Abstract
The nonnucleoside reverse transcriptase inhibitor UC781 is under development as a potential microbicide to prevent sexual transmission of human immunodeficiency virus type 1 (HIV-1). Two gel formulations of UC781 (0.1% and 1.0%) were evaluated in a range of preclinical safety assessments, including systemic absorption analysis following topical application in the pig-tailed macaque models for vaginally and rectally applied topical microbicides. High-sensitivity high-performance liquid chromatography analysis of serum samples showed that no systemic absorption of UC781 was detected after repeated vaginal or rectal application of either product. However, high levels of UC781 were detectable in the cervicovaginal lavage samples up to 6 h after product exposure. Both formulations were safe to the vaginal microenvironment, even with repeated daily use, as evidenced by colposcopy, cytokine analysis, and lack of impact on vaginal microflora. By contrast, rectal application of the 1.0% UC781 formulation caused an increased expression of numerous cytokines not observed after rectal application of the 0.1% UC781 formulation. These results provide additional support for the continued development of UC781 formulations as anti-HIV microbicides.
Nearly 40 million people are currently living with human immunodeficiency virus (HIV)/AIDS worldwide. In 2006, an estimated 4.3 million people became newly infected with HIV, the greatest number in any single year since the beginning of the epidemic. Additionally, the overall proportion of HIV-positive women has steadily increased. In 1997, women accounted for 41% of people living with HIV; by 2005, this figure rose to almost 50% (13).
While the development of an effective vaccine has the potential to slow the spread of HIV, it is now recognized that developing a sterilizing vaccine that will prevent infection presents a considerable challenge. Therefore, there is a need to identify and develop new tools for the prevention of HIV that can be used as an adjunct to vaccine-based approaches. One such complementary approach is the use of topically applied microbicidal agents that inactivate and/or block HIV without affecting the sensitive mucosal surfaces or microbiological ecosystems of vaginal and rectal environments with repeated use. Various agents have been proposed as topical anti-HIV microbicides (5, 10, 11), including reverse transcriptase inhibitors. UC781 is a nonnucleoside reverse transcriptase inhibitor and has been shown to be a potent inhibitor of HIV-1 in cell culture at approximately 3 ng/ml, which is equivalent to 8.9 nM (1).
In this study, two gel formulations of UC781 (0.1% and 1.0% provided by Biosyn, Inc.) were assessed for pharmacokinetic and preclinical safety screening after repeated vaginal and rectal applications in the pig-tailed macaque models (7-9). Blood samples from test animals were assessed for systemic absorption of UC781. Vaginal and rectal lavage samples were assessed for local detection of UC781 in the anatomic sites of product delivery. Finally, safety profiles were determined for 0.1% and 1.0% UC781 formulations after repeated vaginal and rectal use.
MATERIALS AND METHODS
Test products.
Water-based gel formulations containing 0.1% and 1.0% UC781 and a placebo gel were provided by Biosyn, Inc. (Huntingdon Valley, PA). The placebo gel used in these studies is the HEC gel (12), which contains no HIV microbicide drugs, also provided by Biosyn, Inc. The excipients include carbomer 974PNF, methylcellulose USP, glycerin USP, methylparaben USP, propylparaben USP, purified water USP, and hydrochloric acid or sodium hydroxide as needed to adjust the pH.
In vitro toxicity evaluations. (i) Tissue specimens.
Freshly excised human ectocervical tissue was obtained from the Tissue Procurement Facility at Magee-Womens Hospital per the institutional review board-approved protocol. Tissue was obtained from women undergoing hysterectomy for benign conditions. No specimens were used when there was evidence of tissue abnormality that may influence the state of the mucosa. Specimens were obtained within 1 hour of surgical excision. Tissue samples were retained from each specimen for histological evaluation for comparison in before and after exposure studies.
(ii) Exposure studies.
Tissue exposure studies were conducted using a Franz cell system. This is a two-compartment system consisting of an upper chamber (donor compartment) and a lower chamber (receiver compartment) separated by a hydrophobic membrane in which tissue exposure can be isolated to the epithelial surface. The Franz cells used a water jacket to maintain the temperature at 37°C throughout the experiments via a circulating water bath. Dulbecco modified Eagle medium (DMEM) was used in the receiver chamber. The tissue sample was placed on the top of a 7-mm Franz cell opening, which provided an exposure area of 0.385 cm2. The tissue sample was sandwiched between the two compartments with the epithelial side facing the donor compartment. Test 0.1% UC781 gel product was placed in the donor compartment, and tissue samples were exposed to the test product for a period of 2 or 24 h. DMEM in the donor compartment served as the control. Following the exposure period, excess gel was removed from the donor compartment using a syringe. The tissue was then removed and either evaluated for viability using the 1-(4,5-dimethylthiazol-2-y1)-3,5-diphenylformazan (MTT) assay (see below) or fixed in an acidic alcohol solution for histological evaluation. Studies were also conducted to evaluate the potential for systemic uptake of UC781 following exposure in which the receptor solution was assayed for UC781 content using the quantitative high-performance liquid chromatography (HPLC) methods described below.
(iii) MTT assay.
The viability of cells can be evaluated by the MTT assay described by Greenhead et al. (4). This method has been used for human tissue.
(iv) Histological evaluations.
Histological evaluation was conducted on all tissue specimens tested. Retained tissue from each test specimen was fixed for histology and processed prior to the experiment in order to compare with postexperimental histology. Tissue was fixed in Clark's solution (ethanol-acetic acid [75:25]) for 24 h, transferred to ethanol for 24 h, and subsequently embedded in paraffin. Five-micron sections were cut and stained with hematoxylin and eosin. Histology was conducted to evaluate gross alterations in tissue morphology caused by exposure to test gel product.
In vivo safety studies. (i) Animals.
A total of 20 sexually mature Macaca nemestrina (12 females and 8 males) were obtained from a colony of animals at the Washington National Primate Research Center. Prior approval for use of monkeys in this protocol was obtained from the Institutional Animal Care and Use Committee at the University of Washington. Animals were handled humanely, and experiments were performed within the National Institutes of Health's laboratory animal use guidelines.
In order to conserve resources and most effectively use the limited number of research animals available for study, all of the in vivo experiments discussed here were conducted with macaques randomly selected from a pool of 20 animals over a 2.5-year period. Naturally, female animals were used for all studies of vaginal product use, while males were made available for random selection into rectal product use studies (Table 1).
TABLE 1.
Animal enrollment in the various UC781 studies
| Animal sex and ID codea | Animal enrollment in the following studyb:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Systemic uptake (single exposure)
|
Safety evaluation
|
Local detection (secretion) (1.0% UC781, V) | |||||||
| 0.1% UC781
|
1.0% UC781
|
0.1% UC781
|
1.0% UC781
|
||||||
| Vc | Rc | V | R | V | R | V | R | ||
| Females | |||||||||
| CMU01 | X | X | X | ||||||
| CMU02 | X | X | X | ||||||
| CMU03 | X | X | X | ||||||
| CMU04 | X | X | X | X | |||||
| CMU05 | X | X | X | ||||||
| CMU06 | X | X | X | X | X | ||||
| CMU07 | X | X | X | ||||||
| CMU08 | X | X | |||||||
| CMU09 | X | X | X | X | |||||
| CMU10 | X | X | X | X | X | ||||
| CMU11 | X | X | X | X | |||||
| CMU12 | X | X | X | ||||||
| Males | |||||||||
| CMU14 | X | ||||||||
| CMU15 | X | ||||||||
| CMU16 | X | X | X | ||||||
| CMU18 | X | ||||||||
| CMU24 | X | X | |||||||
| CMU25 | X | ||||||||
| CMU26 | X | ||||||||
| CMU27 | X | X | |||||||
ID code, identification code.
An X indicates that the particular animal was used for that study (e.g., animal CMU01 was used for a systemic uptake experiment of 1.0% UC781 applied to the vagina, for a safety evaluation of 0.1% UC781 applied to the vagina, and for local detection [secretion] of 1.0% UC781).
The UC781 formulation was applied to the vagina (V) or rectum (R).
(ii) Vaginal safety.
Two concentrations of UC781 (0.1% and 1.0% gel formulations) were assessed for safety with vaginal application and compared to a placebo formulation (HEC gel). Six animals were randomly assigned to each formulation's safety evaluation. In the two-arm, crossover design studies, each animal controls for itself by completing both arms of the study (test gel and placebo gel). Two experimental runs, each lasting 8 days and evaluating three animals per arm, were conducted with a 2-week recovery time scheduled between runs. Initially, the 0.1% gel was assessed for safety. One of the six animals randomly assigned to this evaluation had been exposed 5 months earlier to a single intravaginal exposure with the 0.1% UC781 gel.
A second series of experiments conducted 5 to 6 months after the 0.1% gel evaluation assessed the 1.0% gel formulation controlled again by the HEC placebo arm. Five of the six animals randomly enrolled in this study had been previously exposed to UC781 gel. These exposures occurred 2 1/2 to 6 months prior to the experiment described here. None of the assays conducted could document any residual effects of previous UC781 exposures.
The same protocol was followed for each safety evaluation (Table 2). A baseline blood sample and vaginal lavage sample (for pharmacokinetic analyses) were collected from each macaque. On study days 1 to 4, colposcopic assessments, swabs for cervical cytokines, vaginal pH, and microflora were obtained. Immediately following specimen collections, an intravaginal application of 1.5 ml of test or placebo gel was administered to each animal. Thirty minutes after gel application, repeat blood samples and vaginal swabs for pH assessment were collected. On study days 5 and 8, colposcopy, swabs for cervical cytokine analysis, vaginal pH, and microflora were collected to document recovery. In addition, blood samples and vaginal lavage samples were collected for systemic and local detection of UC781.
TABLE 2.
Vaginally applied UC781 gel safety study designa
| Procedure or measurement | Procedure or measurement performed at the following timeb:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 1
|
Day 2
|
Day 3
|
Day 4
|
Follow-up
|
||||||
| 0 min | 30 min | 0 min | 30 min | 0 min | 30 min | 0 min | 30 min | Day 5 | Day 8 | |
| Colposcopy | X | X | X | X | X | X | ||||
| Microflora | X | X | X | X | X | X | ||||
| Vaginal pH | X | X | X | X | X | X | X | X | X | X |
| Cervical cytokines | X | X | X | X | X | X | ||||
| Vaginal lavage | X | X | X | |||||||
| Blood draw | X | X | X | X | X | X | X | |||
After each sampling at 0 min, 1.5 ml UC781 or HEC gel was applied intravaginally.
An X indicates that the procedure or measurement was performed (e.g., colposcopy was performed at 0 min on days 1 to 4 and on days 5 and 8 [follow-up]). Repeat blood samples and vaginal swabs for pH assessment were collected 30 min after gel application.
Standardized colposcopic assessments were conducted by a team of three cross-trained individuals. In order to document the appearance of the cervicovaginal tissues prior to potential tissue perturbation caused by sampling, colposcopy took place immediately after speculum placement before any swab collections. Vaginal pH was determined by rolling a swab of vaginal secretions onto a pH indicator strip with a resolution of 0.5 pH unit. A second vaginal swab was collected and immersed in a transport tube (Port-a-Cul; Becton Dickinson Microbiology Systems, Cockeysville, MD) for quantitative microbiologic characterization. Cytokine presence was assessed from additional swab samples collected from the cervical os.
(iii) Rectal safety.
Both the 0.1% and 1.0% UC781 formulations were assessed in the rectal application safety model (Table 3). For each formulation, six animals were randomly selected for inclusion in the rectal safety experiment. Each animal served as its own control in this two-arm (test versus placebo) crossover study. As in the vaginal safety studies, each animal underwent the experimental protocol two times, separated by a 2-week window for macaque recovery, crossing test arms for each repetition. In the first experiment, one arm tested four daily intrarectal applications of 2.5 ml 0.1% UC781 formulation and one tested four daily intrarectal applications of 2.5 ml HEC placebo gel. None of the macaques randomly assigned to this study had previous exposure to UC781. In the second series of experiments conducted 8 months after the 0.1% formulation rectal assessment, the same protocol was followed, but test animals were exposed to four daily intrarectal applications of 2.5 ml of the 1.0% UC781 formulation controlled again by HEC gel. Each of the six animals randomly assigned to this study had been previously exposed to UC781 gels. These exposures occurred 2 1/2 to 13 months prior to this rectal safety experiment.
TABLE 3.
Rectally applied UC781 gel safety study designa
| Procedure or measurement | Procedure or measurement performed at the following timeb:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 1
|
Day 2
|
Day 3
|
Day 4
|
Day 5 (follow-up) | |||||
| 0 min | 30 min | 0 min | 30 min | 0 min | 30 min | 0 min | 30 min | ||
| Blood draw | X | X | X | X | X | X | |||
| Microflora | X | X | X | X | X | ||||
| Rectal pH | X | X | X | X | X | X | X | X | X |
| Rectal cytokines | X | X | X | X | X | ||||
| Rectal lavage | X | X | X | X | X | X | X | X | X |
After each sampling at 0 min, 2.5 ml UC781 or HEC gel was applied intrarectally.
An X indicates that the procedure or measurement was performed (e.g., blood was drawn at 0 and 30 min on day 1, at 30 min on days 2, 3, and 4, and on day 5 [follow-up]). Blood was drawn, and swabs were taken for assessment of microflora, rectal pH, and rectal cytokines 30 min after application of the gel.
Prior to each gel application (days 1 to 4), blood samples (pharmacokinetic), rectal swabs (for assessments of rectal pH, cytokines, and microflora), and rectal lavage specimens were collected. These sample collections were followed by an application of 2.5 ml gel (test or placebo) past the anal sphincter into the rectum. Thirty minutes later, repeat samples were collected. On the fifth day, a final blood sample, rectal pH swab, cytokine swab, microbiology swab, and lavage specimen were collected from each animal.
Rectal pH, cytokine, and microbiology sample collections were performed as described above for vaginal safety studies. Rectal microbiology, however, was characterized by semiquantitative measures. The rectal lavage was performed with a 10-ml syringe attached to an infant feeding tube. The tube was carefully inserted 3 to 5 cm beyond the anal sphincter (into the rectum), and 8 ml of saline wash was expelled. Twenty seconds later, the saline wash was recovered and deposited into a vial for characterization.
Safety measures. (i) Colposcopic assessment.
Colposcopy was conducted following the World Health Organization/Contraceptive Research and Development Program Manual for the Standardization of Colposcopy for the Evaluation of Vaginal Products (14). Vaginal and ectocervical mucosal surfaces were evaluated for erythema, vasculature pattern, epithelial integrity, and any exceptional findings. Observations were noted on daily exam records and documented by digital photography.
(ii) Vaginal and rectal microbiologic characterization.
Each swab collected for microbiologic assessment was individually placed in a Port-a-Cul tube, designed for transport of anaerobic, facultative, and aerobic specimens on swabs. This transport device has been shown to preserve viability of aerobic and anaerobic bacteria in clinical specimens (2). Within 24 h of collection, samples were delivered to the Reproductive Infectious Disease Laboratory at Magee-Womens Research Institute (Pittsburgh, PA) for analysis.
Microbiologic swab specimens from all animals were evaluated for aerobic and anaerobic microorganisms with assays previously described (8, 10). Quantitative analyses were performed for all vaginal specimens. Semiquantitative analyses were performed for each organism detected in rectal specimens, with results reported on a scale of 0 to 4 (0 for not detected and 4 for growth on all quadrants of a plate). Species belonging to the genera Bacteroides, Porphyromonas, and Prevotella were grouped together as anaerobic gram-negative rods (black or nonpigmented) for ease of presentation.
(iii) Cytokine analysis.
Cervical and rectal swabs were collected with Dacron-tipped swabs and immediately placed into aliquoted samples of Dulbecco's phosphate-buffered saline, without calcium or magnesium, and stored at −80°C. Samples were batched for overnight delivery to the Infectious Disease Laboratory at Magee-Womens Research Institute in Pittsburgh, PA, for cytokine detection. Commercially available multiplexed fluorescent bead-based immunoassays (Upstate, Lake Placid, NY) were used to determine cytokine levels following the package insert. The panel of cytokines included interleukin 1β (IL-1β), IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), macrophage inflammatory protein 1α (MIP-1α), and IL-12p40. Briefly, standards were diluted, and 50-μl amounts of standards and samples were then added in duplicate to a 96-well multiscreen plate (Millipore, Billerica, MA). Capture beads were diluted to 1× and added to the plate. The plate was incubated overnight in the dark at 4°C on a plate shaker. Following incubation, a vacuum manifold was applied to the filter plate to remove buffers, and the plate was washed with assay buffer. The plate was vortexed and vacuumed again. The beads were resuspended in 75 μl of assay buffer. Dilute reporter solution was added, and the plate was incubated for 1.5 h in the dark at room temperature. Diluted streptavidin-phycoerythrin solution was added. After a 30-min incubation, the plate was vacuumed and the beads were resuspended in 125 μl assay buffer. The results were read on a Luminex 100 instrument (Luminex Co., Austin, TX). Concentrations were extrapolated from an eight-point standard curve using the StarStation software (Applied Cytometry Systems, Sacramento, CA).
(iv) Rectal lavage.
Two to four hours after sample collections, rectal lavage washes were individually examined under a dissecting microscope at ×7 magnification. At low-power examination, each rectal lavage was evaluated for evidence of fecal matter, cellular debris, epithelial desquamation (epithelial sheets are defined as measuring ≥3 mm in at least one dimension), and stroma and/or blood associated with epithelial sheets. After gross examination, epithelial sheets were measured, and lavage samples were photographed for documentation.
Statistical analyses.
While it is understood that experimental test groups of these described macaque studies are quite small, statistical analysis of repeated application safety measures was performed using SPSS version 10.0.7 (Chicago, IL). The Mann-Whitney test was used to compare continuous covariates in the treatment arms with their respective placebo. The Wilcoxon signed-rank test was used to examine pre- and postapplication changes within each treatment arm. Friedman's test was used to assess differences over the duration of the experiment within each treatment arm. Differences in the prevalence between the treatment group and placebo group were assessed using Fisher's exact test. Pre- and postapplication changes in prevalence within each treatment arm were assessed using McNemar's test. The placebo groups were combined (n = 12) for analysis.
Pharmacokinetics protocols. (i) Serum samples.
Systemic absorption of UC781 was initially measured in a preliminary study of six animals. A rigorous test for systemic uptake of UC781 was designed, where a larger than anticipated volume of test gel was applied either vaginally or rectally. A baseline blood sample was collected from each animal, followed by a single application of the 0.1% UC781 gel formulation. Three animals received an intravaginal application of 2 ml; three others each received a 3-ml intrarectal application. One animal from each study group provided a single postapplication blood sample at one of three time points: 1 h, 3 h, or 5 h.
A second experiment was conducted to assess systemic absorption of UC781 after topical exposure to the 1.0% formulation. This study followed the same protocol as described above, except that a total of 12 macaques (different animals than those that completed the 0.1% UC781 gel systemic uptake study) were used, with two animals giving blood samples at each postapplication time point. Six animals received a single vaginal application (2 ml) of the 1.0% UC781 gel, and the other six received a single rectal application (3 ml) of the same formulation. Serum separations were frozen (−80°C) and delivered to the pharmacokinetic laboratory (M. A. Parniak) for HPLC analysis.
Additional serum UC781 concentration analyses were conducted after repeated daily product use. These sample collections were incorporated in standardized safety evaluation protocols described above. Six macaques were assessed for systemic uptake of UC781 after multiple vaginal exposures to the 0.1% formulation. Briefly, serum samples from each of six animals were assessed for the presence of UC781 at baseline (day 1, time 0: prior to product exposure), 30 minutes after each daily intravaginal product exposure (one product exposure per day on days 1 through 4), 24 hours after the fourth (final) product exposure (day 5), and on day 8 (96 hours after the final product exposure). Another group of six macaques were assessed in the same fashion after multiple rectal exposures to the same 0.1% UC781 formulation.
(ii) Vaginal lavage samples.
Local cellular and secretion levels of UC781 were also assessed after four daily vaginal exposures (1.5 ml/exposure) to the 0.1% gel formulation by analyzing vaginal lavage specimens from six animals. Two of the six macaques had a previous exposure to 0.1% UC781 gel (a single vaginal exposure 4 months prior to this study). The other four macaques were UC781 naïve. In this repeated vaginal exposure study, baseline vaginal lavage samples were compared to test lavage samples collected 24 hours and 96 hours after the final product application to assess persistent UC781 presence/bioavailability (experiment days 1, 5, and 8).
In a separate study, baseline vaginal lavage samples were compared to test lavage samples collected at 30, 60, 90 min and 2, 4, and 6 h after a single vaginal application (1.5 ml) of the 1.0% UC781 gel to assess the presence of UC781. Each of the 12 macaques used for this study had not been exposed to UC781 gel for a minimum of 6 months prior to this study.
Finally, vaginal lavage samples were assessed for persistence of UC781 after repeated vaginal exposures to the 1.0% UC781 formulation, following the same sample collection schedule described above.
Cervicovaginal lavage samples were collected by instilling 4 ml sterile saline into the vagina and then within 30 seconds of instillation, recovering as much fluid as possible using a transfer pipette. Collected lavage samples ranged in volume from 3.0 to 4.5 ml. Cervicovaginal fluid present at the time of lavage collections and lavage saline composed collection samples. Lavage samples were collected into cryovials and then immediately frozen and stored (−80°C) for subsequent shipment on dry ice in batches to the Reproductive Infectious Disease Laboratory (Pittsburgh, PA) for sample processing prior to HPLC analysis.
Quantitative analysis of UC781. (i) Serum samples.
Thawed serum samples were centrifuged at 1,000 × g for 15 min to remove particulate matter, and then a 2.5-ml aliquot of the clarified sample was subjected to solid-phase extraction (SPE) using Prevail C18 extraction columns (130 mg matrix/4-ml column; Alltech, Deerfield, IL). After application of the serum sample, the column was washed with 4 column volumes of water. Excess water was removed under gentle vacuum, and the column was then eluted with 1 ml acetonitrile. The eluate was stored at 4°C overnight, centrifuged at 13,000 × g to remove any particulate material, and the clarified acetonitrile sample was dried in a SpeedVac. The dried residue was redissolved in 60 μl acetonitrile, and aliquots were assessed for UC781 content by HPLC. Control experiments using authentic UC781 (50 to 5,000 pmol) added to control serum samples showed that the recovery of UC781 following SPE was 48% ± 4% over the range of concentrations tested. SPE, rather than direct solvent extraction of serum (the method used for analysis of lavage samples [see below]) was used, as SPE treatment removed potentially interfering substances found in some serum samples.
(ii) Vaginal lavage samples.
Vaginal lavage samples were sterilized by gamma irradiation to remove potential microbial contamination in a J. L. Shepherd and Associates model 143 Cs gamma irradiator. In order to determine the optimal time of irradiation needed to sterilize the samples, a control lavage sample was divided into several aliquots that were again frozen at −80°C and then irradiated. At various times of irradiation, aliquots were removed and thawed, and residual viable microbes were quantified by inoculating serial 10-fold dilutions of each sample onto 5% sheep blood agar plates. Plates were incubated anaerobically and in air plus 5% CO2. After 24 to 48 h of incubation under aerobic and anaerobic conditions, the plates were examined and the number of viable organisms was determined by plate count. The data obtained were used to calculate the decimal reduction value (D value), a parameter that defines the condition required to inactivate 90% of the viable organisms present. This value was used to calculate the time necessary to irradiate the experimental lavage samples to reduce the number of viable bacteria to less than one.
For HPLC quantification of UC781 levels, aliquots of the irradiated lavage samples were extracted with 3 volumes of acetonitrile with vigorous vortexing. The samples were stored overnight at 4°C, and subjected to centrifugation (13,000 × g for 15 min) to remove any precipitated material. The clarified supernatant was dried in a SpeedVac. The dried residue was dissolved in 60 μl of acetonitrile, and aliquots were assessed for UC781 content by HPLC.
(iii) HPLC analysis of UC781.
UC781 was quantified by HPLC using a Gilson system (model 306 pumps, UV/visible 152 detector, UniPoint version 3.2 system and analysis software). Twenty-microliter samples were injected and resolved using an Alltima HP C18 column (3 μm; 2.1 × 150 mm; Alltech, Deerfield IL) and an isocratic mobile phase of 25% 10 mM potassium phosphate (pH 7.0)-75% acetonitrile with a flow rate of 0.2 ml/mi, with detection at 300 nm. Under these conditions, UC781 eluted at 6.3 min, and the limit of quantification (LOQ) was 5 pmol. However, we chose 10 pmol for our LOQ to improve the accuracy and reliability of the measurements. The sensitivity of the method is a combination of the accuracy and stability of the UV detector and the UniPoint method software package. The extraction/analysis protocols described above enabled reliable detection of levels of UC781 equivalent to 8 pmol/ml in serum samples and 30 pmol/ml in lavage samples.
RESULTS
In vitro toxicity evaluations for UC781 gel product.
In vitro toxicity evaluations conducted with the 0.1% UC781 gel product showed that exposure of excised human ectocervical tissues to the product resulted in no significant toxicity. No gross morphological changes in tissue morphology were observed following exposure as evaluated by comparison of histological evaluations of tissues pre- and postexposure (Fig. 1). Tissue viability as assessed by the MTT assay following both a 2-hour and 24-h exposure period showed no significant viability differences between tissues exposed to medium control versus UC781 product (Fig. 2). Tissue viability was affected after nonoxynol-9 exposure in this model. Additionally, Franz cell studies did not indicate likely systemic uptake of UC781, as none was detected from receptor well solutions after tissue exposure.
FIG. 1.
(a to c) Ectocervical tissue prior to UC781 gel exposure (a) and 2 hours after exposure (2-hrs Post Exposure) to control DMEM (b) and 2 h after exposure to 0.1% UC781 (c). No histologic changes were noted. (d) Pretreatment control for 2 h after exposure to 4.0% nonoxynol-9 (Non-9) (e).
FIG. 2.
Tissue viability as assessed using the MTT assay. N-9, nonoxynol-9.
Macaque vaginal safety studies. (i) Colposcopy.
Colposcopic examinations of macaque cervicovaginal tissues revealed no discernible irritation following repeated applications of either 0.1% or 1.0% UC781 products or placebo. Vasculature was noted intermittently in animals of both test groups and the placebo group. These findings were considered to fall within the range of normal appearing tissues. Epithelial integrity was maintained in all study animals.
(ii) Vaginal pH.
Throughout all of the experiments, preapplication vaginal pH values were similar among the 0.1% UC781, 1.0% UC781, and placebo groups. After product application (30 min after application), vaginal pH increased minimally on days 1 and 2 with 1.0% UC781. After day 2, the vaginal pH decreased. Vaginal pH decreased after each application of 0.1% UC781 (Fig. 3a). On days 5 and 8, when no product was applied, vaginal pH values in both test groups had recovered and were no longer significantly different from those of the placebo group.
FIG. 3.
(a) Vaginal application. Mean vaginal pH in the macaque showed increased variability after product use compared to placebo but consistently remained within the normal range for macaques. The vaginal pH values at 0 and 30 minutes on days 1 to 4 are shown. Vaginal pHs that were significantly different from the values for the placebo group are indicated by asterisks. (b) Rectal application. Mean rectal pH in the macaque showed transient pH shifts with product use. There were no significant differences between the baseline pH and follow-up pH in any group. The rectal pH values at 0 and 30 minutes on days 1 to 4 are shown.
Analysis within each study arm revealed decreases in vaginal pH after 0.1% UC781 product applications, a transient increase in vaginal pH after the first two 1.0% UC781 product applications, and nonsignificant decreases in vaginal pH after each placebo gel application. All measures of vaginal pH remained within the normal range for the pig-tailed macaque; no measures fell below pH 4.5.
(iii) Vaginal microflora.
The production of H2O2 by normal vaginal microflora (lactobacilli and viridans streptococci in pig-tailed macaques) is paramount to a healthy vaginal ecosystem. When comparing vaginal microflora among the three treatment groups (0.1% UC781, 1.0% UC781, and placebo), no significant differences in the prevalence of either H2O2-producing Lactobacillus or viridans streptococci was noted in any study arm. Likewise, comparing all vaginal microorganisms among treatment groups, there were no significant differences.
Macaque rectal safety studies. (i) Rectal lavage examinations.
Rectal lavage samples were characterized as described above and compared to preapplication lavage samples within each treatment arm and to samples collected after exposure to the placebo gel. Within both the 0.1% and 1.0% UC781 treatment groups and the placebo group, there were no significant differences in either the number of epithelial sheets or the presence of blood/stroma after product application on each day, nor was there a difference at follow-up compared to baseline. Likewise, there were no significant differences noted when comparing either test arm to the placebo arm of each study.
(ii) Rectal pH.
There were no significant differences between the two UC781 test arms and the placebo regarding pre- and postapplication pH (Fig. 3b). Within each treatment group, there were significant differences between pre- and postapplication rectal pH. Despite the acidic pH of the product and placebo gels, rectal pH increased after product application on all four days in the placebo group and in both UC781 test groups. Though there were transient pH shifts with product application, there were no significant differences between the baseline pH and follow-up pH in any group.
(iii) Rectal microbiology.
Semiquantitative analysis of the prevalence of rectal microflora showed no significant differences in the prevalence of microflora when assessed daily or pre- and postapplication of 0.1% or 1.0% UC781 or placebo. In addition, there were no significant differences in the prevalence of microflora at follow-up compared to the baseline value in either group. Rectal use of 0.1% or 1.0% UC781 did not significantly alter the presence of H2O2-producing Lactobacillus or viridans streptococci.
Detection of cytokines.
Cervical and rectal samples were evaluated against a panel of seven proinflammatory cytokines using the Luminex system. The cytokines assessed in this study were IL-1β, IL-6, GM-CSF, TNF-α, IFN-γ, MIP-1α, and IL-12p40. No significant differences or cytokine patterns were noted after daily vaginal exposure to either 0.1% or 1.0% UC781. The same was true for repeated rectal applications of the 0.1% UC781 product. However, rectal exposure to 1.0% UC781 resulted in significantly increased levels of IL-6, IFN-γ, and MIP-1α over the course of the experiment. When comparing the final levels (day 5) to baseline levels (day 0) of all seven cytokines assessed, a statistical increase was noted for each cytokine after repeated rectal applications of the 1.0% UC781 product (Table 4).
TABLE 4.
Cytokines in the rectums of six pig-tailed macaques after exposure to 1.0% UC781
| Cytokine | Rectal cytokine level (pg/ml)
|
P valuea | |||
|---|---|---|---|---|---|
| Day 1
|
Day 5
|
||||
| Median | Range | Median | Range | ||
| IL-1β | 52 | 7-113 | 199 | 59-461 | 0.028 |
| IL-6 | 264 | 7-785 | 1,448 | 524-2,629 | 0.028 |
| GM-CSF | 196 | 7-866 | 2,001 | 7-3,046 | 0.043 |
| TNF-α | 73 | 7-204 | 998 | 67-2,217 | 0.043 |
| IFN-δ | 12 | 12-501 | 3,789 | 12-9,218 | 0.043 |
| MIP-1α | 1,070 | 180-1,560 | 3,565 | 1,656-5,549 | 0.028 |
| IL-12p40 | 12 | 12-171 | 661 | 12-1,253 | 0.046 |
The P values for the day 5 median value compared to the day 1 median value by the Wilcoxon signed-rank test are shown.
Detection of UC781. (i) Systemic uptake of UC781.
No UC781 was detected in any of the serum samples analyzed, including those obtained from animals receiving repeated exposure to 1.0% UC781 formulations. An average 10-kg pig-tailed macaque has a blood volume of approximately 800 ml. Complete absorption of a single maximum dose of formulated UC781 (3 ml of 1.0% UC781) into the systemic circulation would theoretically provide a serum level of approximately 110 μM. The HPLC analytical method described in this paper could reliably detect 10 pmol of UC781 (LOQ was 5 pmol [see Materials and Methods]), equivalent to 8 pmol per ml of serum. With this sensitivity, as little as 0.02% absorption of UC781 following application in the monkeys would be detectable. It thus appears that no significant systemic absorption of UC781 was detected from vaginal or rectal topical administration of formulated UC781, even after multiple administrations.
It should be noted that only serum was analyzed. We were unable to quantify UC781 in the separated red blood cells due to interfering components that coeluted with UC781 in our HPLC analysis method.
(ii) Cervicovaginal concentrations of UC781.
Eighteen vaginal lavage samples obtained from the six monkeys included in the 0.1% gel safety study were analyzed for UC781 content and antiviral activity. These samples were obtained prior to exposure and at 24 h and 96 h after the final 0.1% UC781 application. Only five samples (four samples at 24 h and one sample at 96 h) showed any detectable UC781 at levels less than 1% of the applied dose. The extraction and analytical methods used could reliably detect UC781 in lavage samples equivalent to 30 pmol of UC781 per ml of lavage fluid.
Parallel samples were collected and assessed from the six animals enrolled in the 1.0% gel safety study. Of these 18 samples, UC781 was again detected in five samples, representing four of six animals. UC781 was detected at levels less than 1% of the applied dose in this experiment as well. The two highest concentrations were calculated to be 10.2 nmol and 24.9 nmol per 4-ml sample. However, it is important to note that these samples were clarified by centrifugation prior to extraction. Thus, it is possible that UC781 associated with particulate matter, such as cells, may have been lost.
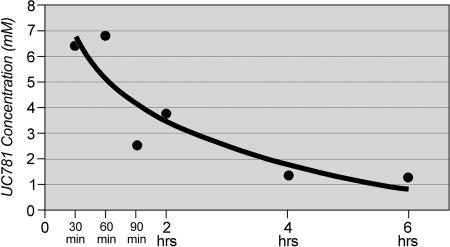
In order to estimate the concentration of UC781 present in the cervicovaginal lavage fluid shortly after product exposure, each of 12 animals was exposed to a single 1.5-ml intravaginal application of the 1% UC781 gel. Vaginal lavage samples were obtained on day 0 (baseline) and then at 0.5, 1, 1.5, 2, 4, or 6 h after product application. Two animals provided a lavage sample for each time point. The lavage samples were evaluated for UC781 presence by HPLC. These analyses were conducted on nonclarified samples, which would include particulate matter. As shown in Fig. 4, the concentration of UC781 in the cervicovaginal lavage was highest at the earliest sampling interval and dropped rapidly. However, it is important to note that the detectable levels of UC781 at 6 h after application were still in excess of 1 mM, which is 10,000-fold greater than the 50% inhibitory concentration (3, 6).
FIG. 4.
Detection of UC781 in macaque vaginal lavage samples. The concentration of 1.0% UC781 in cervicovaginal lavage samples was highest at the 1-hour (60-min) sampling interval, although at 6 h postexposure, detectable levels were still at a high concentration of >1 mM or 10,000 times greater than the 50% inhibitory concentration.
These data suggest that UC781 persists in the vagina at concentrations greatly in excess of those needed for activity against HIV for at least 6 h. However, by 24 h after application, the level of UC781 detectable in the cervicovaginal lavage samples had fallen to barely detectable levels.
DISCUSSION
Women make up approximately half of all people living with HIV worldwide. Most cases of new HIV infection in women are the result of heterosexual intercourse. Correct condom usage and sexual abstinence provide two methods of HIV/sexually transmitted infection prevention. In many cultures, women are unable to negotiate condom use during sexual activity. The development of topical microbicides will provide a new prevention strategy that will be under the control of women and therefore may be more likely to be used. These topical products must be safe for repeated use.
As preventive products, topical microbicides must demonstrate safety to a stricter standard than that of therapeutic products, necessitating extensive and careful preliminary evaluation in nonhuman models and in vitro studies. Results from both in vivo and in vitro testing of nonoxynol-9 and other candidate microbicides suggest that correlates with the potential to predict epithelial damage and/or high-risk proinflammatory changes in human vaginal and rectal mucosa exist. Successful product development will depend in part on studies designed to assess systemic and cellular absorption of active compounds, tissue responses to product exposure, and adverse effects to vaginal or rectal microflora ecosystems. The pig-tailed macaque model has provided the opportunity to explore the possibility of mucosal irritation and systemic absorption prior to the design of a phase I clinical trial that will also utilize colposcopy, lavage, and pharmacokinetic studies to evaluate safety of the 0.1% concentration of formulated UC781 gel. In this study, the nonhuman primate model was used to evaluate the safety profiles of two water-based gel formulations of a nonnucleoside reverse transcriptase inhibitor, UC781, after repeated applications in both the vagina and rectum.
Topical microbicides that can provide a chemical barrier to infection locally without provoking systemic exposure to the drug may allow for the broad use of antiretroviral-based microbicides. In this study, no detectable UC781 was found in serum samples from animals following vaginal or rectal administration, even in samples obtained from animals receiving repeated administration of the 1.0% formulation of UC781. This was analogous to results found in in vitro exposure studies conducted using freshly excised human tissues in which no detectable amount of UC781 was found in the receptor compartment following exposure to the UC781 gel product. The sensitivity of our analytical method is such that as little as 0.02% of the administered dose of the 1.0% UC781 gel (assuming complete absorption) would be detectable. It thus appears that no significant systemic absorption of UC781 occurs from vaginal or rectal administration of formulated UC781, even after multiple administrations. This is an important observation that further highlights the potential utility of UC781 for use as a topical anti-HIV microbicide. Even though no acute toxicity has been associated with UC781 (1), significant systemic absorption of the drug following topical administration could result in undue exposure of healthy individuals to the active agent or its metabolites, especially if the drug accumulated with repeated exposure. Importantly, our data show that repeated vaginal or rectal administration of UC781 gel does not lead to any systemic accumulation of the drug.
UC781 levels were analyzed only in serum. We were unable to quantify UC781 in the separated blood cells due to interfering components that coeluted with UC781 in our HPLC analysis method. It is recognized that this is a limitation of the present study. UC781 may be localizing in some as yet unidentified cellular compartment. It is possible that any UC781 that may have been absorbed into the systemic circulation may have localized in the blood cells and thus would not have been detected in our analytical analyses. We are currently developing an analytical method appropriate for detection of UC781 in blood cells in order to explore this possibility. Blood partition studies may be needed.
The potential cellular localization of UC781 is also of interest in the context of our vaginal lavage data. UC781 has very poor aqueous solubility, and the aqueous gel formulations contain UC781 in the form of a particulate dispersion. In this study, we detected the presence of high concentrations of UC781 in cervicovaginal lavage samples up to 6 h after 1.0% gel application, suggesting a window of protection from acquisition of HIV when used as a topical microbicide. It is important to note that these studies used a lavage sample that had not had substrate products removed prior to extraction and analysis. In contrast, the lavage samples assessed at 24 h utilized samples which had been clarified prior to extraction. In the planned human studies of UC781, nonclarified vaginal lavage samples will be used and cellular components will be analyzed for UC781 separately. Sampling will occur over time points up to 24 h. Thus, these animal model studies were invaluable in optimizing the analytical protocols to be used in the human clinical trials.
Occasionally, petechial hemorrhage and vascular patterns of either intact or diffuse vessels were noted during colposcopic examination in each arm of the study (0.1% or 1.0% UC781 or placebo). By day 8, the follow-up colposcopic observations were normal in all animals after vaginal applications of either 0.1% or 1.0% UC781 or placebo gel. The vaginal pH underwent a transient decrease 30 minutes after gel application with 0.1% UC781. Despite transient shifts in vaginal pH with product application, there was no significant difference in the follow-up (day 8) vaginal pH compared to the baseline value.
No trends or patterns were detectable in regards to cervical cytokine expression after vaginal exposure to either 0.1% or 1.0% UC781. A significant increase in cytokine expression was detected within the rectally applied 1.0% UC781 treatment arm. The clinical significance of this finding is unknown. Cytokine data are difficult to interpret, particularly with small numbers of animals and a relatively large range for cytokine levels detected. These data indicate that repeated rectal applications of the 1.0% UC781 formulation may affect local cytokine expression, which may in turn impact the immunologic response to repeat exposures to the higher concentration of UC781.
We have demonstrated that repeated daily use of two gel formulations of UC781 did not result in detectable systemic absorption in pig-tailed macaques and that the UC781 product was safe to the vaginal and rectal microenvironments. The data indicate a somewhat reduced safety profile for the 1.0% UC781 formulation when applied rectally.
Optimized preclinical testing increases the likelihood that only products with data indicating superior safety profiles will progress to clinical (human) trials and that these products will be administered in human trials at concentrations, doses, and frequencies unlikely to result in potentially harmful changes. The sensitive mucosal surfaces of the human vagina and rectum present a significant challenge to those who design clinical trials of microbicides. Avoiding the paradoxical effect of actually increasing risk for HIV acquisition at these sites remains a primary goal for microbicide development. Safety data gained from the evaluations described here will help to guide the design of safe and efficient human trials further exploring the feasibility of UC781 as a topical microbicide.
Acknowledgments
This work was supported by Public Health Service grants AI-051661 and WaNPRC RR00166.
The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the Division of AIDS.
We thank Peter Cummings, Ray Angeles, Karin Butler, Kathleen Paul, Lisa Cosentino, Lorna Rabe, Eva Nagy, Lena Miller, and Lisa Noguchi for their excellent assistance in performing these studies.
Footnotes
Published ahead of print on 12 March 2007.
REFERENCES
- 1.Balzarini, J., L. Naesens, E. Verbeken, M. Laga, L. V. Damme, M. Parniak, L. V. Mellaert, J. Anne, and E. DeClercq. 1998. Preclinical studies on thiocarboxanilide UC-781 as a virucidal agent. AIDS 121129-1138. [DOI] [PubMed] [Google Scholar]
- 2.Baron, E. J., C. A. Strong, M. McTeague, M.-L. Vaisanen, and S. M. Finegold. 1995. Survival of anaerobes in original specimens transported by overnight mail services. Clin. Infect. Dis. 20(Suppl. 2)S174-S177. [DOI] [PubMed] [Google Scholar]
- 3.Borkow, G., D. Arion, M. A. Wainberg, and M. A. Parniak. 1999. The thiocarboxanilide nonnucleoside inhibitor UC781 restores antiviral activity of 3′-azido-3′-deoxythymidine (AZT) against AZT-resistant human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 43259-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenhead, P., P. Hayes, P. S. Watts, K. G. Liang, G. E. Griffin, and R. J. Shattock. 2000. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J. Virol. 745577-5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCormack, S., R. Hayes, C. J. Lacey, and A. M. Johnson. 2001. Microbicides in HIV prevention. BMJ 322410-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motakis, D., and M. A. Parniak. 2002. A tight-binding mode of inhibition is essential for anti-human immunodeficiency virus type 1 virucidal activity of nonnucleoside reverse transcriptase inhibitors. Antimicrob. Agents Chemother. 461851-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patton, D. L., Y. T. Cosgrove Sweeney, L. K. Rabe, and S. L. Hillier. 1996. The vaginal microflora of pig-tailed macaques and the effects of chlorhexidine and benzalkonium on this ecosystem. Sex. Transm. Dis. 23489-493. [DOI] [PubMed] [Google Scholar]
- 8.Patton, D. L., G. M. Ganzle, Y. T. Cosgrove Sweeney, L. K. Rabe, A. M. Clarke, and S. L. Hillier. 1996. Effects of nonoxynol-9 on vaginal microflora and chlamydial infection in a monkey model. Sex. Transm. Dis. 23461-464. [DOI] [PubMed] [Google Scholar]
- 9.Patton, D. L., Y. T. Cosgrove Sweeney, L. K. Rabe, and S. L. Hillier. 2001. The pig-tailed macaque rectal model: microflora and chlamydial infection. Sex. Transm. Dis. 28363-366. [DOI] [PubMed] [Google Scholar]
- 10.Pauwels, R., and E. DeClercq. 1996. Development of vaginal microbicides for the prevention of heterosexual transmission of HIV. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 11211-221. [DOI] [PubMed] [Google Scholar]
- 11.Rosenthal, S. L., S. S. Cohen, and L. R. Stanberry. 1998. Topical microbicides. Current status and research considerations for adolescent girls. Sex. Transm. Dis. 25368-377. [DOI] [PubMed] [Google Scholar]
- 12.Tien, D., R. L. Schnaare, F. Kang, G. Cohl, T. J. McCormick, T. R. Moench, G. Doncel, K. Watson, R. W. Buckheit, M. G. Lewis, J. Schwartz, K. Douville, and J. W. Romano. 2005. In vitro and in vivo characterization of a potential universal placebo designed for use in vaginal microbicide clinical trials. AIDS Res. Hum Retrovir. 21845-853. [DOI] [PubMed] [Google Scholar]
- 13.United Nations. May 2006. Report on the Global AIDS Epidemic 2006. UNAIDS, the Joint United Nations Programme on HIV/AIDS. http://www.unaids.org/en/HIV_data/2006GlobalReport/default.asp.
- 14.World Health Organization/Contraceptive Research and Development Program. 2000. Manual for the standardization of colposcopy for the evaluation of vaginal products: update 2000. World Health Organization (WHO)/Contraceptive Research and Development Program (CONRAD), Geneva, Switzerland.