Abstract
The efficacy of a novel synthetic antimicrobial peptide (WLBU2) was evaluated against three oral microorganisms (grown planktonically): Streptococcus gordonii, Fusobacterium nucleatum, and Porphyromonas gingivalis. WLBU2 killed all three species, with F. nucleatum being the most susceptible. WLBU2 also reduced the bacterial burden of S. gordonii and F. nucleatum biofilms.
With increasing evidence for links between infectious burden and systemic diseases such as coronary artery disease (6, 15, 18), the oral cavity has been shown to be an important contributor to this systemic microbial bioburden (4, 5, 7, 8, 12). The microcosm found in the oral cavity comprises more than 700 species of microorganisms (1) arranged as both single-species and multispecies biofilms. The tooth-associated biofilm (dental plaque) is the primary etiologic factor associated with dental caries and periodontal disease. Dental plaque biofilm formation is a sequential process initiated by adherence of gram-positive early colonizers to the tooth surface, followed by a shift to a predominance of more pathogenic gram-negative anaerobic species (late colonizers) as the biofilm matures. Importantly, Fusobacterium nucleatum serves as an important coaggregation bridge between early and late colonizers (9).
The use of topical chemotherapeutic agents that inhibit plaque formation is an important adjunct in the prevention of oral diseases such as dental caries and periodontal disease. Therefore, the development of novel antimicrobial compounds that are effective against oral microorganisms is an important aspect of both basic science and commercial oral health research. The lentivirus lytic peptides are one example of this class of novel compounds (11, 13, 17). A de novo-engineered derivative of lentivirus lytic peptide, WLBU2, is a 24-amino-acid peptide that is effective against a broad spectrum of gram-positive and gram-negative isolates (2). WLBU2 eliminates Pseudomonas aeruginosa in coculture with human skin fibroblasts without detectable adverse effects to the host cells, is active against Staphylococcus aureus and P. aeruginosa at physiologic NaCl concentrations, and kills P. aeruginosa in human serum. WLBU2 also eradicates bacteria from ex vivo samples of whole blood, suggesting it may be suitable for use as a topical or systemic chemotherapeutic agent in the prevention and treatment of medically important infections (3). Based on the need for the development of chemotherapeutics against oral pathogens and the demonstrated antimicrobial activity of WLBU2, we hypothesized that oral bacteria would be sensitive to this peptide.
Using a standard broth dilution assay (17), the potency of WLBU2 was evaluated against planktonic cultures of Streptococcus gordonii, an early colonizer of dental plaque; F. nucleatum, the important coaggregation “bridging” colonizer; and Porphyromonas gingivalis, a late colonizer associated with multiple forms of periodontal disease (9, 14). P. aeruginosa was used as a positive bacterial control. Bacterial cultures were propagated in liquid media under appropriate growth conditions (Table 1) to mid-log phase, washed with phosphate buffer (PB), and suspended in PB such that upon dilution, 105 to 106 CFU/ml was tested in the bacterial killing assay. The bacteria were incubated with twofold dilutions of the peptide (100 μM to 0.39 μM) in 96-well plates in PB at 37°C under appropriate growth conditions (Table 1). Although WLBU2 reduces viable counts of P. aeruginosa in seconds in PB (17), a minimum of 20 min is required for killing in serum (3). Since the subgingival environment contains a serum transudate, a standard 30-min peptide exposure was selected for this pilot project. Quantification of bacterial survival post-peptide exposure was evaluated using serial 10-fold dilutions of control and test wells. Bacterial colonies were counted at 24 h for S. gordonii and P. aeruginosa and at 48 h for F. nucleatum and P. gingivalis and were compared to counts of non-peptide-treated controls to determine the amount of WLBU2 that reduced the bacterial counts by 3 orders of magnitude. This level of killing defined the minimum bactericidal concentration (MBC), assessed in micromolar concentrations of peptide. The results were expressed as averages of MBCs obtained from three independent experiments.
TABLE 1.
Bacterial strains and growth conditions
| Bacterial strain | Medium | Growth conditions |
|---|---|---|
| Pseudomonas aeruginosa ATCC 47085 | Todd-Hewitt broth | Aerobic |
| Streptococcus gordonii ATCC 49818/DL1 | Todd-Hewitt broth | Aerobic |
| Fusobacterium nucleatum ATCC 49256, ATCC 25586 | Tryptic soy broth + 0.6% yeast extract | Anaerobic (5% CO2, 10% H2, 85% N2) |
| Porphyromonas gingivalis ATCC BAA0308/W83 | Mycoplasma broth + hemin (5 μg/ml) + menadione (1 μg/ml) | Anaerobic (5% CO2, 10% H2, 85% N2) |
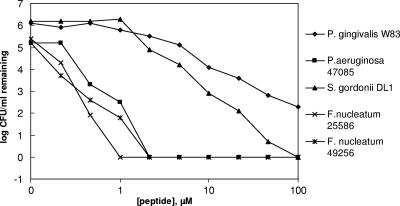
All three species of planktonic oral bacteria were killed by WLBU2 (Fig. 1). The MBC was between 1 and 2 μM for P. aeruginosa and F. nucleatum, between 12.5 and 25 μM for S. gordonii, and between 50 and 100 μM for P. gingivalis. Viable counts also were assessed for S. gordonii and F. nucleatum following exposure to comparable micromolar concentrations of amoxicillin or metronidazole, antibiotics frequently used in treating periodontal disease (16). There were no significant decreases in the viable cell counts of either bacterium following these treatments (Tables 2 and 3).
FIG. 1.
Dose-dependent killing of oral bacteria and P. aeruginosa (positive control) by WLBU2. Bacterial cultures (0.5 × 106 to 1 × 106 CFU/ml) were treated with twofold dilutions of the peptide in phosphate buffer; the log of the number of CFU/ml remaining upon treatment is plotted as a function of peptide concentration.
TABLE 2.
Viable counts of untreated (PBS) versus treated S. gordonii cells
| Method of cell growth | Treatmenta | Total viable count | Pb |
|---|---|---|---|
| Planktonic | PBS | (1.29 ± 0.25) × 104 | |
| WLBU2 | (0.00 ± 0.00) × 104 | <0.001 | |
| Amoxicillin | (1.35 ± 0.41) × 104 | NS | |
| Metronidazole | (1.22 ± 0.11) × 104 | NS | |
| Biofilm | PBS | (2.01 ± 0.08) × 107 | |
| WLBU2 | (0.21 ± 0.02) × 107 | <0.0001 | |
| Amoxicillin | (1.63 ± 0.11) × 107 | NS | |
| Metronidazole | (1.8 ± 0.14) × 107 | NS |
All antimicrobials were used at a concentration of 25 μM.
NS, not significant; P > 0.05 by a t test that was adjusted when necessary for unequal variance.
TABLE 3.
Viable counts of untreated (PBS) versus treated F. nucleatum cells
| Method of cell growth | Treatmenta | Total viable count | Pb |
|---|---|---|---|
| Planktonic | PBS | (1.04 ± 0.08) × 107 | |
| WLBU2 | (0.00 ± 0.00) × 107 | <0.0001 | |
| Amoxicillin | (1.00 ± 0.15) × 107 | NS | |
| Metronidazole | (1.02 ± 0.07) × 107 | NS | |
| Biofilm | PBS | (1.75 ± 0.13) × 104 | |
| WLBU2 | (0.20 ± 0.27) × 104 | NS | |
| Amoxicillin | (2.75 ± 0.07) × 104 | NS | |
| Metronidazole | (2.86 ± 0.57) × 104 | NS |
All antimicrobials were used at a concentration of 2 μM.
NS, not significant; P > 0.05 by a t test that was adjusted when necessary for unequal variance.
The high concentration required for killing P. gingivalis strain W83 may be due to the encapsulated nature of this strain or the presence of the numerous proteolytic enzymes released by this bacterium. This will be assessed in future studies. However, the MBCs for two different strains of F. nucleatum were between 1 and 2 μM, consistent with results evaluating the specificity of WLBU2 against numerous P. aeruginosa strains (2). Interestingly, both strains of F. nucleatum were far more susceptible to killing by this novel peptide than either S. gordonii or P. gingivalis when grown as planktonic cells. Because F. nucleatum is recognized as an important “bridging” bacterium between the less pathogenic early colonizers and the more pathogenic late colonizers of the plaque biofilm, antimicrobial agents disrupting this bridge before the development of a mature biofilm could interfere with plaque maturation.
The effectiveness of WLBU2 was assessed against single-species oral biofilms developed on individual rigid gas-permeable lens (RGPL) material (unpublished data). Replicate RGPLs were coated with fetal bovine serum, washed with sterile phosphate-buffered saline (PBS), and incubated with overnight cultures of S. gordonii or F. nucleatum for 24 h under appropriate conditions (Table 1). After 24 h, the culture medium was carefully removed without displacing the developing biofilm and was replaced with an equal volume of fresh medium daily for 3 days (S. gordonii) or 4 days (F. nucleatum). The RGPL material was then washed with sterile PBS to remove nonadherent cells and treated with either WLBU2, amoxicillin, or metronidazole (25 μM for S. gordonii; 2 μM for F. nucleatum) for 30 min under appropriate conditions (Table 1). Additional replicates treated with PBS served as controls. Treated and control biofilms were released from the RGPL into PBS with a sterile cell scraper and disrupted by vortexing. Serial dilutions were spread onto blood agar plates in triplicate for determination of CFU.
The results of three replicate experiments demonstrated that treatment of S. gordonii biofilms with WLBU2 resulted in a statistically significant decrease in mean cell counts compared to those for PBS-treated controls. Mean cell counts did not decrease significantly following treatment with either amoxicillin or metronidazole (Table 2). Similarly, treatment of F. nucleatum biofilms with these antibiotics did not decrease viable cell counts, while treatment with WLBU2 did lead to decreased counts. However, the results with WLBU2 were not statistically significant (Table 3). Taken together, these results indicate a trend toward the ability of WLBU2 to impact viable counts of both S. gordonii and F. nucleatum cells grown as biofilms.
These studies demonstrate activity of WLBU2 against early and bridging bacteria grown as planktonic cells and known to be part of oral biofilms. Our results also demonstrate that WLBU2 can potentially contribute to a lessening of the bioburden of a mature single-species biofilm. We hypothesize that the somewhat limited effect of the peptide on biofilm cells was due to inefficient penetration beyond the surface layer of bacteria in the mature biofilm. Consistent with our findings and this hypothesis, it has been demonstrated previously that mechanical disruption or adjunctive exposure to a surface-active agent can enhance peptide killing of biofilm cells (10). Therefore, future studies will evaluate the efficacy of WLBU2 in combined therapeutic strategies with a focus on the peptide's impact on F. nucleatum as a coaggregating bridge microorganism in multispecies biofilms.
Acknowledgments
This work was supported by a University of Kentucky research support grant and by Kentucky Science and Technology Corporation/Kentucky Science and Engineering Foundation grant KSEF-47-RDE-004.
We thank Jeff Mattingly for valuable technical assistance with this project, Malini Bharadwaj for assistance with manuscript preparation, and Kazi Islam at the University of Pittsburgh Molecular Medicine Institute Peptide Synthesis Facility for assistance in peptide preparation.
Footnotes
Published ahead of print on 26 February 2007.
REFERENCES
- 1.Aas, J. A., B. J. Paster, L. N. Stokes, I. Olsen, and F. E. Dewhirst. 2005. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 435721-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deslouches, B., S. M. Phadke, V. Lazarevic, M. Cascio, K. Islam, R. C. Montelaro, and T. A. Mietzner. 2005. De novo generation of cationic antimicrobial peptides: influence of length and tryptophan substitution on antimicrobial activity. Antimicrob. Agents Chemother. 49316-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deslouches, B., K. Islam, J. K. Craigo, S. M. Phadke, R. C. Montelaro, and T. A. Mietzner. 2005. Activity of the de novo engineered antimicrobial peptide WLBU2 against Pseudomonas aeruginosa in human serum and whole blood: implications for systemic applications. Antimicrob. Agents Chemother. 493208-3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desvarieux, M., R. T. Demmer, T. Rundek, B. Boden-Albala, D. R. Jacobs, Jr., R. L. Sacco, and P. N. Papapanou. 2005. Peridontal microbiota and carotid intima-media thickness: the Oral Infections and Vascular Disease Epidemiology Study (INVEST). Circulation 111576-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engebretson, S. P., I. B. Lamster, M. S. Elkind, T. Rundek, N. J. Serman, R. T. Demmer, R. L. Sacco, P. N. Papapanou, and M. Desvarieux. 2005. Radiographic measures of chronic periodontitis and carotid artery plaque. Stroke 36561-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espinola-Klein, C., H. J. Rupprecht, S. Blankenberg, C. Bickel, H. Kopp, G. Rippin, A. Victor, G. Hafner, W. Schlumberger, and J. Meyer. 2002. Impact of infectious burden on extent and long-term prognosis of atherosclerosis. Circulation 10515-21. [DOI] [PubMed] [Google Scholar]
- 7.Gatz, M., J. A. Mortimer, L. Fratiglioni, B. Johansson, S. Berg, C. A. Reynolds, and N. L. Pedersen. 2006. Potentially modifiable risk factors for dementia: evidence from identical twins. Alzheimer's Dementia 2110-117. [DOI] [PubMed] [Google Scholar]
- 8.Goepfert, A. R., M. K. Jeffcoat, W. W. Andrews, O. Faye-Petersen, S. P. Cliver, R. L. Goldenberg, and J. C. Hauth. 2004. Periodontal disease and upper genital tract inflammation in early spontaneous preterm birth. Obstet. Gynecol. 104777-783. [DOI] [PubMed] [Google Scholar]
- 9.Kolenbrander, P. E., R. N. Andersen, D. S. Blehert, P. G. Egland, J. S. Foster, and R. J. Palmer, Jr. 2002. Communication among oral bacteria. Microbiol. Mol. Biol. Rev. 66486-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung, K.-P., T. D. Crowe, J. J. Abercrombie, C. M. Molina, C. J. Bradshaw, C. L. Jensen, Q. Luo, and G. A. Thompson. 2005. Control of oral biofilm formation by an antimicrobial decapeptide. J. Dent. Res. 841172-1177. [DOI] [PubMed] [Google Scholar]
- 11.Miller, M. A., M. W. Cloyd, J. Liebmann, C. R. Rinaldo, Jr., K. R. Islam, S. Z. Wang, T. A. Mietzner, and R. C. Montelaro. 1993. Alterations in cell membrane permeability by the lentivirus lytic peptide (LLP-1) of HIV-1 transmembrane protein. Virology 19689-100. [DOI] [PubMed] [Google Scholar]
- 12.Offenbacher, S., K. A. Boggess, A. P. Murtha, H. L. Jared, S. L. Lieff, R. G. McKaig, S. M. Mauriello, K. L. Moss, and J. D. Beck. 2006. Progressive periodontal disease and risk for very preterm delivery. Obstet. Gynecol. 10729-36. [DOI] [PubMed] [Google Scholar]
- 13.Phadke, S. M., V. Lazarevuc, C. C. Bahr, K. Islam, D. B. Stolz, W. Watkins, S. B. Tencza, H. J. Vogel, R. C. Montelaro, and T. A. Mietzner. 2002. Lentivirus lytic peptide 1 perturbs both the outer and inner membranes of Serratia marcescens. Antimicrob. Agents Chemother. 462041-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosan, B., and R. J. Lamont. 2000. Dental plaque formation. Microbes Infect. 21599-1607. [DOI] [PubMed] [Google Scholar]
- 15.Rupprecht, H. J., S. Blankenberg, C. Bickel, G. Rippin, G. Hafner, W. Prellwitz, W. Schlumberger, and J. Meyer. 2001. Impact of viral and bacterial infectious burden on long-term prognosis in patients with coronary artery disease. Circulation 10425-31. [DOI] [PubMed] [Google Scholar]
- 16.Ryan, M. E. 2005. Nonsurgical approaches for the treatment of periodontal diseases. Dent. Clin. N. Am. 49611-636. [DOI] [PubMed] [Google Scholar]
- 17.Tencza, S. B., J. P. Douglass, D. J. Creighton, R. C. Montelaro, and T. A. Mietzner. 1997. Novel antimicrobial peptides derived from human immunodeficiency virus type 1 and other lentivirus transmembrane proteins. Antimicrob. Agents Chemother. 412394-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu, J., A. A. Quyyumi, J. E. Norman, G. Csako, M. A. Waclawiw, G. M. Shearer, and S. E. Epstein. 2000. Effects of total pathogen burden on coronary artery disease risk and C-reactive protein levels. Am. J. Cardiol. 85140-146. [DOI] [PubMed] [Google Scholar]