Abstract
Infection caused by Mycobacterium avium complex (MAC) is common in patients with immunosuppression, such as AIDS, and deficiencies of gamma interferon and interleukin-12, as well as patients with chronic lung diseases. Treatment of MAC disease is limited since few drugs show in vivo activity. We tested a new bridged bicyclic macrolide, EDP-420, against MAC in vitro and in beige mice. EDP-420 was inhibitory in vitro at a concentration ranging from 2 to 8 μg/ml (MIC50 of 4 μg/ml and MIC90 of 8 μg/ml). In macrophages, EDP-420 was inhibitory at 0.5 μg/ml, suggesting that the drug concentrates intracellularly. Mice infected with macrolide-susceptible MAC strain 101 were given 100 mg of EDP-420/kg of body weight daily for 4 weeks and showed a significant reduction in the number of bacteria in both liver and spleen which was greater than the reduction observed with clarithromycin treatment at the same dose (P < 0.05). However, macrolide-resistant MAC 101 did not respond to EDP-420 treatment. A combination of EDP-420 with mefloquine was shown to be indifferent; mefloquine alone was active against macrolide-resistant MAC. The frequency of resistance to EDP-420 in MAC 101 was 10−9, which is significantly less than the emergence of resistance to clarithromycin, ∼10−7 (P < 0.05). Further evaluation of EDP-420 in the treatment of MAC disease is warranted.
The mainstays of current therapy for disease caused by the Mycobacterium avium complex (hereafter referred to as MAC) are macrolide agents (2, 4, 12, 24), while ethambutol and rifabutin are also used as part of a macrolide-containing regimen (15, 21). Moxifloxacin and ketolides, including telithromycin, have been shown in experimental animal systems to be active against MAC (3-6). Amikacin is an effective alternative for patients that failed oral therapy (13), and mefloquine has been shown to be bactericidal in vitro in animal models and in isolated human cases (6, 7, 18).
MAC organisms cause disseminated infections in immunosuppressed patients, such as AIDS patients, and in individuals with gamma interferon deficiency and mutations in the interleukin-12 receptor (11, 16). In addition, MAC affects populations with chronic lung diseases, chiefly patients with bronchiectasis, cystic fibrosis, and chest wall anomalies (1, 19).
The therapy of MAC infections is complicated by the nature of the predisposing disease states and the need for prolonged administration of antibiotics. Further, it appears that respiratory infections caused by MAC might be linked to the presence of biofilm in the airways, which creates additional challenges (9, 10, 23). One of the major concerns is the development of resistance to therapy. Since all current regimens contain a macrolide (clarithromycin, azithromycin, or roxithromycin) as the main component, the development of macrolide-resistant strains during the course of treatment is clearly undesirable. Therefore, the establishment of alternative regimens, preferably containing one or two bactericidal compounds, is the present goal.
As part of an ongoing effort, we evaluated a new bridged bicyclic macrolide, EDP-420. EDP-420 has a long half-life and achieves high concentrations in tissues (20, 22). In this report, we show that EDP-420 is active against MAC in vitro and in mice and is associated with an emergence of resistance during treatment that is significantly lower than that with clarithromycin.
MATERIALS AND METHODS
Bacteria.
MAC strains were obtained from the blood of patients with AIDS. Clarithromycin-resistant MAC 101 was isolated from the spleen of a mouse infected with MAC 101 treated with clarithromycin, as previously described (8). All MAC strains are susceptible to macrolides, with the exception of clarithromycin-resistant 101, 511, 512, 513, JSL, and JWT. MAC strains were grown in 7H10 Middlebrook agar, and pure colonies were selected for the experiment. Mouse experiments were carried out using MAC strain 101 and clarithromycin-resistant MAC 101.
In vitro susceptibility testing.
MICs were determined by a radiometric broth nanodilution method and the T100 method of data analysis (17). The inoculum for the susceptibility testing was prepared by obtaining 5 to 10 colonies from a 7H11 agar plate and placing them into 7H9 broth; the samples were then tested directly or frozen at −70°C. The inoculum was adjusted to approximately 5 × 104 CFU/ml by comparison with a McFarland no. 1 turbidity standard. Isolates that clumped and could not be easily dispersed were shaken with glass beads. Controls included the inoculum undiluted without drug, the inoculum diluted 1:100 (99% control), and the inoculum diluted 1:1,000 (99.9% control). In addition, one vial was inoculated with a suspension of mycobacteria which was boiled for 5 min in order to monitor a non-growth-related release of carbon dioxide in the BACTEC system. The period of observation was approximately 7 days for most isolates.
Mouse experiments.
C57BL/6 beige female mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice used for the experiments were accustomed to the environment and weighed approximately 20 g. Mice were infected intravenously with approximately 3 × 107 bacteria. The inoculum was adjusted using the McFarland turbidity standard. The inoculum was plated onto 7H10 agar to confirm the number of viable organisms. Some infected mice were harvested after 1 week to establish the numbers of bacteria in the organs. Treatment was then initiated with each drug alone or in combinations: EDP-420 (Enanta Pharmaceuticals, Inc., Watertown, MA), 100 mg/kg of body weight; clarithromycin (Abbott Pharmaceuticals, Abbott Park, IL), 100 μg/ml; and mefloquine (Sigma Chemicals, St. Louis, MO) 40 mg/kg. Mefloquine was chosen because it is quite active against MAC in experimental models and in isolated human cases (6, 7, 18). Mefloquine is now in clinical trial for the therapy of macrolide-resistant infection (L. Bermudez, unpublished data). Furthermore, a mefloquine-resistant MAC strain has not been reported. The regimen was administered once a day, 6 days a week, for 4 weeks, and then the mice were harvested and livers and spleens were homogenized, diluted, and plated onto 7H10 agar for quantification for the numbers of CFU, as previously described (4, 5). After 10 days at 37°C, the numbers of CFU were determined. Untreated control mice (diluent treated) were run in parallel.
Macrophage assay.
Macrophage assays were carried out as previously reported (4, 5). Briefly, U937 mononuclear phagocytes were purchased from the American Type Culture Collection (Manassas, VA). Cells were adjusted to 105/ml and seeded onto a 24-well tissue culture plate (Costar, Cambridge, MA) in the presence of RPMI 1640 supplemented with 5% heat-inactivated fetal bovine serum. The U937 cells in suspension were then treated with phorbol myristate acetate, 1 μg/ml overnight, in order to differentiate the macrophages that subsequently bound to the plastic. Monolayers were washed three times and then infected with MAC strain 101 (1 × 106 bacteria) for 2 h. Monolayers were then washed with Hank's balanced salt solution to remove extracellular bacteria (4, 5). Some monolayers were lysed, and the lysate was plated onto 7H10 agar for quantification of the number of intracellular bacteria, according to the method described previously (4, 5). Monolayers were then treated with EDP-420 (0.5 to 8 μg/ml) daily. The medium was replenished daily, and the numbers of cells on the monolayers were monitored as described previously (4, 5). At day 4, the monolayers were lysed and the lysate was plated onto 7H10 agar to determine the number of viable bacteria (4, 5).
Emergence of resistance.
Detection of emergence of resistance was determined in mice as described previously (8). Briefly, mice infected with 3 × 107 bacteria were treated with EDP-420 daily and harvested at weeks 8 and 12. The spleens were removed and cultured to determine the number of viable bacteria. The spleens were also plated onto 7H11 agar without antibiotic and 7H11 agar containing EDP-420, 32 μg/ml.
To confirm the initial observation in vivo, the bactericidal activity of EDP-420 was determined, as previously reported (17, 24), and the concentration was used in vitro to determine the frequency of natural resistance to EDP-420 in a population of MAC. Inocula of 107 to 1011 bacteria were incubated with 32 μg/ml of EDP-420 in 7H9 broth for one week to select for resistant bacteria, and then bacteria were plated onto 7H10 agar with or without 32 μg/ml of EDP-420 to isolate resistant mutants.
Statistical analysis.
The differences between results for untreated control and experimental groups in the macrophage experiments at the same time point were determined by the Mann-Whitney nonparametric test. The differences between organisms recovered from spleens and livers of mice were evaluated by the Student's t test.
RESULTS
Previous studies showed the pharmacokinetics of EDP-420 compared with the pharmacokinetics of telithromycin in mice (20, 22). The maximum concentration of drug in serum (μg/ml) following a dose of 15 mg/kg per os was 5.5 for telithromycin and 2.5 for EDP-420. The time to maximum concentration of drug in serum (h) was 1 for telithromycin and 1.5 for EDP-420. The half-life (h) was 1.5 for telithromycin and 3.3 for EDP-420. Oral bioavailabilities were comparable among the compounds. The concentration within macrophages is not available.
Susceptibility in vitro.
EDP-420 has a MIC50 of 4 μg/ml and a MIC90 of 8 μg/ml against a number of MAC strains. For the macrolide-susceptible mouse challenge MAC strain 101, the MIC of clarithromycin was 4 μg/ml and the MIC of EDP-420 was 8 μg/ml. EDP-420, like telithromycin, a ketolide, is not active against macrolide-resistant M. avium strains (Table 1), with MICs of 32 to 64 μg/ml (telithromycin MICs are >128 μg/ml). Clarithromycin-resistant MAC strains 101 (EDP-420 MIC of 64 μg/ml), 511, 512, 513, JSL, and JWT are either mouse generated or clinical isolates resistant to clarithromycin.
TABLE 1.
In vitro susceptibility of M. avium strains to EDP-420, azithromycin, clarithromycin, and telithromycin
| Drug | MIC for M. avium macrolide-susceptible strains (n = 24) (μg/ml)
|
MIC range for M. avium macrolide-resistant strains (n = 6) (μg/ml) | ||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| EDP-420 | 2-8 | 4 | 8 | 32-64 |
| Azithromycin | 8-16 | 16 | 16 | >512 |
| Clarithromycin | 0.5-4 | 2 | 4 | >64 |
| Telithromycin | 32-64a | 32 | >64 | NAb |
The MIC range for telithromycin reported here is one dilution different from previous results, probably due to different lots of medium and albumin.
NA, not available.
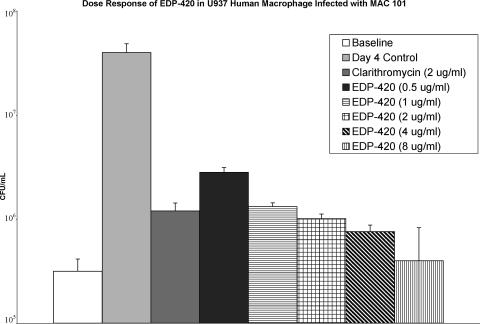
To determine whether EDP-420 had activity within macrophages, U937 mononuclear phagocytes were infected with MAC strain 101 (ratio, 10 bacteria to 1 macrophage) and treated with a range of concentrations of EDP-420 for 4 days. As shown in Fig. 1, 0.5 μg/ml of EDP-420 had a significant inhibitory effect against intracellular MAC. In addition, 2 μg/ml of clarithromycin, a compound with known activity against MAC in vivo, had an inhibitory activity comparable to 2 μg/ml of EDP-420.
FIG. 1.
Activity of EDP-420 against M. avium strain 101 in macrophages. The U937 monocyte cell line was infected with MAC strain 101 as described in Materials and Methods, and then the macrophage monolayers were treated with a range of concentrations of EDP-420. Clarithromycin at 2 μg/ml (serum concentration) was used as a standard. After 4 days, macrophages were lysed and the number of bacteria was quantified. A P value of <0.05 was obtained for the comparisons between all of the concentrations and the day 4 control.
Treatment of mice with EDP-420.
EDP-420 was compared with clarithromycin at 100 mg/kg/day (for both agents) for treatment of MAC infection in mice. As shown in Table 2, EDP-420 was significantly more active than clarithromycin in both liver and spleen (P < 0.05). Both antibiotics had bactericidal activity in mice. Table 3 demonstrates that, in contrast with the treatment of the susceptible MAC strain, macrolide-resistant MAC 101 was not affected by either clarithromycin or EDP-420.
TABLE 2.
Effect of the treatment of MAC 101-infected beige mice with EDP-420
| Regimena | Doseb | Bacterial load (CFU/g) in:
|
|
|---|---|---|---|
| Liver | Spleen | ||
| Clarithromycin | 100 mg/kg | 2.8 × 107 ± 0.6 × 107c | 6.6 × 107 ± 1.2 × 107c |
| EDP-420 | 100 mg/kg | 7.1 × 106 ± 1.4 × 106c,d | 1.2 × 107 ± 0.3 × 107c,d |
| Untreated control | 8.0 × 108 ± 1.8 × 108 | 2.7 × 109 ± 0.4 × 109 | |
| Baseline control (1 week) | 2.4 × 108 ± 1.4 × 108 | 7.0 × 108 ± 3.1 × 108 | |
Mice were treated daily for 6 days during 4 weeks.
Mice were infected intravenously, and 1 week after infection 10 mice were harvested and the level of infection before therapy was established. Twenty mice were used for the experimental group.
P < 0.05 compared with untreated control.
P < 0.05 compared with clarithromycin.
TABLE 3.
Effect of treatment of clarithromycin-resistant M. avium strain 101 with EDP-420
| Regimenb | Dose | Bacterial load (CFU/g ± SEM) ina:
|
|
|---|---|---|---|
| Liverc | Spleenc | ||
| Clarithromycin | 100 mg/kg | 2.8 × 108 ± 0.4 × 108 | 8.0 × 108 ± 1.3 × 108 |
| EDP-420 | 100 mg/kg | 1.7 × 108 ± 0.2 × 108 | 5.7 × 108 ± 0.8 × 108 |
| Untreated control | 2.4 × 108 ± 0.5 × 108 | 6.2 × 108 ± 1.3 × 108 | |
| Baseline control (1 week) | 8.7 × 107 ± 1.5 × 107 | 2.1 × 108 ± 0.5 × 108 | |
Mice were infected intravenously, and 1 week after infection 10 mice were harvested and the level of infection before therapy was established. Twenty animals were used in each experimental group.
Mice were treated once a day, 6 days a week, for 4 weeks.
P > 0.05 for all comparisons.
The use of EDP-420 in combination with mefloquine, an agent known to have anti-MAC activity, showed that both drugs were bactericidal in vivo (mefloquine was only in the spleen), but the combination of both compounds was not more active than either alone (Table 4). When both compounds were employed to treat infection with macrolide-resistant MAC, EDP-420 was inactive, while mefloquine had bactericidal activity (Table 5).
TABLE 4.
Activity of EDP-420 in combination with mefloquine against M. avium in mice
| Regimena | Dose (mg/kg) | Mice (n) | Bacterial load (CFU/g ± SEM) in:
|
|
|---|---|---|---|---|
| Liver | Spleen | |||
| Mefloquine | 40 | 13 | 1.8 × 107 ± 0.7 × 107b | 4.8 × 107 ± 2.2 × 107b |
| EDP-420 | 100 | 15 | 1.2 × 107 ± 0.4 × 107b | 1.7 × 107 ± 0.5 × 107b |
| Mefloquine + EDP-420 | 40 + 100 | 14 | 1.3 × 107 ± 0.4 × 107b | 1.6 × 107 ± 0.6 × 107b |
| Untreated control | 15 | 1.1 × 109 ± 0.4 × 109 | 2.4 × 109 ± 0.9 × 109 | |
| Baseline (7 days) | 9 | 2.5 × 107 ± 1.3 × 107 | 1.2 × 108 ± 0.9 × 108 | |
Mice were treated daily, 6 days a week, for 4 weeks.
P < 0.05 compared with untreated control.
TABLE 5.
Activity of EDP-420 in combination with mefloquine against clarithromycin-resistant M. avium 101 in mice
| Regimena | Dose (mg/kg) | Mice (n) | Bacterial load (CFU/g ± SEM) in:
|
|
|---|---|---|---|---|
| Liver | Spleen | |||
| Mefloquine | 40 | 22 | 2.9 × 107 ± 0.9 × 107b | 4.6 × 107 ± 0.6 × 107b |
| EDP-420 | 100 | 15 | 2.5 × 108 ± 1.6 × 108 | 2.7 × 108 ± 1.0 × 108 |
| Mefloquine + EDP-420 | 40 + 100 | 14 | 2.9 × 107 ± 1.9 × 107b | 4.9 × 107 ± 2.0 × 107b |
| Untreated control | 15 | 1.4 × 108 ± 0.4 × 108 | 5.6 × 108 ± 1.5 × 108 | |
| Baseline (7 days) | 8 | 4.5 × 107 ± 1.9 × 107 | 9.5 × 107 ± 3.0 × 107 | |
Mice were treated once a day, 6 days a week, for 4 weeks.
P < 0.5 compared with untreated control.
Emergence of resistance.
To verify the frequency of resistance of MAC to EDP-420, mice were infected intravenously and treated with EDP-420 (100 mg/kg) alone or EDP-420 plus mefloquine (40 mg/kg) for several weeks. At weeks 8 and 12, mice were harvested, and the spleens were plated onto both 7H10 agar and 7H10 agar containing either EDP-420 (32 μg/ml) or EDP-420 and mefloquine (32 μg/ml), to determine the frequency of resistance. No resistant colonies, either to EDP-420 alone or in combination with mefloquine, were identified at 8 weeks and 12 weeks (Table 6). To determine if this observation was related to the low number of bacteria in the spleen at weeks 8 and 12, we exposed an inoculum of 107 to 1011 MAC 101 organisms in vitro (7H9 broth) to 32 μg/ml of EDP-420 for 7 days and then plated the suspension onto 7H10 and 7H10 with 32 μg/ml of EDP-420. The frequency of resistance to EDP-420 was one organism in 109 bacteria, which can explain the failure to isolate resistant mutants in vivo.
TABLE 6.
Frequency of resistance to EDP-420 and EDP-420/mefloquine
| Experimental group | Mice (n) | CFU/g of spleen | Frequency of resistance to EDP-420 |
|---|---|---|---|
| 8 week control | 24 | 7.0 × 109 ± 1.6 × 109 | ∼10−9 |
| 8 week EDP-420 | 27 | 2.3 × 105 ± 0.8 × 105 | Undetected |
| 8 week EDP-420 + mefloquine | 22 | 1.2 × 105 ± 0.3 × 105 | Undetected |
| 12 week control | 21 | 4.6 × 109 ± 1.5 × 109 | ∼10−9 |
| 12 week EDP-420 | 27 | 2.5 × 105 ± 1.1 × 105 | Undetected |
| 12 week EDP-420 + mefloquine | 20 | 1.1 × 105 ± 0.5 × 105 | Undetected |
DISCUSSION
EDP-420, a new bicyclolide (bridged bicyclic macrolide), is active against MAC both in vitro and in vivo. This work also demonstrated that EDP-420 is not active against macrolide-resistant MAC. More recently, a related group of compounds, the ketolides, have shown limited activity for the treatment of MAC. One of the first ketolides to be evaluated against MAC, telithromycin, was shown to be bacteriostatic in vivo (5) but was not active against clarithromycin- and azithromycin-resistant strains.
The frequency of resistance to EDP-420 was evaluated in mice in vivo and determined to be greater than 5 × 10−8. To confirm the observation, the minimal bactericidal concentration was determined (32 μg/ml) and several inocula (from 107 to 1011) were incubated with 32 μg/ml of EDP-420, both in liquid media and in solid media, to isolate naturally resistant mutants. It was observed that mutants naturally resistant to EDP-420 were observed only with an inoculum of 109 bacteria/ml, in contrast to 107 bacteria/ml for clarithromycin. This indicates that resistant clones were not observed in mice due to the relatively low bacterial burden in tissues.
Standard therapy of MAC infection includes a modern macrolide, such as clarithromycin, azithromycin, or roxithromycin (2, 12), in combination with ethambutol and rifabutin (15, 21). However, once resistance to macrolides develops, no alternative agent of the macrolide class is available. Resistance to macrolides is not a rare event, having been described both in humans and in experimental models of infection (8, 12). Therefore, the development of an alternative therapeutic regimen that does not contain a macrolide is desirable. In mice, a regimen with mefloquine, moxifloxacin, and ethambutol is a bactericidal regimen and has been shown to be as effective against disseminated infection as regimens containing macrolides (6). Studies in animal systems appear to show that the quinolone moxifloxacin is very effective against MAC (4). Experimental studies also suggest that mefloquine, an antimalarial, is active in vivo against MAC strains (6, 7). In addition, mefloquine-resistant strains have not been identified either in vitro or in vivo (14).
EDP-420 appears to achieve a high concentration within cells, a pharmacokinetic property shared with telithromycin and macrolides (5, 12). The dose-response study of macrophages suggests that EDP-420 concentrates within the phagocyte, leading to an approximately 1.7 log reduction following exposure to 1 μg/ml. However, the effect up to 8 μg/ml was bacteriostatic. The discrepancy between the activity in the macrophage system and the results in mice can be due to the period of treatment (4 days versus 4 weeks), the intracellular concentration, and the potential activation of macrophages in vivo.
While there is cross-resistance between EDP-420 and clarithromycin, at least two lines of evidence indicate important differences between the two macrolides: (i) while the MIC of clarithromycin (4 μg/ml) has been consistently half that of EDP-420 (8 μg/ml), the latter agent is significantly more active in vivo; and (ii) EDP-420 selects resistant mutants significantly less frequently than clarithromycin. Thus, bridged bicyclic macrolides, such as EDP-420, may have a more complex mechanism of effect than conventional macrolides or azalides.
EDP-420 is currently in human clinical trials and offers promise over conventional macrolide therapy for MAC disease.
Acknowledgments
This work was supported by NIH contract no. NO 1-AI-25468.
We are grateful for the technical assistance of Karen Allen and Denny Weber in the preparation of the manuscript. We thank Christopher Lambros for his support during this work.
Footnotes
Published ahead of print on 12 February 2007.
REFERENCES
- 1.Aksamit, T. R. 2002. Mycobacterium avium complex pulmonary disease in patients with pre-existing lung disease. Clin. Chest Med. 23643-653. [DOI] [PubMed] [Google Scholar]
- 2.Benson, C. A., P. L. Williams, D. L. Cohn, S. Becker, P. Hojczyk, T. Nevin, J. A. Korvick, L. Heifets, C. C. Child, M. M. Lederman, R. C. Reichman, W. G. Powderly, G. F. Notario, B. A. Wynne, R. Hafner, et al. 2000. Clarithromycin or rifabutin alone or in combination for primary prophylaxis of Mycobacterium avium complex disease in patients with AIDS: a randomized, double-blind, placebo-controlled trial. J. Infect. Dis. 1811289-1297. [DOI] [PubMed] [Google Scholar]
- 3.Bermudez, L., C. B. Inderlied, P. Kolonoski, M. Wu, and L. S. Young. 1998. Activity of HMR3004 against Mycobacterium avium complex in vitro, in human macrophages and in beige mice. Clin. Microbiol. Infect. 4325-331. [Google Scholar]
- 4.Bermudez, L. E., C. B. Inderlied, P. Kolonoski, M. Petrofsky, P. Aralar, M. Wu, and L. S. Young. 2001. Activity of moxifloxacin by itself and in combination with ethambutol, rifabutin, and azithromycin in vitro and in vivo against Mycobacterium avium. Antimicrob. Agents Chemother. 45217-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bermudez, L. E., C. B. Inderlied, P. Kolonoski, M. Wu, P. Aralar, and L. S. Young. 2001. Telithromycin is active against Mycobacterium avium in mice despite lacking significant activity in standard in vitro and macrophage assays and is associated with low frequency of resistance during treatment. Antimicrob. Agents Chemother. 452210-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bermudez, L. E., P. Kolonoski, M. Petrofsky, M. Wu, C. B. Inderlied, and L. S. Young. 2003. Mefloquine, moxifloxacin, and ethambutol are a triple-drug alternative to macrolide-containing regimens for treatment of Mycobacterium avium disease. J. Infect. Dis. 1871977-1980. [DOI] [PubMed] [Google Scholar]
- 7.Bermudez, L. E., P. Kolonoski, M. Wu, P. A. Aralar, C. B. Inderlied, and L. S. Young. 1999. Mefloquine is active in vitro and in vivo against Mycobacterium avium complex. Antimicrob. Agents Chemother. 431870-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bermudez, L. E., M. Petrofsky, P. Kolonoski, and L. S. Young. 1998. Emergence of Mycobacterium avium populations resistant to macrolides during experimental chemotherapy. Antimicrob. Agents Chemother. 42180-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter, G., M. Wu, D. C. Drummond, and L. E. Bermudez. 2003. Characterization of biofilm formation by clinical isolates of Mycobacterium avium. J. Med. Microbiol. 52747-752. [DOI] [PubMed] [Google Scholar]
- 10.Carter, G., L. S. Young, and L. E. Bermudez. 2004. A subinhibitory concentration of clarithromycin inhibits Mycobacterium avium biofilm formation. Antimicrob. Agents Chemother. 484907-4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casanova, J. L., and L. Abel. 2002. Genetic dissection of immunity to mycobacteria: the human model. Annu. Rev. Immunol. 20581-620. [DOI] [PubMed] [Google Scholar]
- 12.Chaisson, R. E., C. A. Benson, M. P. Dube, L. B. Heifets, J. A. Korvick, S. Elkin, T. Smith, J. C. Craft, F. R. Sattler, et al. 1994. Clarithromycin therapy for bacteremic Mycobacterium avium complex disease: a randomized, double-blind, dose-ranging study in patients with AIDS. Ann. Intern. Med. 121905-911. [DOI] [PubMed] [Google Scholar]
- 13.Chiu, J., J. Nussbaum, S. Bozzette, J. G. Tilles, L. S. Young, J. Leedom, P. N. Heseltine, J. A. McCutchan, et al. 1990. Treatment of disseminated Mycobacterium avium complex infection in AIDS with amikacin, ethambutol, rifampin, and ciprofloxacin. Ann. Intern. Med. 113358-361. [DOI] [PubMed] [Google Scholar]
- 14.Danelishvili, L., M. Wu, L. S. Young, and L. E. Bermudez. 2005. Genomic approach to identifying the putative target of and mechanisms of resistance to mefloquine in mycobacteria. Antimicrob. Agents Chemother. 493707-3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dube, M. P., F. R. Sattler, F. J. Torriani, D. See, D. V. Havlir, C. A. Kemper, M. G. Dezfuli, S. A. Bozzette, A. E. Bartok, J. M. Leedom, J. G. Tilles, J. A. McCutchan, et al. 1997. A randomized evaluation of ethambutol for prevention of relapse and drug resistance during treatment of Mycobacterium avium complex bacteremia with clarithromycin-based combination therapy. J. Infect. Dis. 1761225-1232. [DOI] [PubMed] [Google Scholar]
- 16.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 1993-129. [DOI] [PubMed] [Google Scholar]
- 17.Inderlied, C. B., L. S. Young, and J. K. Yamada. 1987. Determination of in vitro susceptibility of Mycobacterium avium complex isolates to antimycobacterial agents by various methods. Antimicrob. Agents Chemother. 311697-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nannini, E. C., M. Keating, P. Binstock, G. Samonis, and D. P. Kontoyiannis. 2002. Successful treatment of refractory disseminated Mycobacterium avium complex infection with the addition of linezolid and mefloquine. J. Infect. 44201-203. [DOI] [PubMed] [Google Scholar]
- 19.Prince, D. S., D. D. Peterson, R. M. Steiner, J. E. Gottlieb, R. Scott, H. L. Israel, W. G. Figueroa, and J. E. Fish. 1989. Infection with Mycobacterium avium complex in patients without predisposing conditions. N. Engl. J. Med. 321863-868. [DOI] [PubMed] [Google Scholar]
- 20.Scorneaux, B., A. Arya, A. Polemeropoulos, M. Lillard, F. Han, K. Amsler, A. J. Sonderfan, G. Wang, Y. C. Wang, Y. Peng, G. Xu, H. Kim, T. Lien, L. Phan, and Y. S. Or. 2003. In vitro and in vivo evaluation of EP-13420: a novel ketolide highly active against resistant pathogens and having exceptional pharmacokinetic properties in the dog. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-1191.
- 21.Sullam, P. M., F. M. Gordin, B. A. Wynne, et al. 1994. Efficacy of rifabutin in the treatment of disseminated infection due to Mycobacterium avium complex. Clin. Infect. Dis. 1984-86. [DOI] [PubMed] [Google Scholar]
- 22.Tsuji, M., H. H. Miwa, M. Takema, E. Kanaoka, T. Yoshikawa, J. Shimada, and S. Kuwahara. 2005. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-2035.
- 23.Yamazaki, Y., L. Danelishvili, M. Wu, E. Hidaka, T. Katsuyama, B. Stang, M. Petrofsky, R. Bildfell, and L. E. Bermudez. 2006. The ability to form biofilm influences Mycobacterium avium invasion and translocation of bronchial epithelial cells. Cell. Microbiol. 8806-814. [DOI] [PubMed] [Google Scholar]
- 24.Young, L. S., L. Wiviott, M. Wu, P. Kolonoski, R. Bolan, and C. B. Inderlied. 1991. Azithromycin for treatment of Mycobacterium avium-intracellulare complex infection in patients with AIDS. Lancet 3381107-1109. [DOI] [PubMed] [Google Scholar]