4.3 |
Includes 150 B. caccae isolates, 34 B. eggerthii isolates, 2 B. merdae isolates, 15 B. stercoris isolates, and 1 nonidentified isolate.
The breakpoints for resistance are given in parentheses. The breakpoints for resistance for ertapenem, imipenem, meropenem, piperacillin-tazobactam, ampicillin-sulbactam, cefoxitin, and clindamycin are those recommended by NCCLS (13). The breakpoints for resistance for moxifloxacin and tigecycline are FDA recommendations (Tygacil package insert [Wyeth Pharmaceuticals] and Avelox package insert [Bayer Pharmaceuticals]).
Analysis of the non-B. fragilis species showed that the GM MICs of all the β-lactams agents (carbapenems, inhibitor combinations, cefoxitin) and tigecycline for B. distasonis were generally the highest. In addition, B. distasonis was the most resistant of all the species to ampicillin-sulbactam (resistance rate of 16.8% compared to an average resistance rate of 1.8% for all the other species combined). Approximately one-third or more of the B. ovatus, B. thetaiotaomicron, and B. uniformis isolates were resistant to clindamycin. Among the isolates of these three species, high rates of resistance to moxifloxacin were also observed (38.3%, 26.3%, and 38.6%, respectively).
Over half the B. vulgatus isolates were resistant to moxifloxacin (54.7%), and more than a third were resistant to clindamycin (35.3%). However, all isolates of this species were highly susceptible to the other antibiotics tested.
Isolates in the group Bacteroides “other” (B. caccae, B. eggerthii, B. merdae, and B. stercoris) showed relatively high rates of resistance to tigecycline (7.2%) compared to the rates for the other species (≤5%). Within this group, we also observed high rates of resistance to moxifloxacin (36.4%) and clindamycin (28.2%).
In general, the GM MICs of all the antibiotics against all the species were below their breakpoints for resistance; however, the MIC90s of clindamycin and moxifloxacin against all the species were at or above the breakpoints for resistance. The MIC90 of ampicillin-sulbactam was at the breakpoint for resistance for B. distasonis (32 μg/ml), while the MIC90s of cefoxitin were at the breakpoint for resistance (64 μg/ml) for B. ovatus, B. thetaiotaomicron, and Bacteroides “other” and were above this value for B. distasonis. By comparison, the MIC90s of the three carbapenems as well as those of piperacillin-tazobactam and tigecycline were below the breakpoint for resistance for all the species.
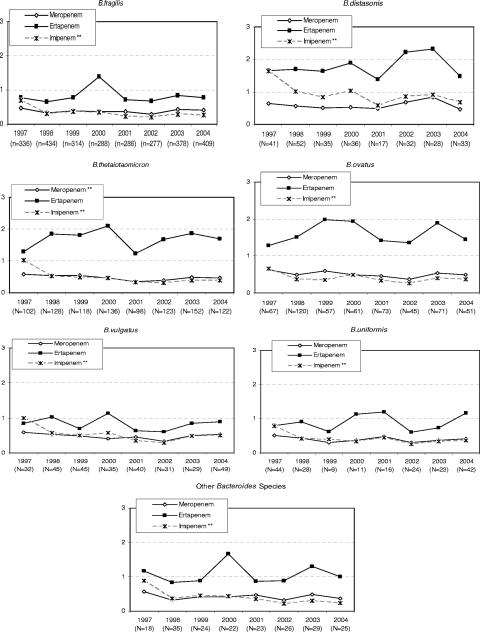
Since very few isolates were resistant to the carbapenems, we chose to examine the GM MIC over time (Fig. 1). Susceptibility trends from 1997 to 2004 (Fig. 1) showed an overall significant decrease in the GM MIC for imipenem against all the species of the group. A significant decrease in the GM MIC of meropenem for B. thetaiotaomicron was also observed. The GM MICs for ertapenem remained virtually unchanged against all the species within the group.
FIG. 1.
Change in GM MICs of the carbapenems over time (1997 to 2004) for Bacteroides species. **, statistically significant trend over time at the P < 0.05 level.
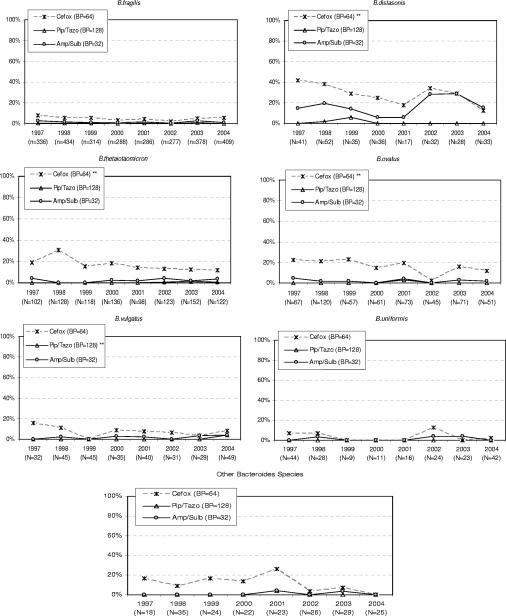
Figure 2 illustrates the trend over time in the percent resistance to piperacillin-tazobactam, ampicillin-sulbactam, and cefoxitin against Bacteroides species. With the exception of the rates for B. distasonis in 1999, the rates of resistance to piperacillin-tazobactam were ≤1% for all the years of the study. A significant decrease in percent resistance to piperacillin-tazobactam was noted against B. vulgatus. In contrast, the rates of resistance ampicillin-sulbactam were ≥20% in 2002 and 2003. At a breakpoint of 64 μg/ml, a significant decrease in the percent resistance to cefoxitin was noted for B. distasonis, B. ovatus, and B. thetaiotaomicron. The trends in the GM MICs of the inhibitor combinations and cefoxitin are not shown; however, we noted that the GM MIC of piperacillin-tazobactam against all species except B. thetaiotaomicron and B. uniformis declined significantly, while the GM MIC of ampicillin-sulbactam against B. ovatus increased significantly (P = 0.012). With the exception of B. uniformis, cefoxitin also showed a significant decrease in its GM MICs against all the non-Bacteroides species.
FIG. 2.
Change in percent resistant at the specified breakpoints (BP) for Bacteroides species over time (1997 to 2004) for inhibitor combinations and cefoxitin (Cefox). Pip/Tazo, piperacillin-tazobactam; Amp/Sulb, ampicillin-sulbactam; **, statistically significant trend over time at the P < 0.05 level.
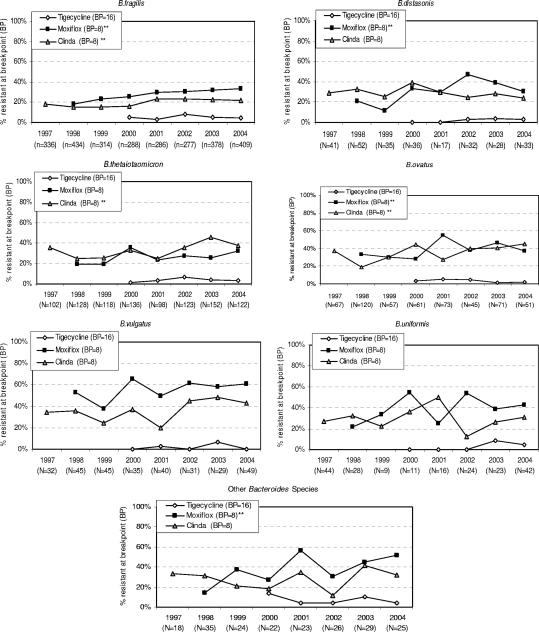
The trends in resistance to clindamycin, moxifloxacin, and tigecycline are illustrated in Fig. 3. The rate of resistance of B. fragilis, B. ovatus and B. thetaiotaomicron to clindamycin increased significantly. In addition, the increased percent resistance to clindamycin by these species was accompanied by significant increases in the GM MICs. An increase in the rate of resistance to moxifloxacin was observed for B. fragilis, B. distasonis, B. ovatus, and “other” Bacteroides spp. For moxifloxacin, significant increases in the GM MICs for the following species were also noted: B. distasonis, B. fragilis, B. ovatus, B. thetaiotaomicron, and “other” Bacteroides spp. (data on GM MICs not shown).
FIG. 3.
Change in percent resistant at the specified breakpoints (BP) for Bacteroides species over time (1997 to 2004) for clindamycin (Clinda), moxifloxacin (Moxiflox), and tigecycline. **, statistically significant trend over time at the P < 0.05 level.
During the 5 years that tigecycline was studied (2000 to 2004), the percent resistant as well as the GM MICs remained relatively stable against all species. Among all the species, the resistance rates varied from a low of 1.6% for B. vulgatus to a high of 7.2% for “other” Bacteroides spp.
Because of the excellent activities of metronidazole and chloramphenicol against the B. fragilis group isolates, the data for these two agents are not shown. All isolates were susceptible to chloramphenicol at concentrations of <8 μg/ml; however, the first confirmed metronidazole-resistant isolate (MIC, 64 μg/ml) in the United States was tested in 2002; none were noted in 2003 or 2004.
DISCUSSION
As in previous reports, the present study shows the variability of resistance patterns among the species of the B. fragilis group (21, 22). To facilitate the comparison of trends over time with the trends described in our previous reports as well as with those from surveillance studies performed by other investigators, the results are presented as changes in the percent resistance of the various antibiotics evaluated rather than percent susceptible. Emphasis through the use of percent resistance also highlights the importance of selecting an effective and active agent for the treatment of B. fragilis infections (20, 21).
Our results confirmed some interesting trends noticed previously (22). There is a continuing trend toward significantly lower MICs of the carbapenems. The exception to this trend is ertapenem; however, the rate of resistance to this drug is still very low (1.4%).
Piperacillin-tazobactam remains the most active β-lactamase inhibitor combination. This class of antibiotics remains very active against the B. fragilis group. Nevertheless, an interesting trend is observed with the bug-drug combination of B. distasonis and ampicillin-sulbactam; indeed, the rate of resistance to this combination increased from less than 10% during the initial years of the study to approximately 20% during the later years. This cautionary statement also applies to B. distasonis and cefoxitin since the 8-year resistance rate of this species was very high (36.3%). The rates of resistance to clindamycin remained high, and as reported previously, resistance is higher among the non-B. fragilis species (22).
At the FDA-established breakpoint for resistance of 8 μg/ml (currently, CLSI has not published susceptibility criteria for this agent), significantly increased rates of resistance to moxifloxacin were noted for most species. In a previous publication released in 2003, we pointed out that the increasing resistance rates might reflect the frequent use of quinolones (7). Two studies in Spain showed similar trends of increased resistance to quinolones (5, 16); however, recent data from Goldstein et al. (9) showed lower rates of resistance to moxifloxacin, particularly for B. vulgatus. The study by Goldstein et al. (9) may reflect a marked difference in the population from whom the isolates were obtained, such as patients with community-acquired intra-abdominal infections, without much prior antibiotic exposure, and the population in tertiary-care medical centers. The isolates referred from the R. M. Alden Research Laboratories for this analysis may differ from those included in the recently cited publication (9; data not shown). It is likely that regional or institutional rates may vary considerably throughout the United States and the world and that factors such as previous antibiotic use or the site of isolation may play a role in the selection of resistant isolates (10). Unfortunately, we do not have data on the use of antibiotics prior to isolation, and our analysis by center or site of isolation is currently being completed for publication (8).
The 5-year data for tigecycline show that at a breakpoint for resistance of 16 μg/ml, this agent has very good in vitro activity against most of the species within the group. As with moxifloxacin, the breakpoint for resistance of tigecycline used in the analysis was that established by FDA; currently, CLSI has not established susceptibility criteria for this agent. The less susceptible isolates were among the B. fragilis and the “other” Bacteroides isolates (mostly B. caccae). Similar activity was reported by Betriu et al. (4).
It is also important to note the isolation of a metronidazole-resistant isolate of Bacteroides fragilis in this survey. Such isolates have been noted in Europe (6) but have not previously occurred in the present survey. The emergence of resistance to metronidazole in the United States has significant therapeutic implications.
This survey presents the most comprehensive report on the susceptibility trends for the B. fragilis group over time. Other surveillance studies, which have used smaller numbers of isolates, have shown similar results (3, 10, 12, 17, 18). Many investigators have joined us in emphasizing the need for monitoring the susceptibility patterns by using a standardized methodology (2, 9, 10, 17).
There is also a need for rapid and less expensive methods for the determination of the species of the isolates tested as well as identification of specific resistance determinants (17). The information is pivotal in the decision making and empirical treatment of these very important anaerobic pathogens, since susceptibility has been shown to be related to outcome in Bacteroides infections (19, 20).
Acknowledgments
This study was supported with major funding from Astra-Zeneca, Merck & Company, and Wyeth-Ayerst. Additional support was also provided by the Pfizer and Bayer Corporations.
Footnotes
Published ahead of print on 5 February 2007.
REFERENCES
- 1.Agresti, A. 1990. Categorical data analysis. John Wiley & Sons, Inc., New York, NY.
- 2.Aldridge, K. E., D. Ashcraft, K. Cambre, et al. 2001. Multicenter survey of the changing in vitro antimicrobial susceptibilities of clinical isolates of Bacteroides fragilis group, Prevotella, Fusobacterium, Porphyromonas, and Peptostreptococcus species. Antimicrob. Agents Chemother. 451238-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldridge, K. E., and M. O'Brien. 2002. In vitro susceptibilities of the Bacteroides fragilis group species: change in isolation rates significantly affects overall susceptibility data. J. Clin. Microbiol. 404349-4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betriu, C., E. Culebras, M. Gomez, I. Rodriguez-Avial, and J. J. Picazo. 2005. In-vitro activity of tigecycline against Bacteroides species. J. Antimicrob. Chemother. 56349-352. [DOI] [PubMed] [Google Scholar]
- 5.Betriu, C., I. Rodriguez-Avial, M. Gomez, E. Culebras, E., and J. J. Picazo. 2005. Changing pasterns of fluoroquinolone resistance among Bacteroides fragilis group organisms over a 6-year period (1997-2002). Diagn. Microbiol. Infect. Dis. 53221-223. [DOI] [PubMed] [Google Scholar]
- 6.Brazier, J. S., S. L. Stubbs, and B. I. Duerden. 1999. Metronidazole resistance among clinical isolates belonging to the Bacteroides fragilis group: time to be concerned? J. Antimicrob. Chemother. 44580-581. [DOI] [PubMed] [Google Scholar]
- 7.Golan, Y., L. A. McDermott, N. V. Jacobus, et al. 2003. Emergence of fluoroquinolone resistance among the Bacteroides species. J. Antimicrob. Chemother. 52208-213. [DOI] [PubMed] [Google Scholar]
- 8.Golan, Y., et al. 2001. Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. K1209.
- 9.Goldstein, E. J. C., D. M. Citron, Y. A. Warren, K. L. Tyrell, C. Vreni Merriam, and H. Fernandez. 2006. In vitro activity of moxifloxacin against 923 anaerobes isolated from human intra-abdominal infections. Antimicrob. Agents Chemother. 50148-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hedberg, M., C. E. Nord, and the ESCMID Study Group on Antimicrobial Resistance in Anaerobic Bacteria. 2003. Antimicrobial susceptibility of Bacteroides fragilis group isolates in Europe. Clin. Microbiol. Infect. 9475-488. [DOI] [PubMed] [Google Scholar]
- 11.Holdeman, L. V., and E. C. Moore. 1977. Anaerobic laboratory manual, 4th ed. Virginia Polytechnic Institute and State University, Blacksburg.
- 12.Nakano, V., and M. J. Avila-Campos. 2004. Survey of antimicrobial susceptibility patterns of the bacteria of the Bacteroides fragilis group isolated from the intestinal tract of children. Mem. Inst. Oswaldo Cruz 99319-324. [DOI] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. 2000. Methods for antimicrobial susceptibility testing of anaerobic bacteria, 5th ed. Approved standard. NCCLS publication no. M11-A5. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 14.Nguyen, M. H., V. L. Yu, A. J. Morris, L. A. McDermott, M. W. Wagener, L. Hareell, and D. R. Snydman. 2000. Antimicrobial resistance and clinical outcome of Bacteroides bacteremia: findings of a multicenter prospective observational trial. Clin. Infect. Dis. 30870-876. [DOI] [PubMed] [Google Scholar]
- 15.Nichols, R. L., and J. W. Smith. 1993. Wound and intra-abdominal infections: microbiological considerations and approaches to treatment. Clin. Infect. Dis. 16(Suppl. 4)S266-S272. [DOI] [PubMed] [Google Scholar]
- 16.Oteo-Iglesias, J., J. I. Alos, and J. L. Gomez-Garces. 2002. Increase in resistance to new fluoroquinolones from 1998 to 2001 in the Bacteroides fragilis group. J. Antimicrob. Chemother. 501055-1057. [DOI] [PubMed] [Google Scholar]
- 17.Paula, G. R., L. S. Falcao, E. N. Antunes, K. E. Avelar, F. N. Reis, M. A. Maluhy, M. C. Ferreira, and R. M. Dominguez. 2004. Determinants of resistance in Bacteroides fragilis strains according to recent Brazilian profiles of antimicrobial susceptibility. Int. J. Antimicrob. Agents 2453-58. [DOI] [PubMed] [Google Scholar]
- 18.Rokosz, A., J. Pawlowska, A. Sawicka-Grzelak, and M. Luczak. 2004. ESBL-positive strains of Bacteroides fragilis group isolated from patients at the regional hospital center in Plock (Poland). Med. Dosw. Mikrobiol. 56245-253. [PubMed] [Google Scholar]
- 19.Rosenblatt, J. E., and I. Brook. 1993. Clinical relevance of susceptibility testing of anaerobic bacteria. Clin. Infect. Dis. 16(Suppl. 4)S446-S448. [DOI] [PubMed] [Google Scholar]
- 20.Snydman, D. R., G. J. Cuchural, L. McDermott, and M. Gill. 1992. Correlation of various in vitro testing methods with clinical outcomes in patients with Bacteroides fragilis group infections treated with cefoxitin: a retrospective analysis. Antimicrob. Agents Chemother. 36540-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snydman, D. R., N. V. Jacobus, L. A. McDermott, et al. 1999. Multicenter study of in vitro susceptibility of Bacteroides fragilis group, 1995 to 1996, with comparison of resistance trends from 1990 to 1996. Antimicrob. Agents Chemother. 432417-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snydman, D. R., N. V. Jacobus, L. A. McDermott, et al. 2002. National survey on the susceptibility of the Bacteroides fragilis group: report and analysis of trends for 1997-2000. Clin. Infect. Dis. 35(Suppl. 1)S126-S134. [DOI] [PubMed] [Google Scholar]
- 23.Summanen, P., E. J. Baron, D. M. Citron, C. A. Strong, H. Wexler, and S. M. Finegold. 1993. Wadsworth anaerobic bacteriology manual, 5th ed. Star Publishing, Belmont, CA.