Abstract
Dalbavancin is a lipoglycopeptide antibiotic with broad-spectrum activity against gram-positive cocci and a markedly prolonged serum elimination half-life. We used the neutropenic murine thigh and lung infection models to characterize the pharmacodynamics of dalbavancin. Single-dose pharmacokinetic studies demonstrated linear kinetics and a prolonged elimination half-life which ranged from 7.6 to 13.1 h over the dose range of 2.5 to 80 mg/kg of body weight. The level of protein binding in mouse serum was 98.4%. The time course of in vivo activity of dalbavancin over the same dose range was examined in neutropenic ICR Swiss mice infected with a strain of either Streptococcus pneumoniae or Staphylococcus aureus by using the thigh infection model. The burden of organisms for S. pneumoniae was markedly reduced over the initial 24 h of study, and organism regrowth was suppressed in a dose-dependent fashion for up to the entire 96 h of study following dalbavancin doses of 2.5 mg/kg or greater. Dalbavancin doses of 20 mg/kg or greater resulted in less killing of S. aureus but were still followed by a prolonged suppression of regrowth. Multiple-dosing-regimen studies with the same organisms were used to determined which of the pharmacodynamic indices (maximum concentration in serum [Cmax]/MIC, area under the concentration-versus-time curve [AUC]/MIC, or the duration of time that levels in serum exceed the MIC) best correlated with treatment efficacy. These studies used a dose range of 3.8 to 480 mg/kg/6 days fractionated into 2, 4, 6, or 12 doses over the 144-h dosing period. Nonlinear regression analysis was used to examine the data fit with each pharmacodynamic index. Dalbavancin administration by the use of large, widely spaced doses was the most efficacious for both organisms. Both the 24-h AUC/MIC and the Cmax/MIC parameters correlated well with the in vivo efficacy of treatment against S. pneumoniae and S. aureus (for 24-h AUC/MIC, R2 = 78 and 77%, respectively; for Cmax/MIC, R2 = 90 and 57%, respectively). The free-drug 24-h AUC/MICs required for a bacteriostatic effect were 17 ± 7 for five S. pneumoniae isolates. A similar treatment endpoint for the treatment against five strains of S. aureus required a larger dalbavancin exposure, with a mean free-drug 24-h AUC/MIC of 265 ± 143. Beta-lactam resistance did not affect the pharmacodynamic target. The dose-response curves were relatively steep for both species; thus, the pharmacodynamic target needed to achieve organism reductions of 1 or 2 log10 in the mice were not appreciably larger (1.3- to 1.6-fold). Treatment was similarly efficacious in neutropenic mice and in the lung infection model. The dose-dependent efficacy and prolonged elimination half-life of dalbavancin support the widely spaced regimens used in clinical trials. The free-drug 24-h AUC/MIC targets identified in these studies should be helpful for discerning rational susceptibility breakpoints. The current MIC90 for the target gram-positive organisms would fall within this value.
The increasing rates of resistance among both hospital- and community-acquired bacterial pathogens such as Staphylococcus aureus, coagulase-negative staphylococci, and enterococcci have prompted attempts to discover new antimicrobials with activities against multidrug-resistant gram-positive pathogens (4, 13, 14, 21, 26, 30). Dalbavancin is a new lipoglycopeptide antibiotic with broad-spectrum activity against multidrug-resistant gram-positive organisms (12, 13, 14, 26, 30, 32). In addition to enhanced antimicrobial potency, the compound possesses a unique pharmacokinetic (PK) profile that includes an extremely prolonged elimination half-life of more than 1 week (2). Clinical development of the compound has thus far been successful for the treatment of skin and soft tissue and of catheter-related bloodstream infections (15, 27, 31). Once-weekly administration of the dosing regimens used in these trials has been shown to produce free-drug trough concentrations exceeding the MIC90s of gram-positive pathogens from large surveillance databases.
The current studies were designed to characterize the in vivo pharmacodynamic (PD) characteristics of this new compound. We first examined the impact of the dalbavancin concentration on in vivo antimicrobial killing activity over time. Studies were then performed to determine (i) which PK parameter (the peak concentration in serum [Cmax/MIC], the area under the concentration-versus-time curve [AUC/MIC], or the duration of time that levels in serum exceed the MIC [T > MIC]) best predicts the efficacy of dalbavancin and (ii) whether the magnitude of the PK/PD parameter required for efficacy is similar among common gram-positive bacteria, including penicillin-resistant pneumococci and methicillin-resistant S. aureus (MRSA). Lastly, we determined the effect of the infection site on the activity of dalbavancin against both Streptococcus pneumoniae and S. aureus in both the thigh and pneumonia infection models. The results from these studies provide a PD rationale in support of the current clinical dosing regimens. Furthermore, the data provide a starting point for the development of susceptibility breakpoints for this new compound.
MATERIALS AND METHODS
Bacteria, media, and antibiotic.
Five strains of Streptococcus pneumoniae with various levels of resistance to penicillin (one penicillin-susceptible S. pneumoniae strain, one penicillin-intermediate S. pneumoniae strain, and three penicillin-resistant S. pneumoniae strains) were used. Six strains of Staphylococcus aureus (three methicillin-susceptible S. aureus strains and three MRSA strains) were also used for these experiments. All organisms except S. pneumoniae were grown, subcultured, and quantified in Mueller-Hinton broth (Difco Laboratories, Detroit, MI) and Mueller-Hinton agar (Difco Laboratories). Sheep blood agar plates (Remel, Milwaukee, WI) were used for S. pneumoniae. Dalbavancin was supplied by Vicuron. Penicillin and methicillin were purchased from Sigma.
In vitro susceptibility studies.
The MICs and minimal bactericidal concentrations (MBCs) of dalbavancin, penicillin, and methicillin for the various isolates were determined in duplicate on at least two occasions by standard Clinical and Laboratory Standards Institute (formerly NCCLS) microdilution methods (23).
Murine thigh infection model.
Animals were maintained in accordance with the criteria of the American Association for Accreditation of Laboratory Animal Care (24). All animal studies were approved by the Animal Research Committee of the William S. Middleton Memorial VA Hospital. Six-week-old, specific-pathogen-free, female ICR/Swiss mice (Harlan Sprague-Dawley, Indianapolis, IN) weighing 23 to 27 g were used for all studies. A neutropenic model was used for all studies, with the exception of a single substudy for determination of the impact of leukocytes. The mice were rendered neutropenic (neutrophils, <100/mm3) by injecting them with cyclophosphamide (Mead Johnson Pharmaceuticals, Evansville, IN) intraperitoneally 4 days (150 mg/kg of body weight) and 1 day (100 mg/kg) before the thigh infection study and 100 mg/kg every 48 h (q24h) after the start of infection until the end of the study. Previous studies have shown that this regimen produces neutropenia in this model for more than 7 days (1, 20, 33). Broth cultures of freshly plated bacteria were grown to logarithmic phase overnight to an absorbance at 580 nm of 0.3 (Spectronic 88; Bausch & Lomb, Rochester, NY). After dilution 1:10 in fresh Mueller-Hinton broth, the bacterial counts of the inoculum ranged from 106.6 to 107.6 CFU/ml. Thigh infections with each of the isolates were produced by injection of 0.1 ml of inoculum into the thighs of halothane-anesthetized mice 2 h before therapy with dalbavancin. At the end of the study period, the thighs were processed for CFU determination as described previously (1, 20, 33).
Murine lung infection model.
Stationary-phase broth cultures of S. pneumoniae strain ATCC 10813 were obtained by overnight incubation. The cultures were centrifuged at 10,000 × g for 20 min and washed twice in 0.9% saline before being resuspended in saline. Diffuse pneumonia was induced in three mice per treatment regimen by intranasal inoculation of 50 μl of an inoculum of 108.3 to 108.6 CFU/ml. Antimicrobial therapy was initiated 2 h after the infection procedure. Treatment was continued for 72 h. At the end of the study period the animals were euthanized and their lungs were removed, homogenized, diluted, and plated to determine the viable organism burden. The results are expressed as the mean ± standard deviation log10 CFU/lungs.
Drug PKs.
Single-dose serum PK studies were performed with thigh-infected mice given intraperitoneal doses (0.2 ml/dose) of dalbavancin (2.5, 5, 10, 20, 40, and 80 mg/kg). Blood was removed from groups of three mice by retroorbital aspiration and placed into heparinized capillary tubes at 0.5, 1, 2, 4, 6, 24, 48, 72, and 96 h after dosing. The plasma was separated by centrifugation, and dalbavancin plasma concentrations were measured by a microbiologic assay with Bacillus subtilus as the test organism. The lower limit of detection of the assay was 0.10 mg/liter, and the interday variation was less than 6%. Pharmacokinetic constants, including the elimination half-life, AUC, and Cmax, were calculated by using a noncompartmental model. The half-lives of dalbavancin were determined by linear least-squares regression. The AUC was calculated from the mean concentrations by use of the trapezoidal rule. The AUC was estimated at 24, 36, 48, 72, and 96 h and was extrapolated to infinity. An accumulation factor was considered for the shorter-dosing-interval studies (q12h and q24h).
Protein binding.
The impact of serum protein binding was assessed by examining the impact of 95% mouse serum on the activity of dalbavancin in vitro (7). The dalbavancin MICs for S. aureus ATCC 25923 and S. aureus MRSA were determined in broth, heat-treated (100°C for 30 min) 95% mouse serum, and 95% serum ultrafiltrate by using arithmetic dilutions of 0.02 mg/liter per tube. A reduced potency (higher MIC) in serum was presumed due to drug binding to serum protein. The difference in potency was used to estimate the percentage of protein binding by the following equation: (MIC in 95% serum − MIC in serum ultrafiltrate)/MIC in 95% serum. Previous studies have demonstrated that these staphylococcal isolates grow well in the presence of mouse serum. Other methodologies by filtration and dialysis were attempted (7, 18). However, nonspecific binding of dalbavancin to device material made interpretation difficult.
Treatment protocols. (i) In vivo time-kill study.
Two hours after infection with S. pneumoniae strain ATCC 10813 or S. aureus strain ATCC 29213, neutropenic mice (two mice per time point) were treated with one of five twofold-escalating single intraperitoneal doses of dalbavancin (for S. pneumoniae, 0.625, 1.25, 2.5, 5, and 10 mg/kg; for S. aureus, 5, 10, 20, 40, and 80 mg/kg). Treatment was initiated 2 h after infection. Groups of two treated mice (sampled at 0.5, 1, 2, 4, 6, 24, 48, 72, and 96 h) and two untreated control mice (sampled at 0, 1, 2, 4, 6, 24, and 48 h) were each killed at sampling intervals ranging from 0.5 to 24 h. The thighs were removed at each time point and immediately processed for CFU determination. Data are expressed as the mean ± standard deviation log10 CFU/thigh.
(ii) PK/PD index determination.
Neutropenic mice were infected with a strain of either penicillin-susceptible S. pneumoniae ATCC 10813 or MRSA strain ATCC 29213. Treatment with dalbavancin was initiated 2 h after infection. Groups of two mice were treated for 6 days with 20 different dosing regimens by using twofold-increasing total doses divided into 2, 4, 6, or 12 doses (q72h, q36h, q24h, and q2h, respectively). The total doses of dalbavancin ranged from 3.8 to 60 mg/kg/6 days for S. pneumoniae and 30 to 480 mg/kg/6 days for S. aureus. The drug doses were administered intraperitoneally in 0.2-ml volumes. The mice were killed after 144 h of therapy, and the thighs were removed and processed for CFU determination. Untreated control mice were killed just before treatment and after 48 h.
(iii) PK/PD index magnitude studies.
Similar dosing studies with six fourfold-increasing dalbavancin doses administered q24h or q72h were used to treat thigh-infected neutropenic animals with five strains of S. pneumoniae (one penicillin-susceptible strain, one penicillin-intermediate strain, three penicillin-resistant strains) and six strains of S. aureus (three methicillin-susceptible strains and three MRSA strains). The dalbavancin MICs for the organisms studied varied 30-fold. The animals were treated for a period of 144 h. The total dose of dalbavancin used in these studies varied from 0.625 to 960 mg/kg/6 days.
(iv) Impact of host infection site and immune status.
Two additional dosing studies were designed to determine the impacts of the infection site and host immune state. In the first of those studies, the in vivo efficacies of dalbavancin in the pneumonia and thigh infection models by using S. pneumoniae strain ATCC 10813 were compared. In the second of those studies, the activity of dalbavancin in neutropenic mice was compared to that in nonneutropenic mice infected with S. pneumoniae ATCC 10813 in the thigh infection model.
Data analysis.
The results of these studies were analyzed by using the sigmoid dose-effect model (5). The model, as follows, is derived from the Hill equation: E = (Emax × DN)/(ED50N × DN), where E is the effect or, in this case, the log10 change in CFU per thigh or lung between treated mice and untreated controls after the 144-h period of study; Emax is the maximum effect; D is the 144-h total dose; ED50 is the dose required to achieve 50% of Emax; and N is the slope of the dose-effect curve. The indices Emax, ED50, and N were calculated by using nonlinear least-squares regression. The correlation between efficacy and each of the three PK/PD indices studied (T > MIC, AUC/MIC, Cmax/MIC) was determined by nonlinear least-squares multivariate regression (Sigma Stat; Jandel Scientific Software, San Rafael, CA). The coefficient of determination (R2) was used to estimate the variance that could be due to regression with each of the PK/PD indices. We used the 72-h static dose as well as the doses necessary to achieve both a 1-log10 reduction and a 2-log10 reduction in colony counts compared to the numbers at the start of therapy to compare the impact of the dosing interval on treatment efficacy. If these dose values remained similar among each of the dosing intervals, this would suggest that AUC/MIC is the predictive index. If the dose values increased as the dosing interval was lengthened, this would suggest that T > MIC is the predictive parameter. Lastly, if the dose value decreased as the dosing interval was increased, this would suggest that Cmax/MIC is the pharmacodynamically important index.
To allow a comparison of the potency of dalbavancin against a variety of organisms, we used similar dosing endpoints (72-h static dose and the doses required to achieve a 1-log10 reduction and a 2-log10 reduction in colony counts). The magnitude of the PK/PD index associated with each endpoint dose was calculated from the following equation: log10 D = log10 [E/(Emax − E)]/(N + log10 ED50), where E is the control growth for the static dose (D), E is the control growth − 1 log unit for a D of 1-log killing, and E is the control − 2 log units for a D of 2-log killing. The significance of differences among the various dosing endpoints was determined by using analysis of variance.
RESULTS
Study organisms and dalbavancin MICs.
The study organisms and the MICs and MBCs of dalbavancin are listed in Table 1. The dalbavancin MICs for the pneumococci ranged from 0.004 to 0.03 mg/liter. The range of MICs for the S. aureus isolates were narrower and higher than those for the pneumococci, ranging from 0.06 to 0.12 mg/liter. MBCs were 1 to 2 tube dilutions higher than the MIC for the S. aureus isolates studied. Conversely, for the S. pneumoniae strains the MICs and the MBCs were the same. Beta-lactam resistance did not affect the in vitro potency of dalbavancin.
TABLE 1.
In vitro activity of dalbavancin against S. pneumoniae and S. aureus strains
| Organism | Dalbavancin MIC/MBC (mg/liter) | MIC (mg/liter)
|
|
|---|---|---|---|
| Penicillin | Methicillin | ||
| S. pneumoniae strain | |||
| 1199 | 0.004/0.004 | 1.0 | |
| 1293 | 0.004/0.004 | 2.0 | |
| 1325 | 0.008/0.008 | 2.0 | |
| 1329 | 0.008/0.008 | 2.0 | |
| ATCC 10813 | 0.03/0.03 | 0.008 | |
| S. aureus strain | |||
| ATCC 25923 | 0.12/0.50 | 0.12 | |
| 33591 | 0.12/0.25 | >8.0 | |
| 31005 | 0.06/0.12 | 0.12 | |
| MRSA | 0.06/0.25 | >8.0 | |
| Smith | 0.06/0.25 | 0.12 | |
| 307109 | 0.06/0.12 | >8.0 | |
PKs.
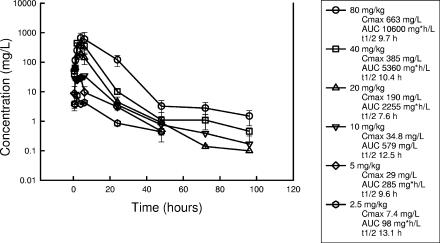
The PKs of dalbavancin in thigh-infected neutropenic Swiss ICR mice are shown in Fig. 1. Peak levels were observed by 4 h. Dalbavancin exhibited linear pharmacokinetics from 2.5 to 40 mg/kg. The half-life was prolonged and varied from 9.6 to 13.1 h. The level of protein binding of dalbavancin in pooled 95% serum from infected mice was 98.4% (Table 2).
FIG. 1.
Serum PKs of dalbavancin in neutropenic infected mice following the administration of six twofold escalating doses ranging from 2.5 to 80 mg/kg intraperitoneally. Each datum point represents the mean and standard deviation for three mice. t1/2, half-life.
TABLE 2.
Impacts of serum, serum ultrafiltrate, and albumin on in vitro activity of dalbavancin against selected S. aureus strains
| S. aureus strain | MIC (mg/liter) in:
|
||
|---|---|---|---|
| Broth | 95% mouse serum | 95% mouse serum ultrafiltrate | |
| ATCC 25923 | 0.12 | 32 | 0.5 |
| MRSA | 0.12 | 32 | 0.5 |
In vivo time-kill study.
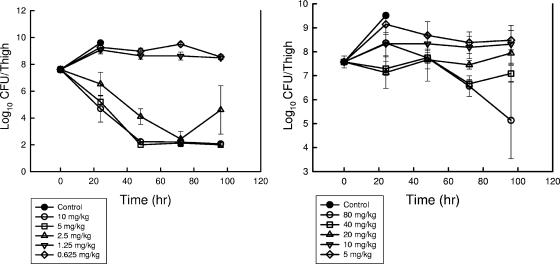
The effect of single doses of dalbavancin on the in vivo killing of a strain of S. pneumoniae and a strain of S. aureus over time are shown in Fig. 2. At the start of therapy mice had 107.6 CFU/thigh of either organism. Both organisms grew nearly 2 log10 units over the first 24 h in the thighs of untreated control mice. The two lowest dose levels of dalbavancin were relatively ineffective at reducing or suppressing organism growth. The extent and the rate of killing of the pneumococcal isolate were greater than those of the staphylococcal strain. Only the two highest doses of dalbavancin resulted in the killing of S. aureus. Three of the five dose levels used in study against S. pneumoniae reduced the burden of organisms nearly 4 log10 units in the thighs of infected mice. Organism reduction continued over the first 48 h of in vivo exposure to S. pneumoniae and over the entire 96-h period for S. aureus. Additional killing may have been produced in the S. pneumoniae model system; however, the reductions were at the lower limit of detection by 48 h. We did not observe appreciable organism recovery or regrowth over the 96-h period of observation.
FIG. 2.
Impacts of single doses of dalbavancin on organism burden in thighs of neutropenic mice infected with either S. pneumoniae ATCC 10813 (left panel) or S. aureus ATCC 29213 (right panel). Mice received either no drug or one of five twofold-increasing dose levels of dalbavancin administered via the intraperitoneal route. The microbiologic burden was determined by plate counts of thigh homogenates at selected time points over 96 h. Each symbol represents a different dose level. Each datum point represents the mean and standard deviation for four thighs.
Dosing regimen studies.
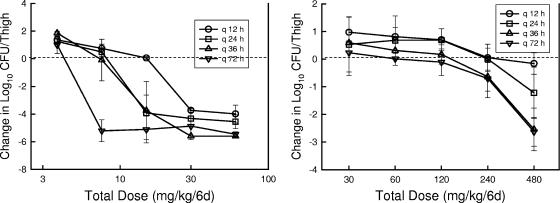
Figure 3 illustrates the dalbavancin dose-response curves for the different dosing intervals against the strains of S. pneumoniae and S. aureus studied in the thighs of neutropenic mice. Each point represents the mean for four thighs. The dotted lines represent the number of bacteria at the start of therapy. In general, increasing the dosing interval resulted in a shift of the dose-response curves to the left, indicating greater efficacy with the regimens for which large doses were administered infrequently. Similar to the single-dose time-kill studies, against S. pneumoniae, we observed more rapid and extensive killing than we did for S. aureus. However, maximal reductions with the largest most infrequently administered regimens in the S. aureus model still resulted in nearly a 3-log10-unit reduction in the thigh organism burden compared to that at the start of therapy.
FIG. 3.
Impact of dalbavancin dosing interval on the in vivo efficacy of dalbavancin against S. pneumoniae ATCC 10813 (left panel) or S. aureus ATCC 29213 (right panel) in neutropenic mice. Five total dose levels were fractionated over a 144-h study period. Each symbol represents one of four dosing intervals. Each datum point represents the mean and standard deviation log10 CFU/thigh for four thighs.
Each of the dose-response curves was also mathematically characterized by using a maximum-effect model. This methodology uses the Hill equation to estimate by nonlinear regression Emax, the ED50, and the slope of the dose-response relationship (1, 5). From these parameters we calculated the dose required to produce a net bacteriostatic effect over the 144-h treatment period as well as the doses necessary to produce a 1- and 2-log reductions in the organism burden. The static dose and the doses associated with 1- and 2-log killing for each of the drug-organism combinations and the various dosing regimens are shown in Table 3.
TABLE 3.
Impact of dalbavancin dosing interval on efficacy against S. pneumoniae and S. aureus
| Organism | Dose (mg/kg [95% CI])a
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| q12h
|
q24h
|
q36h
|
q72h
|
|||||||||
| Static dose | Dose associated with 1-log killing | Dose associated with 2-log killing | Static dose | Dose associated with 1-log killing | Dose associated with 2-log killing | Static dose | Dose associated with 1-log killing | Dose associated with 2-log killing | Static dose | Dose associated with 1 log killing | Dose associated with 2-log killing | |
| S. pneumoniae ATCC 10813 | 2.3 (1.9-2.7) | 2.8 (2.3-3.3) | 3.5 (2.8-4.2) | 1.34 (1.14-1.57) | 1.54 (1.34-1.74) | 1.75 (1.47-2.03) | 1.26 (1.16-1.36) | 1.53 (1.41-1.65) | 1.81 (1.67-1.95) | 0.71 (0.64-0.88) | 0.77 (0.59-.95) | 0.82 (0.62-1.02) |
| S. aureus ATCC 29213 | 80 (8-152) | 26 (0-91) | 267 (0-934) | 15 (0-44) | 36 (0-106) | 122 (0-361) | 10 (0-38) | 31 (0-117) | 128 (0-486) | |||
Dose values are based on dose levels in mg/kg/24 h.
Increasing the frequency of the dosage regimen from 12 to 36 h in study against S. pneumoniae did not result in appreciable changes in the doses associated with the three microbiologic endpoints. However, efficacy with the 72-h dosing interval required less drug. Statistical comparison of these differences were significant (P < 0.05) among all of the regimens (q12h versus q24h, q12h versus q36h, q12h versus q72h, q24h versus q72h, and q36h versus q72h), with the exception of the q24h and q36h intervals. A similar relationship was observed in the study against S. aureus. However, there was more variability in the S. aureus data set. The only regimens for which the differences were statistically significant for S. aureus were the q12h interval versus the other more extended intervals. The only dosing regimens that produced 1- and 2-log killing of S. aureus were the q36h and q72h intervals. Both visual inspection of the dose-response curves in Fig. 2 and these statistical comparisons suggest that large infrequent dosing of dalbavancin was most effective in this model against both bacterial species. This pattern of activity would suggest that either the 24-h AUC/MIC or the Cmax/MIC PD index would be the most important for describing the activity of this lipoglycopeptide (5).
PD parameters correlating with efficacy.
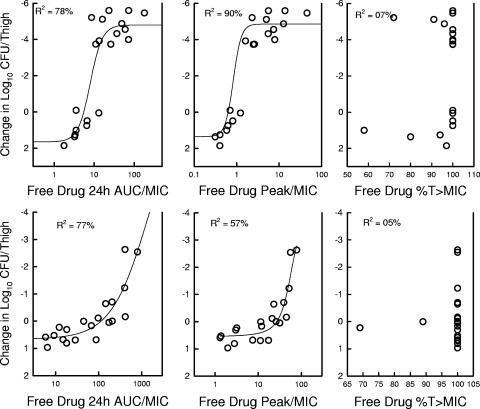
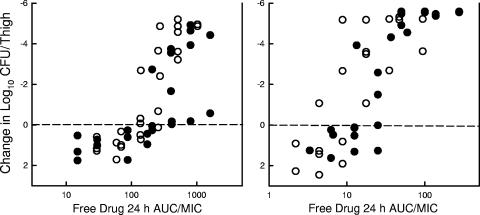
Subsequent analysis of the dose-response data examined the impact of each of the three PD indices by relating the number of bacteria in thigh at the end of 144 h of therapy with (i) the Cmax/MIC ratio, (ii) the 24-h AUC/MIC ratio, and (iii) the T > MIC for each of the dosage regimens studied. The PK/PD index values for those doses not specifically studied were extrapolated from the values of the nearest doses studied. For the AUC/MIC index, we calculated the predicted AUC over time as opposed to the AUC to infinity for all regimens. The dalbavancin accumulation for Cmax was considered in these calculations for the shorter dosing intervals. The relationship between the log10 numbers of CFU per thigh and the Cmax/MIC ratio, the 24-h AUC/MIC ratio, and the T > MIC are illustrated in Fig. 4 for S. pneumoniae and S. aureus. Each point represents the mean for four thighs. For both organisms a strong correlation was observed with the 24-h AUC/MIC and the Cmax/MIC ratios. Regression of the data with the 24-h AUC/MIC ratio resulted in the strongest correlation for S. aureus (R2 = 77% for the 24-h AUC/MIC and 57% for the Cmax/MIC). However, for S. pneumoniae the Cmax regression fit was the strongest (R2 = 78% for the 24-h AUC/MIC and 90% for the Cmax/MIC). Regression of the dose-response data with the T > MIC parameter resulted in a poor fit of the data, with R2 values below 10%.
FIG. 4.
Relationship between the free-drug 24-h AUC/MIC, Cmax/MIC, and T > MIC and the efficacy of dalbavancin against S. pneumoniae ATCC 10813 (top panel) and S. aureus ATCC 29213 (bottom panel) in the thighs of neutropenic mice over a 144-h treatment period. Each symbol represents the mean log10 CFU/thigh values for four thighs.
PK/PD index magnitude or target.
Both the concentration-dependent indices were important predictors of the in vivo efficacy of dalbavancin. For comparison of the index magnitude among strains with various MICs, we chose to examine the 24-h AUC/MIC index due to the extremely prolonged elimination half-life in humans. To determine if the 24-h AUC/MIC (by the use of free drug values) required for a static effect was similar for multiple pathogens, we studied the activities of the q24h and q72h dosing regimens of dalbavancin against five strains of S. pneumoniae and six strains of S. aureus. The dose-response curves for dalbavancin against these various strains are shown in Fig. 5. The growth curves of the five pneumococcal and staphylococcal strains for the thighs of the control animals were similar. At the start of therapy the mice had 6.9 ± 0.21 log10CFU/thigh of pneumococci (range, 6.6 to 7.2 log10CFU/thigh of pneumococci). The organism burdens at the start of therapy were similar for the staphylococci and ranged from 6.3 to 7.3 log10 CFU/thigh. The organisms grew to 2.1 ± 0.5 log10 CFU/thigh in the control mice. In general, the shapes of the dose-response curves were similar for all strains. The location of the dose-response curve was related to the MIC of the organism. However, the dose-response curves for the pneumococcal organisms were shifted slightly to the left. This curve shift suggests that less drug was necessary for the drug to have an effect against pneumococci than against staphylococci. The static dose, the doses associated with 1-log and 2-log killing, and the associated free-drug 24-h AUC/MIC are shown in Table 4. The extent of bacterial killing was relatively similar for most strains. All strains exhibited more than a 4-log10 drop in the numbers of CFU following dalbavancin therapy over the 6-day study compared to the numbers of CFU for the untreated controls.
FIG. 5.
Relationship between free-drug 24-h AUC/MIC and change in log10 CFU/thigh over 6 days. Each datum point represents the mean value for four thighs. Hollow symbols, data from the q72h regimens; solid symbols, data from the q24h regimens; left panel, data for five S. aureus strains; right panel, data for five S. pneumoniae strains.
TABLE 4.
Efficacy of dalbavancin against S. pneumoniae and S. aureus
| Regimen and organism | MIC (mg/liter) | MIC/MBC (mg/liter) | Static effect
|
1-log killing
|
2-log killing
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg/24 h or mg/kg/72 h) | Free-drug 24-h AUC/MIC | Free-drug 24-h AUC/MBC | Dose (mg/kg/24 h or mg/kh/72 h) | Free-drug 24-h AUC/MIC | Free-drug 24-h AUC/MBC | Dose (mg/kg/24 h or mg/kh/72 h) | Free-drug 24-h AUC/MIC | Free-drug 24-h AUC/MBC | |||
| q24h regimen | |||||||||||
| S. pneumoniae | |||||||||||
| 1199 | 0.004 | 0.40 | 16.0 | 0.49 | 19.4 | 0.57 | 22.7 | ||||
| 1293 | 0.004 | 0.44 | 17.4 | 0.55 | 21.7 | 0.65 | 25.9 | ||||
| 1325 | 0.008 | 1.46 | 29.0 | 1.6 | 31.8 | 1.73 | 34.5 | ||||
| 1329 | 0.008 | 0.90 | 18.0 | 1.18 | 23.5 | 1.45 | 26.7 | ||||
| ATCC 10813 | 0.03 | 1.34 | 7.5 | 1.54 | 8.7 | 1.75 | 9.9 | ||||
| Mean ± SD | 0.91 ± 0.44 | 17.6 ± 6.9 | 1.1 ± 0.47 | 21 ± 7.4 | 1.23 ± 0.52 | 24.3 ± 8.2 | |||||
| S. aureus | |||||||||||
| ATCC 25923 | 0.12/0.50 | 42.7 | 216 | 52 | 51.2 | 250 | 60 | 60 | 289 | 69.3 | |
| 33591 | 0.12/0.25 | 22.3 | 96.2 | 46 | 27.7 | 121 | 58.3 | 33.5 | 157 | 74.3 | |
| 31005 | 0.06/0.12 | 49.3 | 483 | 242 | |||||||
| MRSA | 0.06/0.25 | 37.7 | 374 | 93 | 45.3 | 452 | 109 | 53.5 | 519 | 125 | |
| Smith | 0.12/0.25 | 33.5 | 156 | 75 | 50.7 | 248 | 119 | 73.5 | 361 | 173 | |
| Mean ± SD | 37.1 ± 9.1 | 265 ± 143 | 101 ± 72 | 43.7 ± 9.5 | 268 ± 119 | 86.6 ± 27.6 | 55 ± 14 | 332 ± 131 | 110 ± 42 | ||
| q72h regimen | |||||||||||
| S. pneumoniae | |||||||||||
| 1325 | 0.008 | 0.83 | 6.0 | 0.99 | 8.8 | 1.16 | 17.6 | ||||
| 1293 | 0.004 | 1.01 | 14.1 | 1.28 | 18 | 1.68 | 23.8 | ||||
| 1199 | 0.004 | 0.72 | 10.3 | 0.85 | 12.1 | 0.99 | 35.3 | ||||
| 1396 | 0.008 | 0.52 | 4.0 | 0.61 | 4.3 | 0.69 | 4.8 | ||||
| ATCC 10813 | 0.03 | 0.71 | 1.4 | 0.77 | 1.6 | 0.82 | 1.6 | ||||
| Mean ± SD | 0.77 ± 0.18 | 7.2 ± 4.5 | 0.93 ± 0.24 | 9.0 ± 5.8 | 1.13 ± 0.36 | 16.6 ± 12.3 | |||||
| S. aureus | |||||||||||
| ATCC 25923 | 0.12/0.50 | 160 | 274 | 66 | 185 | 317 | 76.1 | 214 | 367 | 88.1 | |
| 33591 | 0.12/0.25 | 74 | 123 | 59 | 93 | 160 | 76.6 | 114 | 195 | 94 | |
| MRSA | 0.06/0.25 | 43 | 147 | 35.3 | 62 | 202 | 48.8 | 85 | 292 | 70 | |
| Smith | 0.12/0.25 | 59 | 96 | 46.1 | 95 | 163 | 78.0 | 148 | 254 | 22 | |
| 307109 | 0.06/0.50 | 31 | 94 | 11.3 | 34 | 108 | 12.9 | 37 | 121 | 14.6 | |
| 31005 | 0.06/0.12 | 67.7 | 223 | 112 | 94.6 | 325 | 163 | 127 | 364 | 217 | |
| Mean ± SD | 72 ± 41 | 160 ± 67 | 55 ± 31 | 94 ± 46 | 213 ± 81.4 | 75.9 ± 45.2 | 120 ± 54 | 266 ± 88 | 101 ± 61 | ||
For the dalbavancin regimens with q24h dosing, the free-drug 24-h AUC/MICs associated with a static effect against S. pneumoniae and S. aureus were 18 ± 7 and 265 ± 143, respectively. Therapy against pneumococci, based upon the 24-h AUC/MIC, required 2.1- to 6.4-fold less drug, as suggested by the dose-response curves, compared to the amount required for S. aureus. When the drug exposure for the S. aureus strains was considered relative to the MBC, the AUC/MICs were lower, yet they were still larger than those for S. pneumoniae. The dose-response curves with both species were relatively steep, and the 24-h AUC/MICs associated with 1- and 2-log killing were not appreciably higher than that associated with a net static effect.
For the regimens that used less frequent dosing (q72h), the free-drug 24-h AUC/MICs associated with a static effect against S. pneumoniae and S. aureus were 7.2 ± 4.52 and 160 ± 67, respectively. The PK/PD magnitudes necessary to achieve the three in vivo microbiologic endpoints (static dose and the doses associated with 1- and 2-log killing) were lower for the more widely spaced dosing regimens. When dalbavancin was dosed every 72 h, the 24-h AUC/MICs associated with the various endpoints were 1.3- to 2.4-fold lower than those found when the drug was dosed q24h. Penicillin resistance in S. pneumoniae and methicillin resistance in S. aureus did not affect the 24-h AUC/MIC required for dalbavancin efficacy.
Impacts of neutrophils and infection site on activity of dalbavancin.
To determine the effects of neutrophils on the activity of dalbavancin, we compared the dose-response curves with dosing of the drug q24h in both healthy (nonneutropenic) and neutropenic mice infected with S. pneumoniae. The static dose and the doses associated with 1- and 2-log killing were calculated from the parameters estimated by nonlinear regression by using the Hill equation, as described above. The doses (mg/kg/6 days) required to achieve these endpoints in both healthy and neutropenic mice are shown in Table 5. The presence of neutrophils resulted in 1.7- to 2.1-fold reductions in the doses necessary for efficacy. However, these differences were not statistically significant.
TABLE 5.
Impact of neutrophils on in vivo efficacy of dalbavancin against S. pneumoniae
| Mouse group | Dose (mg/kg/6 days [95% CI])
|
||
|---|---|---|---|
| Static dose | Dose associated with 1-log killing | Dose associated with 2-log killing | |
| Healthy | 2.03 (1.99-2.07) | 2.35 (2.26-2.44) | 2.66 (2.56-2.76) |
| Neutropenic | 4.13 (0.23-8.0) | 4.38 (0.28-8.50) | 4.62 (0.32-8.90) |
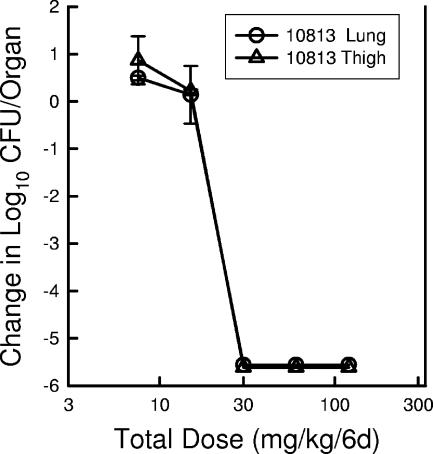
To determine the impact of the infection site on the activity of dalbavancin, we compared the dose-response curves with the q24h dosing of the drug in both the thigh and the lung infection models (Fig. 6). The dose-response curves in the two models with S. pneumoniae were nearly identical. These data suggest that the PD target was independent of infection site (soft tissue sepsis and pneumonia).
FIG. 6.
Dose-response relationship for dalbavancin in the neutropenic murine thigh and lung infection models against S. pneumoniae ATCC 10813 over a 6-day treatment period. Each datum point represents the mean and standard deviation for either two mice (four thighs [triangles]) or three mice (lungs [circles]).
DISCUSSION
A variety of in vitro and in vivo studies have demonstrated that the killing activities of glycopeptide antibiotics, such as vancomycin and teicoplanin, are not enhanced by exposure to drug concentrations far exceeding the MIC (3, 6, 8, 9, 10, 11, 16, 17, 19, 25, 29). However, they have been shown to produce moderately long in vitro postantibiotic effects. The in vivo efficacies of antimicrobial drugs exhibiting these time course characteristics are most often best described by the 24-h AUC/MIC PD parameter. Several dose fractionation studies with vancomycin suggest that the data fit best when they are regressed with either the AUC/MIC or the Cmax/MIC parameter. However, a few studies report contradictory conclusions, in which the T > MIC was believed to better predict treatment results. The current studies have characterized the in vivo PD activity of dalbavancin against a variety of pathogens. This lipoglycopeptide demonstrated in vivo dose-dependent bactericidal activity against both S. pneumoniae and S. aureus. Regression of the dose fractionation studies with each of the PK/PD parameters demonstrated the importance of both the 24-h AUC/MIC and the Cmax/MIC. Correlation of the results of the dose fractionation experiments with the T > MIC parameter resulted in a very poor data fit. These dose-response relationships clearly support the use of large infrequent doses to optimize in vivo treatment efficacy.
The next logical PD-related question is to ask what drug exposure is necessary for treatment effect. More simply put, how much drug relative to the MIC needs to be given for efficacy? A study with vancomycin in the neutropenic murine thigh infection model also reported that AUC/MICs ranging from 80 to 460 were needed to produce 50% of maximum killing over a 24-h treatment period (9). Analysis of the treatment efficacy of vancomycin in patients with ventilator-associated pneumonia caused by S. aureus reported that a similar range of exposure (mean 24-h AUC/MICs of 345 for clinical outcome and 850 for microbiologic cure) was associated with positive patient outcomes (22). These exposure-response relationships suggest that the glycopeptide concentrations associated with in vivo efficacy are larger than those needed for efficacy in vitro. For example, averaging of 1× the MIC over 24 h in vitro would result in a 24-h AUC/MIC ratio of 24. Even if free-drug concentrations are considered, for vancomycin against S. aureus, optimal outcomes in vivo are observed when concentrations exceed the in vitro MIC by a factor of four to eight over the treatment period (corresponding to 24-h AUC/MICs up to several hundred). The dalbavancin in vivo exposures associated with a net static effect for the S. aureus isolates examined in the current study were similar to those values for vancomycin with 24-h AUC/MICs. Interestingly, the dose-response curves were relatively steep, and the exposures necessary to produce significant organism killing (1- and 2-log10 reductions) were not much larger than those associated with a net static effect (less than twofold). The dose-response relationships were fairly similar among the five S. aureus strains examined, and methicillin resistance, not surprisingly, had no impact on the dalbavancin PD target associated with efficacy.
The dose-response curves in studies with each of the five S. pneumoniae isolates was shifted to the left compared to those for the staphylococcal isolates, suggesting that less drug is needed to achieve similar outcomes. In fact, about 10-fold less dalbavancin was needed to achieve each of the microbiologic endpoints for pneumococci in this neutropenic infection model. The in vivo 24-h AUC/MIC exposure values suggest that for S. pneumoniae the in vitro MIC is very near the in vivo MIC (i.e., growth is inhibited in vivo when a concentration near the in vitro MIC is averaged over the treatment period). Beta-lactam resistance in the pneumococcal strains did not affect the dalbavancin PD index required for treatment efficacy.
A number of host, organism, and drug factors have been theorized to affect the PD target necessary for efficacy. For most antimicrobials, in vivo PD studies have demonstrated that these variables do not markedly impact the PD target. However, there are a few situations where experimental observations suggest significant differences. For example, with drugs from the fluoroquinolone class, the presence of neutrophils can enhance antimicrobial activity by up to a factor of four to six (1, 5). Another host variable of demonstrated importance for some antimicrobials is the ability to penetrate into the epithelial lining fluid of the lung. Some antimicrobial compounds, such as the macrolides and the quinolones, achieve relatively high concentrations in this tissue space compared to those achieved in serum. Conversely, some molecules such as vancomycin do not attain high concentrations in the epithelial lining fluid relative to those concentrations observed in serum and other interstitial tissue spaces. In the current investigations we examined the impacts of two host variables on the in vivo efficacy of dalbavancin. In the first study we compared the efficacy of dalbavancin against a strain of S. pneumoniae in both neutropenic and nonneutropenic mice by using the thigh infection model. In the nonneutropenic model, the dose-response curve was slightly shifted to the left, suggesting that less drug was needed when the mice were healthy than when the mice were immunocompromised. For each microbial endpoint examined (static dose and the doses associated with 1- and 2-log10 killing), nearly twofold less dalbavancin was required. However, these differences did not reach statistical significance. We next compared the in vivo activity of dalbavancin against S. pneumoniae in both the thigh infection and the pneumonia models. The dose-response relationship was nearly identical for both infection sites, suggesting that dalbavancin achieves concentrations in the mouse lung that are similar to those achieved in serum and soft tissue. However, for some compounds, studies have suggested that penetration into mouse epithelial lining fluid does not always correlate with the kinetics in this tissue space in humans (W. A. Craig, unpublished data). Thus, it will be important to explore the kinetics in epithelial lining fluid and treatment efficacy for dalbavancin in patients.
In summary, the current studies demonstrate that dalbavancin has dose-dependent in vivo efficacy against pneumococci and staphylococci, independent of beta-lactam resistance, the presence of host neutrophils, or the infection site. The 24-h AUC/MIC parameter was very highly associated with in vivo dalbavancin activity. These PD characteristics support the infrequent administration of large doses. Against pneumococci, PD target studies suggest that achieving concentrations near the in vitro MIC over the dosing period produces optimal efficacy (i.e., a free-drug 24-h AUC/MIC of nearly 25× or 1× the MIC for 24 h). A larger drug exposure was needed for similar efficacy against S. aureus. The 24-h AUC/MIC target associated with efficacy against this bacterial species was in the range of 100 to 300. These observations are similar to those observed for other glycopeptides and S. aureus (8).
Human PK studies with dalbavancin demonstrate that is has an extremely long half-life and serum concentrations that exceed the MIC90 for target gram-positive pathogens more than 1 week following the administration of a single dose (2). If one considers the PD targets identified in the current in vivo models, current dalbavancin dosing regimens would exceed the free-drug 24-h AUC/MICs for both streptococci and staphylococci. For example, the dalbavancin serum exposure following the administration of a single dose of 1 g produces a free-drug AUC of more than 1,500 mg·h/liter. Studies have also examined the trough free-drug concentrations at the end of 1- and 2-week treatment periods; the values exceed 2 μg/ml. When these kinetic values are considered relative to MIC90 values of <0.12 mg/liter, one would anticipate more than adequate PD target attainment.
Acknowledgments
The research was supported by a grant from Vicuron.
Footnotes
Published ahead of print on 16 February 2007.
REFERENCES
- 1.Andes, D., and W. A. Craig. 2002. Animal model pharmacokinetics and pharmacodynamics: a critical review. Int. J. Antimicrob. Agents 19261-268. [DOI] [PubMed] [Google Scholar]
- 2.Buckwalter, M., and J. A. Dowell. 2005. Population pharmacokinetic analysis of dalbavancin, a novel lipopeptide. J. Clin. Pharmacol. 451279-1287. [DOI] [PubMed] [Google Scholar]
- 3.Chambers, H. F., and S. Kennedy. 1990. Effects of dosage, peak and trough concentrations in serum, protein binding, and bactericidal rate on efficacy of teicoplanin in a rabbit model of endocarditis. Antimicrob. Agents Chemother. 34510-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chambers, H. F. 2005. Community-associated MRSA—resistance and virulence converge. N. Engl. J. Med. 3521485-1487. [DOI] [PubMed] [Google Scholar]
- 5.Craig, W. A. 1998. Pharmacokinetics and pharmacodynamics of antibiotics in mice and men. Clin. Infect. Dis. 261-12. [DOI] [PubMed] [Google Scholar]
- 6.Craig, W. A. 2003. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect. Dis. Clin. N. Am. 17479-501. [DOI] [PubMed] [Google Scholar]
- 7.Craig, W. A., and B. Suh. 1996. Protein binding and the antimicrobial effects: methods for the determination of protein binding, p. 367-402. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. The Williams & Wilkins Co., Baltimore, MD.
- 8.Dudley, M., D. Griffith, E. Corcoran, C. Liu, K. Sorensen, V. Tembe, et al. 1999. Pharmacokinetic pharmacodynamic (PK-PD) indices for vancomycin treatment of susceptible (VSSA) and intermediate (VISA) S. aureus in the neutropenic murine thigh model, p. 49. Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 9.Duffull, S. B., E. J. Begg, S. T. Chambers, and M. L. Barclay. 1994. Efficacies of different vancomycin dosing regimens against Staphylococcus aureus determined with a dynamic in vitro model. Antimicrob. Agents Chemother. 382480-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebert, S., J. Leggett, and B. Vogelman. 1987. In vivo cidal activity and pharmacokinetics parameters (PKPs) for vancomycin against methicillin-susceptible (MSSA) and -resistant (MRSA) S. aureus, p. 173. Abstr. 27th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 11.Houlihan, H. H., R. C. Mercier, and M. J. Rybak. 1997. Pharmacodynamics of vancomycin alone and in combination with gentamicin at various dosing intervals against methicillin-resistant Staphylococcus aureus-infected fibrin-platelet clots in an in vitro infection model. Antimicrob. Agents Chemother. 412497-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jabes, D., G. Candiani, G. Romano, C. Brunati, S. Riva, and M. Cavaleri. 2004. Efficacy of dalbavancin against methicillin-resistant Staphylococcus aureus in the rat granuloma pouch infection model. Antimicrob. Agents Chemother. 481118-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones, R. N., T. R. Fritsche, H. S. Safdar, and B. P. Goldstein. 2005. Antimicrobial spectrum and potency of dalbavancin tested against clinical isolates from Europe and North America (2003): initial results from an international surveillance protocol. J. Chemother. 17593-600. [DOI] [PubMed] [Google Scholar]
- 14.Jones, R. N., M. G. Stilwell, H. S. Sader, T. R. Fritsche, and B. P. Goldstein. 2006. Spectrum and potency of dalbavancin tested against 3322 gram-positive cocci isolated in the United States Surveillance Program (2004). Diagn. Microbiol. Infect. Dis. 54149-153. [DOI] [PubMed] [Google Scholar]
- 15.Kairegio, L. E., S. Babazadeh, E. Seltzer, L. Goldberg, D. Krievins, M. Frederick, D. Krause, I. Satilovs, Z. Endzinas, J. Breaux, and W. O'Riordan. 2005. Randomized, double-blind comparison of once-weekly dalbavancin versus twice-daily linezolid therapy for the treatment of complicated skin and skin structure infections. Clin. Infect. Dis. 411407-1415. [DOI] [PubMed] [Google Scholar]
- 16.Knudsen, J. D., K. Fuursted, S. Raber, F. Espersen, and N. Frimodt-Moller. 2000. Pharmacodynamics of glycopeptides in the mouse peritonitis model of Streptococcus pneumoniae or Staphylococcus aureus infection. Antimicrob. Agents Chemother. 441247-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knudsen, J. D., K. Fuursted, F. Espersen, and N. Frimodt-Moller. 1997. Activities of vancomycin and teicoplanin against penicillin-resistant pneumococci in vitro and in vivo and correlation to the pharmacokinetics parameters in the mouse peritonitis model. Antimicrob. Agents Chemother. 411910-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunin, C. M., W. A. Craig, M. Kornguth, and R. Monson. 1973. Influence of binding on the pharmacologic activity of antibiotics. Ann. N. Y. Acad. Sci. 226214-224. [DOI] [PubMed] [Google Scholar]
- 19.Larsson, A. J., K. J. Walker, J. K. Raddatz, and J. C. Rotschafer. 1996. The concentration-independent effect of monoexponential and biexponential decay of vancomycin concentrations on the killing of Staphylococcus aureus under aerobic and anaerobic conditions. J. Antimicrob. Chemother. 38589-597. [DOI] [PubMed] [Google Scholar]
- 20.Leggett, J. E., B. Fantin, S. Ebert, K. Totsuka, B. Vogelman, W. Calamae, H. Mattie, and W. A. Craig. 1989. Comparative antibiotic dose-effect relationships at several dosing intervals in murine pneumonitis and thigh-infection models. J. Infect. Dis. 159281-292. [DOI] [PubMed] [Google Scholar]
- 21.Moellering, R. C. 2006. The growing menace of community-acquired methicillin-resistant Staphyococcus aureus. Ann. Intern. Med. 144368-370. [DOI] [PubMed] [Google Scholar]
- 22.Moise, P. A., A. Forrest, S. M. Bhavnani, M. C. Birmingham, and J. J. Schentag. 2000. Area under the inhibitory curve and a pneumonia scoring systemic for predicting outcomes of vancomycin therapy for respiratory infections by Staphylococcus aureus. Am. J. Health Syst. Pharm. 57(Suppl. 2)4-9. [DOI] [PubMed] [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution susceptibility tests for bacteria that grow aerobically. Approved standard, 5th ed. Document M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 24.National Research Council, Committee on the Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources, and Commission on Life Sciences. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 25.Pavie, J., A. Lefort, M. C. Ploy, L. Massias, F. Chau, L. Garry, et al. 2003. Influence of reduced susceptibility to glycopeptides on activities of vancomycin and teicoplanin against Staphylococcus aureus in experimental endocarditis. Antimicrob. Agents Chemother. 472018-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pope, S. D., and A. M. Roecker. 2006. Dalbavancin: a novel lipoglycopeptide antibacterial. Pharmacotherapy 26908-918. [DOI] [PubMed] [Google Scholar]
- 27.Raad, I., R. Darouiche, J. Vazquez, A. Lentnek, R. Hachem, H. Hanna, B. Goldstein, T. Henkel, and E. Seltzer. 2005. Efficacy and safety of weekly dalbavancin therapy for catheter-related bloodstream infection caused by gram-positive pathogens. Clin. Infect. Dis. 40374-380. [DOI] [PubMed] [Google Scholar]
- 28.Rice, L. B. 2006. Antimicrobial resistance in gram-positive bacteria. Am. J. Infect. Control 34(5 Suppl 1)S11-S19. [DOI] [PubMed] [Google Scholar]
- 29.Rybak, M. J. 2006. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin. Infect. Dis. 42S35-S39. [DOI] [PubMed] [Google Scholar]
- 30.Safdar, N., D. Andes, and W. A. Craig. 2004. In vivo pharmacodynamic activity of daptomycin. Antimicrob. Agents Chemother. 4863-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seltzer, E., M. B. Dorr, B. P. Goldstein, M. Perry, J. A. Dowell, and T. Henkel. 2003. Once-weekly dalbavancin versus standard-of-care antimicrobial regimens for treatment of skin and soft-tissue infections. Clin. Infect. Dis. 371298-1303. [DOI] [PubMed] [Google Scholar]
- 32.Streit, J. M., T. R. Fritsche, H. S. Sader, and R. N. Jones. 2004. Worldwide assessment of dalbavancin activity and spectrum against over 6,000 clinical isolates. Diagn. Microbiol. Infect. Dis. 48137-143. [DOI] [PubMed] [Google Scholar]
- 33.Vogelman, B., S. Gudmundsson, J. Leggett, J. Turnidge, S. Ebert, and W. A. Craig. 1988. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in in animal model. J. Infect. Dis. 158831-847. [DOI] [PubMed] [Google Scholar]