Abstract
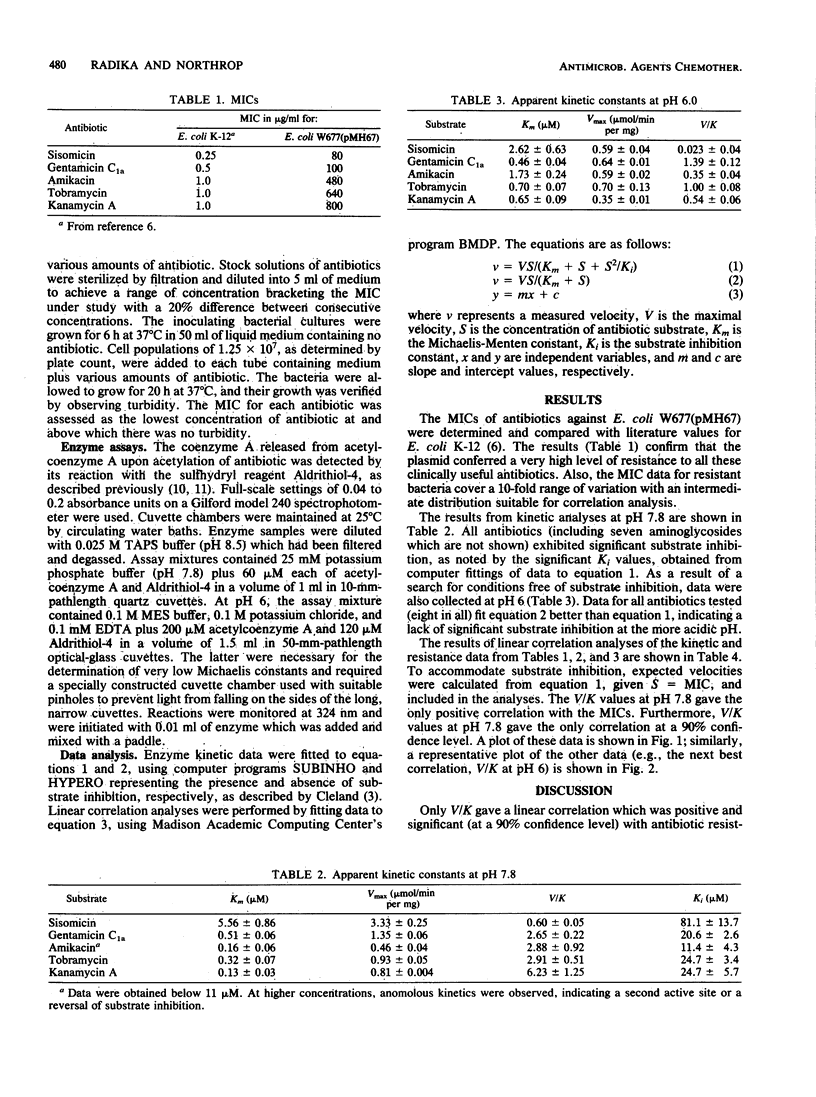
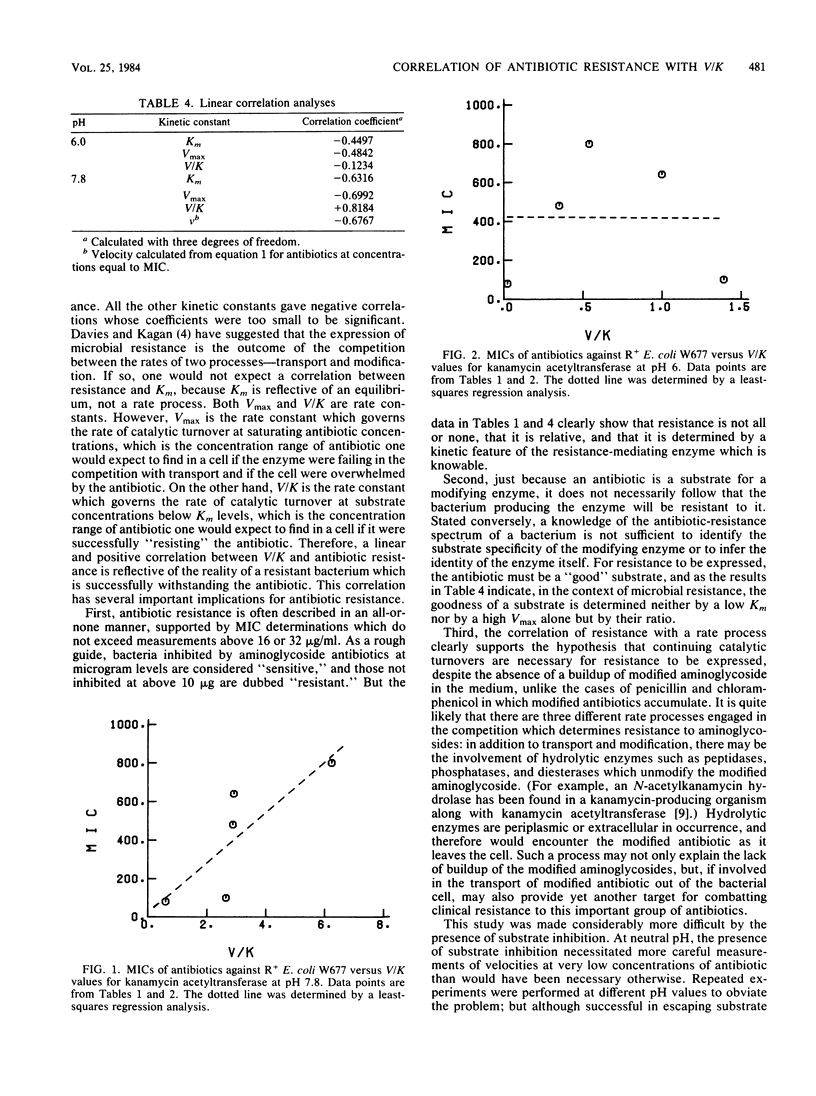
Kinetic data for the antibiotic-modifying enzyme kanamycin acetyltransferase AAC(6')-IV have been determined for five aminoglycoside antibiotics (amikacin, gentamicin C1a, kanamycin A, sisomicin, and tobramycin) and compared with close-interval determinations of the minimal inhibitory concentrations of the same antibiotics against Escherichia coli W677 harboring the resistance plasmid pMH67. These minimal inhibitory concentrations for the resistant bacteria varied from 80 to 800 micrograms/ml. Of the kinetic parameters Vmax, Km, and Vmax/Km ratio only Vmax/Km ratio had a linear correlation with minimal inhibitory concentrations (r = +0.818) at pH 7.8, where all antibiotics produced substrate inhibition, but not at pH 6.0, where they did not. The correlation with only Vmax/Km ratio has implications regarding the expression of resistance within the dynamics of the bacterial cell (i.e., antibiotic uptake versus modification), whereas substrate inhibition presents an opportunity to search for new chemotherapeutic agents which will combat resistance directly.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste R., Davies J. Mechanisms of antibiotic resistance in bacteria. Annu Rev Biochem. 1973;42:471–506. doi: 10.1146/annurev.bi.42.070173.002351. [DOI] [PubMed] [Google Scholar]
- Biddlecome S., Haas M., Davies J., Miller G. H., Rane D. F., Daniels P. J. Enzymatic modification of aminoglycoside antibiotics: a new 3-N-acetylating enzyme from a Pseudomonas aeruginosa isolate. Antimicrob Agents Chemother. 1976 Jun;9(6):951–955. doi: 10.1128/aac.9.6.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- Haas M. J., Davies J. Characterization of the plasmids comprising the "R factor" R5 and their relationships to other R plasmids. Plasmid. 1980 May;3(3):260–277. doi: 10.1016/0147-619x(80)90040-2. [DOI] [PubMed] [Google Scholar]
- Le Goffic F., Martel A., Capmau M. L., Baca B., Goebel P., Chardon H., Soussy C. J., Duval J., Bouanchaud D. H. New plasmid-mediated nucleotidylation of aminoglycoside antibiotics in Staphlococcus aureus. Antimicrob Agents Chemother. 1976 Aug;10(2):258–264. doi: 10.1128/aac.10.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrop D. B., Cleland W. W. The kinetics of pig heart triphosphopyridine nucleotide-isocitrate dehydrogenase. II. Dead-end and multiple inhibition studies. J Biol Chem. 1974 May 10;249(9):2928–2931. [PubMed] [Google Scholar]
- Scarbrough E., Williams J. W., Northrop D. B. Spectrophotometric assay for amikacin using purified kanamycin acetyltransferase. Antimicrob Agents Chemother. 1979 Aug;16(2):221–224. doi: 10.1128/aac.16.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. W., Northrop D. B. Purification and properties of gentamicin acetyltransferase I. Biochemistry. 1976 Jan 13;15(1):125–131. doi: 10.1021/bi00646a019. [DOI] [PubMed] [Google Scholar]
- Williams J. W., Northrop D. B. Substrate specificity and structure-activity relationships of gentamicin acetyltransferase I. The dependence of antibiotic resistance upon substrate Vmax/Km values. J Biol Chem. 1978 Sep 10;253(17):5908–5914. [PubMed] [Google Scholar]


