Abstract
Acyclovir-resistant clinical isolates of herpes simplex virus type 1 (HSV-1) were analyzed in vitro for their susceptibilities to essential oils of ginger, thyme, hyssop, and sandalwood. All essential oils exhibited high levels of virucidal activity against acyclovir-sensitive strain KOS and acyclovir-resistant HSV-1 clinical isolates and reduced plaque formation significantly.
Herpes simplex virus type 1 (HSV-1) is a highly prevalent pathogen among children and adults, causing primary infections which present clinically as herpes labialis or as primary herpetic gingivostomatitis, and is able to establish a latent infection in the nervous system that can be reactivated quite frequently (10, 31, 32). Acyclovir has been widely used for the management of herpes virus infections, and its preferential phosphorylation by the HSV-encoded thymidine kinase (TK) makes it a selective antiviral drug (8, 14). The emergence of virus strains resistant to commonly used antiherpesvirus drugs is a problem in the clinical setting, particularly in immunocompromised patients (3, 4, 6, 19, 30). This trend has led to a search for alternative antiherpetic agents that have a wide range of efficacy without serious adverse effects and are effective for viral strains resistant to current antiviral agents. HSV develops resistance predominantly as a result of mutations in genes that code for TK, but resistance can also result from mutations in DNA polymerase. The antiherpes activities of Australian tea tree oil (16, 23), peppermint oil (25), and manuka oil (17) have previously been published. In the present study, we analyzed the virucidal activities of essential oils derived from ginger, thyme, hyssop, and sandalwood against acyclovir-sensitive and acyclovir-resistant clinical HSV-1 isolates for which therapy with acyclovir failed.
Essential oils from ginger (Zingiber officinale), thyme (Thymus vulgaris), hyssop (Hyssopus officinalis), and sandalwood (Santalum album) were investigated. The main components (composing about 5 to 10%) of ginger oil are sesquiterpenes (e.g., zingiberene, β-bisabolene, sesquiphellandrene, and curcumen), thyme oil consists mainly of thymol and carvacrol, hyssop oil consists mainly of monoterpenes (e.g., 1-pinocamphone, isopinocamphone, pinocarvone, and α-pinene), and sandalwood oil is mainly composed of sesquiterpene alcohols (e.g., santalol, bergamotol, and santalene). Acyclovir-sensitive HSV-1 strain KOS (15) and acyclovir-resistant patient isolates 1246/99 and 496/02 were used for the experiments. Each of the two hospital specimens from infected patients revealed a single-point mutation in the coding sequence of the TK gene which resulted in frameshifts, and probably only truncated, nonfunctional TK was expressed. These mutations were both located in homopolymer stretches of guanines downstream of the ATP-binding site for 1246/99 and cytosines downstream of the nucleoside-binding site for 496/02 and have been reported previously (1, 5, 9, 21, 22). The well-characterized acyclovir-resistant HSV-1 strain Angelotti was also used in the experiments and exhibits a single-point mutation in the DNA polymerase gene (12). Viruses were routinely grown on RC-37 cells as described previously (20). Genomic DNA was extracted from the supernatant of plaque-purified virus and amplified by PCR (5), and PCR products were sequenced as described previously (24). All essential oils were dissolved in ethanol and added to the medium at a final concentration of 1% ethanol for cytotoxicity assays, which determined the viability and proliferation of the cells (25, 29).
The cytotoxic concentration of the drug which reduced viable cell number by 50% (CC50) and the effective concentration of the test compound which inhibited plaque numbers by 50% (EC50) were determined from dose-response curves (Table 1). Selectivity indices for different essential oils were calculated as CC50/EC50 ratios and are given in Table 1. Ginger oil and hyssop oil exhibited selectivity indices of 20 and 75, respectively. The maximum noncytotoxic concentrations of the tested essential oils were determined at 0.003% for ginger oil, 0.005% for thyme oil and hyssop oil, and 0.0006% for sandalwood oil. The dose-response curves shown in Fig. 1 demonstrate dose-dependent activities for the tested essential oils. The inhibitory effects of the essential oils against HSV were tested by adding the oils at different times during the infection cycle of HSV (Table 2). To identify the step at which replication might be inhibited, cells were infected with these HSV-1 strains after preincubation of the cells for 1 h with essential oils; after pretreatment of the virus strains for 1 h with the essential oils prior to infection; after addition of the essential oils, during adsorption; or after adsorption, during the intracellular-replication period. In all experiments, untreated, virus-infected cells were used as controls. Percent reduction was calculated relative to the amount of virus produced in the absence of the compounds. Pretreatment of HSV with the analyzed essential oils prior to infection caused significant reductions of infectivity, ranging from 95.9% to 99.9% for the acyclovir-sensitive and drug-resistant HSV-1 strains.
TABLE 1.
Selectivity indices of essential oils for HSV-1a
| Essential oil | CC50 (%) | EC50 (%) | SI |
|---|---|---|---|
| Ginger | 0.004 ± 0.001 | 0.0002 ± 0.00001 | 20 |
| Thyme | 0.007 ± 0.0003 | 0.001 ± 0.0001 | 7 |
| Hyssop | 0.0075 ± 0.002 | 0.0001 ± 0.00001 | 75 |
| Sandalwood | 0.0015 ± 0.0001 | 0.0002 ± 0.000003 | 7 |
Experiments were repeated independently two times, and data presented are the means for three experiments. SI, selectivity index.
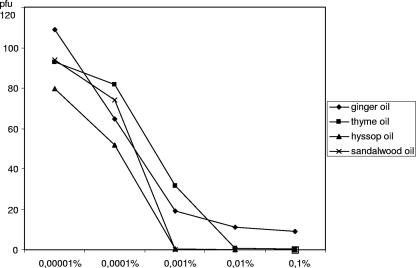
FIG. 1.
Determination of the EC50s of ginger oil, thyme oil, hyssop oil, and sandalwood oil against HSV-1. Viruses were incubated for 1 h at room temperature with increasing concentrations of the essential oils and immediately tested in a plaque reduction assay. Experiments were repeated independently two times, and data presented are the means for three experiments.
TABLE 2.
Virucidal effects of essential oils against acyclovir-sensitive HSV-1 strain KOS, acyclovir-resistant strain Angelotti, and acyclovir-resistant clinical isolates 1246/99 and 496/02a
| Step | Result (%) for indicated oil and strain
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ginger oil
|
Thyme oil
|
Hyssop oil
|
Sandalwood oil
|
|||||||||||||
| KOS | Ang | 1246/99 | 496/02 | KOS | Ang | 1246/99 | 496/02 | KOS | Ang | 1246/99 | 496/02 | KOS | Ang | 1246/99 | 496/02 | |
| Pretreatment of cells | 101.3 ± 8.4 | 99.4 ± 5.5 | 109.3 ± 3.2 | 87.9 ± 4.1 | 101.1 ± 8.0 | 93.9 ± 18.1 | 117.9 ± 10.4 | 89.3 ± 1.0 | 95.7 ± 10.7 | 89.2 ± 6.4 | 104.8 ± 4.8 | 98.6 ± 2.3 | 104.3 ± 2.6 | 97.1 ± 8.5 | 104.7 ± 7.7 | 85.9 ± 1.6 |
| Pretreatment of virus | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.2 | 0.1 ± 0.1 | 3.4 ± 2.9 | 4.1 ± 3.2 | 1.2 ± 0.9 | 0.3 ± 0.3 | 0.1 ± 0.1 | 0.2 ± 0.2 | 0.3 ± 0.3 | 0.1 ± 0.1 | 2.1 ± 2.9 | 0.2 ± 0.2 | 1.1 ± 0.8 | 0.3 ± 0.2 |
| Adsorption | 66.5 ± 4.0 | 70.7 ± 3.1 | 68.2 ± 9.4 | 47.1 ± 0.9 | 92.2 ± 9.2 | 91.3 ± 5.0 | 87.2 ± 8.3 | 61.1 ± 3.3 | 76.1 ± 5.9 | 105.3 ± 12.0 | 80.0 ± 10.0 | 51.2 ± 4.9 | 62.3 ± 12.9 | 104.3 ± 8.2 | 89.8 ± 5.9 | 71.0 ± 5.2 |
| Replication | 87.2 ± 19.5 | 101.6 ± 8.3 | 95.4 ± 6.0 | 100.8 ± 3.3 | 101.5 ± 15.9 | 99.3 ± 0.5 | 99.1 ± 11.3 | 98.4 ± 0.8 | 88.1 ± 10.2 | 97.6 ± 7.0 | 106.8 ± 10.0 | 99.7 ± 4.0 | 87.4 ± 18.9 | 85.0 ± 0.5 | 105.5 ± 2.6 | 93.6 ± 0.1 |
The maximum noncytotoxic concentrations of the essential oils were used for all experiments. Data represent percentages of plaques compared to those for untreated controls. Experiments were repeated independently two times, and data presented are the means ± standard deviations for three experiments. Ang, Angelotti strain.
These results indicate that essential oils derived from ginger, thyme, hyssop, and sandalwood affected the virus before adsorption and in a different manner than acyclovir since plaque formation levels for acyclovir-resistant patient isolates HSV-1 1246/99 and 496/02 were significantly reduced, too. A high level of virucidal activity during the pretreatment of HSV-1 was detected previously by using the essential oil of Salvia fruticosa (28). Essential oils seem to be mostly efficient on cell-free virus but have limited effects on virus replication in cells and on the cell-to-cell spread of the virus (13). These results suggest that the investigated essential oils might interfere with virion envelope structures which are necessary for adsorption to or entry into host cells or might dissolute the HSV envelope. Treatment of HSV-1 with oregano essential oil has been shown to disrupt the viral envelope (27). In preliminary electron microscopical studies, we also demonstrated a disruption of the viral envelope after pretreatment of HSV with essential oils, thereby impairing their abilities to infect host cells. Shogan et al. (26) investigated the antiviral mechanisms of a GT-rich oligonucleotide which potently inhibited attachment of HSV to cells by induction of a conformational change in glycoprotein B, resulting in inactivation of infectivity. The virucidal activity of the GT-rich oligonucleotide is time dependent and causes an irreversible loss of infectivity. A resistant virus with mutations in the UL27 gene was isolated by these authors, and attachment of HSV to cells was not inhibited in the mutant strain. Since lipophilic essential oils inhibit attachment only moderately and most likely exert their virucidal activities by disrupting the viral lipid membrane, resistant strains of HSV could not be detected. After pretreatment of HSV with essential oils, the few remaining infectious viruses are still sensitive to treatment with essential oils. Essential oils are complex mixtures of compounds with low molecular weights, such as monoterpene hydrocarbons, sesquiterpene hydrocarbons, and their corresponding oxidized products (e.g., alcohols, aldehydes, and ketones); homologues of phenylpropanoids; and small amounts of diterpenoids. The active components of essential oils might consist of lipophilic carbohydrates that interact with the lipid membrane (18). These antibacterially active substances (7, 11) might exhibit similar activities against viral envelopes. Interestingly, acyclovir-resistant clinical isolates were significantly inhibited by the essential oils, and the titers of HSV were reduced by 95.9% to 99.9%. Since essential oils are able to inhibit acyclovir-resistant HSV-1 isolates, the mechanism of interaction between these compounds and acyclovir for HSV must be different. Acyclovir inhibits virus replication by interference with the DNA polymerase inside the cell, whereas essential oils probably inactivate HSV before it enters the cell. The effective dosage for a systemic application of essential oils is rather high and leads to cytotoxic effects. Furthermore, a short-term systemic bioavailability makes a systemic application unlikely. Therefore, other antiherpetic agents which are effective against viral mutants resistant to current antiviral agents are of great interest for additional topical treatment of recurrent acyclovir-susceptible and acyclovir-resistant HSV-1 infections, as has been demonstrated by topical application of tea tree oil (2) against recurrent herpes labialis.
Acknowledgments
We thank U. Bahr, University of Heidelberg, for sequencing and E. Daum for technical assistance. We also thank A. Sauerbrei, Institute for Antiviral Chemotherapy, University of Jena, Germany, for kindly providing the HSV-1 clinical isolates 1246/99 and 496/02 and C. W. Knopf for providing HSV-1 strain Angelotti.
Footnotes
Published ahead of print on 12 March 2007.
REFERENCES
- 1.Andrei, G., J. Balzarini, P. Fiten, E. De Clercq, G. Opdenakker, and R. Snoeck. 2005. Characterization of herpes simplex virus type 1 thymidine kinase mutants selected under a single round of high-dose brivudin. J. Virol. 795863-5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carson, C. F., L. Ashton, L. Dry, D. W. Smith, and T. V. Riley. 2001. Melaleuca alternifolia (tea tree) oil gel (6%) for the treatment of recurrent herpes labialis. Antimicrob. Agents Chemother. 48450-451. [DOI] [PubMed] [Google Scholar]
- 3.Chakrabarti, S., D. Pillay, D. Ratcliffe, P. A. Cane, K. E. Collinghan, and D. W. Milligan. 2000. Resistance to antiviral drugs in herpes simplex virus infections among allogeneic stem cell transplant recipients: risk factors and prognostic significance. J. Infect. Dis. 1812055-2058. [DOI] [PubMed] [Google Scholar]
- 4.Chen, Y., V. Scieux, V. Garrait, G. Socié, V. Rocha, J. M. Molina, D. Thouvenot, F. Morfin, I. Hocqueloux, L. Garderei, H. Esperou, F. Selimi, A. Dervergie, G. Lelen, M. Aymard, F. Morinet, E. Gluckman, and P. Ribaud. 2000. Resistent herpes simplex virus type 1 infection: an emerging concern after allogeneic stem cell transplantation. Clin. Infect. Dis. 31927-935. [DOI] [PubMed] [Google Scholar]
- 5.Chibo, D., J. Druce, J. Sasadeusz, and C. Birch. 2004. Molecular analysis of clinical isolates of acyclovir-resistant herpes simplex virus. Antivir. Res. 6183-91. [DOI] [PubMed] [Google Scholar]
- 6.Christophers, J., J. Clayton, J. Craske, R. Ward, P. Collins, M. Trowbridge, and G. Darby. 1998. Survey of resistance of herpes simplex virus to acyclovir in northwest England. Antimicrob. Agents Chemother. 42868-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox, S. D., C. M. Mann, J. L. Markham, H. C. Bell, J. E. Gustafson, J. R. Warmington, and S. G. Wyllie. 2000. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J. Appl. Microbiol. 88170-175. [DOI] [PubMed] [Google Scholar]
- 8.De Clercq, E. 2004. Antiviral drugs in current clinical use. J. Clin. Virol. 30115-133. [DOI] [PubMed] [Google Scholar]
- 9.Gaudreau, A., E. L. Hill, H. H. Balfour, Jr., A. Erice, and G. Boivin. 1998. Phenotypic and genotypic characterization of acyclovir-resistant herpes simplex viruses from immunocompromised patients. J. Infect. Dis. 178297-303. [DOI] [PubMed] [Google Scholar]
- 10.Hill, E. L., G. A. Hunter, and M. N. Ellis. 1991. In vitro and in vivo characterization of herpes simplex virus clinical isolates recovered from patients infected with human immunodeficiency virus. Antimicrob. Agents Chemother. 352322-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inoue, Y., A. Shiraishi, T. Hada, K. Hirose, H. Hamashima, and J. Shimada. 2004. The antibacterial effects of terpene alcohols on Stapylococcus aureus and their mode of action. FEMS Microbiol. Lett. 237325-331. [DOI] [PubMed] [Google Scholar]
- 12.Knopf, C. W. 1987. The herpes simplex virus type 1 DNA polymerase gene: site of phosphonoacetic acid resistance mutation in strain Angelotti is highly conserved. J. Gen. Virol. 681429-1433. [DOI] [PubMed] [Google Scholar]
- 13.Koch, C., J. Reichling, and P. Schnitzler. Essential oils inhibit the replication of herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2). In R. R. Watson and V. R. Preedy (ed.), The encyclopedia of herbal medicine in clinical practice, in press. CAB International, Wallingford, United Kingdom.
- 14.Morfin, F., G. Souillet, K. Bilger, T. Ooka, M. Aymard, and D. Thouvenot. 2000. Genetic characterization of thymidine kinase from acyclovir-resistant and acyclovir-susceptible herpes simplex virus type 1 isolated from bone marrow transplant recipients. J. Infect. Dis. 182290-293. [DOI] [PubMed] [Google Scholar]
- 15.Parris, D. S., R. A. Dixon, and P. A. Schaffer. 1980. Physical mapping of herpes simplex virus type 1 ts mutants by marker rescue: correlation of the physical and genetic maps. Virology 100275-287. [DOI] [PubMed] [Google Scholar]
- 16.Reichling, J. 2001. Australian and New Zealand tea tree oil as the source of noval antiinfective agents, p. 69-80. In E. Wildi and M. Wink (ed.), Trends in medicinal plant research. Romneya-Verlag, Dossenheim, Germany.
- 17.Reichling, J., C. Koch, E. Stahl-Biskup, C. Sojka, and P. Schnitzler. 2005. Virucidal activity of a beta-triketone-rich essential oil of Leptospermum scoparium (manuka oil) against HSV-1 and HSV-2 in cell culture. Planta Med 711123-1127. [DOI] [PubMed] [Google Scholar]
- 18.Reichling, J., U. Suschke, J. Schneele, and H. K. Geiss. 2006. Antibacterial activity and irritation potential of selected essential oil components—structure-activity relationship. Nat. Prod. Commun. 11003-1012. [Google Scholar]
- 19.Reusser, P. 1996. Herpesvirus resistance to antiviral drugs: a review of the mechanisms, clinical importance and therapeutic options. J. Hosp. Infect. 3235-248. [DOI] [PubMed] [Google Scholar]
- 20.Rösen-Wolff, A., T. Ben-Hur, Y. Becker, and G. Darai. 1988. Comparative analysis of the transcripts mapped in the BamHI DNA fragment B of avirulent HSV-1 HFEM, virulent HSV-1 F, and their intratypic recombinant viruses. Virus Res. 10315-324. [DOI] [PubMed] [Google Scholar]
- 21.Saijo, M., T. Suzutani, E. De Clercq, M. Niikura, A. Maeda, S. Morikawa, and I. Kurane. 2002. Genotypic and phenotypic characterization of the thymidine kinase of ACV-resistant HSV-1 derived from an acyclovir-sensitive herpes simplex virus type 1 strain. Antiviral Res. 56253-262. [DOI] [PubMed] [Google Scholar]
- 22.Sasadeusz, J. J., F. Tufuro, S. Safrin, K. Schubert, M. M. Hubinette, P. K. Cheung, and S. L. Sacks. 1997. Homopolymer mutational hot spots mediate herpes simplex virus resistance to acyclovir. J. Virol. 713872-3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schnitzler, P., K. Schön, and J. Reichling. 2001. Antiviral activity of Australian tea tree oil and eucalyptus oil against herpes simplex virus in cell culture. Pharmazie 56343-347. [PubMed] [Google Scholar]
- 24.Schöndorf, E., U. Bahr, M. Handermann, and G. Darai. 2003. Characterization of the complete genome of the tupaia (tree shrew) adenovirus. J. Virol. 774345-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuhmacher, A., J. Reichling, and P. Schnitzler. 2003. Virucidal effect of peppermint oil on the enveloped viruses herpes simplex virus type 1 and type 2 in vitro. Phytomedicine 10504-510. [DOI] [PubMed] [Google Scholar]
- 26.Shogan, B., L. Kruse, G. B. Mulamba, A. Hu, and D. M. Coen. 2006. Virucidal activity of a GT-rich oligonucleotide against herpes simplex virus mediated by glycoprotein B. J. Virol. 804740-4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siddiqui, Y. M., M. Ettayebi, A. M. Haddad, and M. N. Al-Ahdal. 1996. Effect of essential oils on the enveloped viruses: antiviral activity of oregano and clove oils on herpes simplex virus type 1 and Newcastle disease virus. Med. Sci. Res. 24185-186. [Google Scholar]
- 28.Sivropoulou, A., C. Nikolaou, E. Papanikolaou, S. Kokkini, T. Lanaras, and M. Arsenakis. 1997. Antimicrobial, cytotoxic, and antiviral activities of Salvia fructicosa essential oil. J. Agric. Food Chem. 453197-3201. [Google Scholar]
- 29.Söderberg, T., A. Johannson, and R. Gref. 1996. Toxic effects of some conifer resin acids and tea tree oil on human epithelial and fibroblast cells. Toxicology 10799-109. [DOI] [PubMed] [Google Scholar]
- 30.Stranska, R., R. Schuurman, E. Nienhuis, I. W. Goedegebuure, M. Polman, J. F. Weel, P. M. Wertheim-Van Dillen, R. J. M. Berkhout, and A. M. van Loon. 2005. Survey of acyclovir-resistant herpes simplex virus in the Netherlands: prevalence and characterization. J. Clin. Virol. 327-18. [DOI] [PubMed] [Google Scholar]
- 31.Whitley, R. J. 2001. Herpes simplex virus, p. 2461-2509. In D. M. Knipe, P. M. Howley, and D. F. Griffin (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 32.Whitley, R. J., M. Levin, N. Barton, B. J. Hershey, G. Davis, R. E. Keeney, J. Welchel, A. G. Diethelm, P. Kartus, and S. J. Soong. 1984. Infections caused by herpes simplex in the immunocompromised host: natural history and topical acyclovir therapy. J. Infect. Dis. 150323-329. [DOI] [PubMed] [Google Scholar]