Abstract
We report here the dissemination of a conjugative IncI1 plasmid carrying blaTEM-52 on a Tn3 transposon conferring resistance to extended-spectrum cephalosporins in Salmonella enterica serovar Agona, Derby, Infantis, Paratyphi B dT+, and Typhimurium isolates from poultry and humans in Belgium and France from 2001 to 2005. The most prevalent serovar spreading this resistance was serovar Infantis.
Food-producing animals are the primary reservoir of zoonotic pathogens, and the rate of detection of extended-spectrum β-lactamase (ESBL)-producing Escherichia coli and Salmonella strains has increased in recent years. ESBLs are widely detected in various human medical institutions, but they are not so frequently reported in bacterial populations circulating in animals. In Belgium and France the emergence of resistance to extended-spectrum cephalosporins, such as ceftriaxone and ceftiofur, has been recently reported in Salmonella enterica serovar Virchow isolates from poultry and humans (1, 15). Resistance was due to the ESBL genes blaCTX-M-2 or blaCTX-M-9 carried on large conjugative plasmids.
Since 2001 a large number of strains have been isolated from poultry (more than 150 in Belgium) and a more limited number from humans (n = 15) in Belgium and France showing resistance to extended-spectrum cephalosporins by production of an ESBL not belonging to the CTX-M family and with various additional resistances to other antibiotic families. The serovars concerned were Agona, Derby, Infantis, Paratyphi B dT+, and Typhimurium. In particular, the emergence of extended-spectrum cephalosporin-resistant serovar Infantis with more than 80 strains isolated from poultry and 4 strains from humans caused some concern. The purpose of the present study was to identify the ESBL gene and its location in these strains.
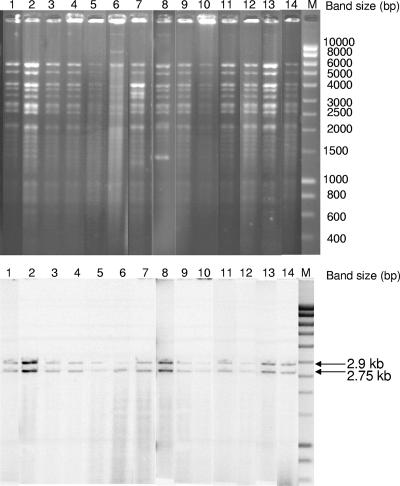
Strains studied are shown in Table 1. Antibiotic susceptibility testing was done by the disk diffusion method, and the MICs of ceftriaxone and ceftiofur were determined as described previously (1, 14, 15). Resistance to extended-spectrum cephalosporins from all Salmonella strains was transferred to an E. coli recipient strain by conjugation as previously described (1, 14, 15), and all E. coli transconjugant strains showed the same antibiotic resistance profile (Table 1). Other resistances from multidrug-resistant strains were not transferred by conjugation. According to the MICs, the levels of resistance to ceftiofur and ceftriaxone were lower in the transconjugant strains than in the parental strains, but this was also observed in a previous study (14). PCR assays to detect ESBL genes (TEM, SHV, and CTX-M) were performed on parental and transconjugant strains using previously described primers (1, 14, 15), and nucleotide sequencing of the amplicons identified the blaTEM-52 resistance gene in all strains. Plasmids extracted from the transconjugants were further characterized by PstI restriction analysis showing that they were all identical and greater than 100 kb in size (Fig. 1). Southern blot hybridization experiment with a blaTEM-52 gene probe was performed as described previously (12). It revealed two PstI fragments of 2.9 and 2.75 kb. In fact, this PstI restriction profile corresponded exactly to that of blaTEM-52-carrying plasmids isolated in 2002 and 2003 from four isolates of S. enterica serovars Typhimurium, Enteritidis, and Panama from French patients with gastroenteritis (14). A study performed in 2001 and 2002 on Salmonella isolated from poultry, poultry products, and human patients in The Netherlands revealed that the TEM-52 variant was the most common ESBL detected in this bacterial collection (6). In particular, TEM-52-producing salmonellae of the serovars Blockley, Virchow, Typhimurium, and Paratyphi B were identified from poultry, and strains of the serovars Thompson, London, Enteritidis, and Blockley were identified from human patients (6). Several sporadic cases of E. coli TEM-52 producers were reported in animals: dogs in Portugal, rabbits in Spain, and beef meat in Denmark (2, 4, 8). These findings suggest a wide dissemination of this ESBLs in Europe in animals and humans. The presence of the blaTEM-52 gene in E. coli, as well as in different Salmonella serovars, strongly indicated that it is not due to the spread of a single clone but to the horizontal transmission of this resistance trait. To better identify the molecular mechanism of dissemination of this ESBL, the blaTEM-52-positive plasmids were typed by the PCR-based replicon typing as previously described (3), demonstrating that they all belong to the IncI1 incompatibility group. IncI1 plasmids were recently described in E. coli and Salmonella strains of different serovars isolated in the United Kingdom associated with relevant β-lactamases such as CMY-2, CMY-7, and CTX-M-15, suggesting a large prevalence of IncI1 plasmids in Europe (7).
TABLE 1.
Characteristics of the Salmonella strains and their transconjugants producing TEM-52 used in this study
| Straina | Serovar | Geographic origin | Animal or human origin | Yr of isolation | Antibiotic resistance profileb | MIC (μg/ml)c
|
SGI1 variantd | |
|---|---|---|---|---|---|---|---|---|
| Xnl | Cro | |||||||
| 777SA01 | Agona | Belgium | Poultry | 2001 | Ap(Caz)CfCm(Cro)FfSmSpSuTcTm(Xnl) | 16 | 16 | SGI1-A |
| 777SA01TC1 | Ap(Caz)Cf(Cro)(Xnl) | 4 | 2 | |||||
| 260SA04 | Agona | Belgium | Poultry | 2004 | ApCazCfCmCroFfSmSuTcTmXnl | 64 | 256 | — |
| 260SA04TC1 | Ap(Caz)Cf(Cro)(Xnl) | 2 | 1 | |||||
| 833SA04 | Agona | Belgium | Poultry | 2004 | ApCazCfCroXnl | 32 | 64 | — |
| 833SA04TC1 | Ap(Caz)Cf(Cro)(Xnl) | 2 | 1 | |||||
| 977SA01 | Derby | Belgium | Poultry | 2001 | ApCazCfCroXnl | 32 | 16 | — |
| 977SA01TC1 | Ap(Caz)Cf(Cro)(Xnl) | 4 | 4 | |||||
| 988SA01 | Infantis | Belgium | Poultry | 2001 | ApCazCfCroXnl | 32 | 32 | — |
| 988SA01TC1 | Ap(Caz)Cf(Cro)(Xnl) | 2 | 1 | |||||
| 2004/10101 | Infantis | Belgium | Poultry | 2004 | ApCazCfCroXnl | 32 | 128 | — |
| 2004/10101TC1 | Ap(Caz)Cf(Cro)(Xnl) | 2 | 4 | |||||
| 2004/10256 | Infantis | Belgium | Poultry | 2004 | ApCazCfCroXnl | 32 | 64 | — |
| 2004/10256TC1 | Ap(Caz)Cf(Cro)(Xnl) | 2 | 2 | |||||
| 05-00001 | Infantis | Belgium | Human | 2005 | ApCazCfCroXnl | 32 | 64 | — |
| 05-00001TC1 | Ap(Caz)Cf(Cro)(Xnl) | 4 | 4 | |||||
| 05-00590 | Infantis | Belgium | Human | 2005 | ApCazCfCroXnl | 32 | 64 | — |
| 05-00590TC1 | Ap(Caz)Cf(Cro)(Xnl) | 2 | 4 | |||||
| 05-00838 | Infantis | Belgium | Human | 2005 | ApCazCfCroXnl | 16 | 64 | — |
| 05-00838TC1 | Ap(Caz)Cf(Cro)(Xnl) | 4 | 4 | |||||
| 1043SA04 | Paratyphi B | Belgium | Poultry | 2004 | ApCazCfCroSmSpSuTmXnl | 32 | 64 | — |
| 1043SA04TC1 | Ap(Caz)Cf(Cro)(Xnl) | 4 | 4 | |||||
| 153SA02 | Typhimurium | Belgium | Poultry | 2002 | ApCazCfCroSmSpSuTmXnl | 32 | 32 | — |
| 153SA02TC1 | Ap(Caz)Cf(Cro)(Xnl) | 2 | 1 | |||||
| 04-3486 | Typhimurium | France | Human | 2004 | ApCazCfCm(Cro)FfSmSpSuTc(Xnl) | 16 | 32 | SGI1 |
| 04-3486TC1 | Ap(Caz)Cf(Cro)(Xnl) | 2 | 2 | |||||
Strains labeled with TC1 are E. coli transconjugant strains.
Antibiotics: Ap, ampicillin; Caz, ceftazidime; Cf, cefalothin; Cm, chloramphenicol; Cro, ceftriaxone; Ff, florfenicol; Sm, streptomycin; Sp, spectinomycin; Su, sulfonamide; Tc, tetracycline; Tm, trimethoprim; Xnl, ceftiofur. Parentheses indicate intermediate resistance according to the breakpoints of the CA-SFM (Comité de l'Antibiogramme de la Société Française de Microbiologie) for Enterobacteriaceae (i.e., susceptible, >20 mm; resistant, <15 mm).
Cro, ceftriaxone; Xnl, ceftiofur.
—, negative for SGI1.
FIG. 1.
Restriction analysis (PstI) (upper panel) and Southern blot hybridization with a blaTEM-52 probe (lower panel) of plasmid DNAs isolated from E. coli transconjugants. Lane 1, E. coli transconjugant 988SA01TC1; lane 2, E. coli transconjugant 2004/10101TC1; lane 3, E. coli transconjugant 2004/10256TC1; lane 4, E. coli transconjugant 05-00001TC1; lane 5, E. coli transconjugant 05-00590TC1; lane 6, E. coli transconjugant 05-00838TC1; lane 7, E. coli transconjugant 1043SA04TC1; lane 8, E. coli transconjugant 777SA01TC1; lane 9, E. coli transconjugant 260SA04TC1; lane 10, E. coli transconjugant 833SA04TC1; lane 11, E. coli transconjugant 153SA02TC1; lane 12, E. coli transconjugant 04-3486TC1; lane 13, E. coli transconjugant 977SA01TC1; lane 14, E. coli transconjugant pPAN-1 (the latter strain served as control and was previously published (14); lane M, markers (Smartladder; Eurogentec, Seraing, Belgium).
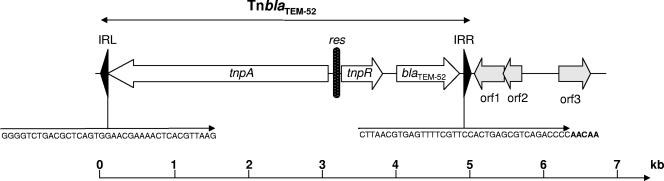
To identify the mobile genetic element carrying the blaTEM-52 gene, the plasmid DNA of E. coli transconjugant 04-3486TC1 extracted with a QIAfilter Midi kit (QIAGEN, Courtaboeuf, France) was digested with ClaI and ligated into the ClaI-restricted phagemid pBK-CMV (Stratagene). Recombinant plasmids were introduced into E. coli DH10B by electroporation (Bio-Rad Gene Pulser II; Bio-Rad, Marnes-La-Coquette, France) and selected on Mueller-Hinton (MH) agar (Bio-Rad) containing kanamycin (30 μg/ml) and ceftazidime (2 μg/ml). Recombinant plasmids that possessed a 4.7-kb insert were selected. Nucleotide sequencing of the insert indicated that the blaTEM-52 gene was located on a Tn3 transposon. To complete the transposon sequence, nucleotide sequencing was further performed by genome walking on the native plasmid. This Tn3 transposon structure is shown in Fig. 2. Its nucleotide sequence is deposited in GenBank under accession number EF141186. Very few sequences of complete Tn3 elements from Salmonella are currently available (10). Complete plasmid-borne Tn3 elements, however, specifying non-ESBLs have recently been described in serovar Typhimurium from a rabbit and in serovar Infantis from poultry and shown to be linked to either the tetracycline resistance gene tet(A) or the quinolone resistance gene qnrS (9, 13).
FIG. 2.
Genetic organization of the blaTEM-52 carrying transposon on a conjugative IncI1 plasmid from serovar Typhimurium strain 04-3486. The position and orientation of the genes are indicated by arrows. Gray arrows correspond to plasmid genes flanking the transposon. IRL and IRR correspond, respectively, to left and right terminal inverted repeats of Tn3 and are indicated as black arrowheads. The sequences of IRL and IRR are also indicated. The 5-bp direct repeat at the integration site of Tn3 downstream IRR is indicated in boldface letters. The black box between tnpA and tnpR indicates the resolution site of Tn3. The nucleotide sequence of TnblaTEM-52 has been deposited under GenBank accession number EF141186. A distance scale in kilobase pairs is given above the map.
Among the extended-spectrum cephalosporin-resistant Salmonella strains studied, five isolates belonging to serovars Agona, Paratyphi B dT+, and Typhimurium, showed an additional multidrug resistance profile with resistances to chloramphenicol, florfenicol, streptomycin, spectinomycin, sulfonamide, tetracycline, and trimethoprim (Table 1). This multidrug resistance profile is characteristic of SGI1 antibiotic resistance gene clusters, which were previously identified in these serovars (11). Identification of SGI1 and mapping of its antibiotic resistance gene cluster performed as described previously (5) showed that two of the five isolates possessed SGI1 and the SGI1-A variant in serovar Typhimurium strain 04-3486 and serovar Agona strain 777SA01, respectively (Table 1). Serovar Agona strains with SGI1-A are frequently isolated from poultry in Belgium (5). The serovar Typhimurium isolate carrying SGI1 was further shown to be of phage type DT104, a dominant multidrug-resistant clone that has spread all over the world (11). To our knowledge, this is the first time that multidrug-resistant strains carrying SGI1 together with a plasmid-borne ESBL gene have been reported, and further surveillance of such strains is thus warranted.
Since most of the strains showing extended-spectrum cephalosporin resistance were of serovar Infantis, these were further investigated for clonality by XbaI and BlnI macrorestriction pulsed-field gel electrophoresis analysis. The Infantis isolates showing the same resistance profile and whatever their origin, poultry or human, showed identical XbaI and BlnI macrorestriction profiles, indicating that these were clonal (data not shown).
In conclusion, the present study showed the spread of an IncI1 plasmid carrying the blaTEM-52 gene among S. enterica serovars Agona, Derby, Infantis, Paratyphi B dT+, and Typhimurium, as well as the spread of a single Infantis clone carrying this plasmid mainly in poultry. It is thus likely that humans infected with these strains were contaminated by ingestion of undercooked poultry products. The further spread of such plasmids in multidrug-resistant strains carrying SGI1 is of concern.
Acknowledgments
We thank C. Mouline and V. Collet for expert technical assistance.
Footnotes
Published ahead of print on 26 February 2007.
REFERENCES
- 1.Bertrand, S., F. X. Weill, A. Cloeckaert, M. Vrints, E. Mairiaux, K. Praud, K. Dierick, C. Wildemauve, C. Godard, P. Butaye, H. Imberechts, P. A. D. Grimont, and J. M. Collard. 2006. Clonal emergence of extended-spectrum β-lactamase (CTX-M-2)-producing Salmonella enterica serovar Virchow isolates with reduced susceptibilities to ciprofloxacin among poultry and humans in Belgium and France (2000 to 2003). J. Clin. Microbiol. 442897-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinas, L., M. A. Moreno, and T. Teshager. 2005. Monitoring and characterization of extended-spectrum beta-lactamases in Escherichia coli strains from healthy and sick animals in Spain in 2003. Antimicrob. Agents Chemother. 491262-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carattoli, A., A. Bertini, L. Villa, V. Falbo, K. L. Hopkins, and E. J. Threlfall. 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63219-228. [DOI] [PubMed] [Google Scholar]
- 4.Costa, D., P. Poeta, L. Brinas, Y. Saenz, J. Rodrigues, and C. Torres. 2004. Detection of CTX-M-1 and TEM-52 beta-lactamases in Escherichia coli strains from healthy pets in Portugal. J. Antimicrob. Chemother. 54960-961. [DOI] [PubMed] [Google Scholar]
- 5.Doublet, B., P. Butaye, H. Imberechts, D. Boyd, M. R. Mulvey, E. Chaslus-Dancla, and A. Cloeckaert. 2004. Salmonella genomic island 1 multidrug resistance gene clusters in Salmonella enterica serovar Agona isolated in Belgium in 1992 to 2002. Antimicrob. Agents Chemother. 482510-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasman, H., D. Mevius, K. Veldman, I. Olesen, and F. M. Aarestrup. 2005. β-Lactamases among extended-spectrum beta-lactamase (ESBL)-resistant Salmonella from poultry, poultry products and human patients in The Netherlands. J. Antimicrob. Chemother. 56115-121. [DOI] [PubMed] [Google Scholar]
- 7.Hopkins, K. L., E. Liebana, L. Villa, M. Batchelor, E. J. Threlfall, and A. Carattoli. 2006. Replicon typing of plasmids carrying CTX-M or CMY β-lactamases circulating among Salmonella and Escherichia coli isolates. Antimicrob. Agents Chemother. 503203-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen, L. B., H. Hasman, Y. Agerso, H. D. Emborg, and F. M. Aarestrup. 2006. First description of an oxyimino-cephalosporin-resistant, ESBL-carrying Escherichia coli isolated from meat sold in Denmark. J. Antimicrob. Chemother. 57793-794. [DOI] [PubMed] [Google Scholar]
- 9.Kehrenberg, C., S. Friederichs, A. de Jong, G. B. Michael, and S. Schwarz. 2006. Identification of the plasmid-borne quinolone resistance gene qnrS in Salmonella enterica serovar Infantis. J. Antimicrob. Chemother. 5818-22. [DOI] [PubMed] [Google Scholar]
- 10.Michael, G. B., P. Butaye, A. Cloeckaert, and S. Schwarz. 2006. Genes and mutations conferring antimicrobial resistance in Salmonella: an update. Microbes Infect. 81898-1914. [DOI] [PubMed] [Google Scholar]
- 11.Mulvey, M. R., D. A. Boyd, A. B. Olson, B. Doublet, and A. Cloeckaert. 2006. The genetics of Salmonella genomic island 1. Microbes Infect. 81915-1922. [DOI] [PubMed] [Google Scholar]
- 12.Olliver, A., M. Vallé, E. Chaslus-Dancla, and A. Cloeckaert. 2005. Overexpression of the multidrug efflux operon acrEF by insertional activation with IS1 or IS10 elements in Salmonella enterica serovar Typhimurium DT204 acrB mutants selected with fluoroquinolones. Antimicrob. Agents Chemother. 49289-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pasquali, F., C. Kehrenberg, G. Manfreda, and S. Schwarz. 2005. Physical linkage of Tn3 and part of Tn1721 in a tetracycline and ampicillin resistance plasmid from Salmonella Typhimurium. J. Antimicrob. Chemother. 55562-565. [DOI] [PubMed] [Google Scholar]
- 14.Weill, F. X., M. Demartin, L. Fabre, and P. A. D. Grimont. 2004. Extended-spectrum-β-lactamase (TEM-52)-producing strains of Salmonella enterica of various serotypes isolated in France. J. Clin. Microbiol. 423359-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weill, F. X., R. Lailler, K. Praud, A. Kérouanton, L. Fabre, A. Brisabois, P. A. D. Grimont, and A. Cloeckaert. 2004. Emergence of extended-spectrum-β-lactamase (CTX-M-9)-producing multiresistant strains of Salmonella enterica serotype Virchow in poultry and humans in France. J. Clin. Microbiol. 425767-5773. [DOI] [PMC free article] [PubMed] [Google Scholar]