Abstract
Asiatic acid and corosolic acid are two natural products identified as biofilm inhibitors in a biofilm inhibition assay. We evaluated the activities of these two compounds on Pseudomonas aeruginosa biofilms grown in rotating disk reactors (RDRs) in combination with tobramycin and ciprofloxacin. To determine the ruggedness of our systems, the antibiotic susceptibilities of these biofilms were assessed with tobramycin and ciprofloxacin. The biofilm bacteria produced in the RDR were shown to display remarkable tolerance to 10 μg/ml of ciprofloxacin, thus mimicking the tolerance observed in recalcitrant bacterial infections. These studies further demonstrate that a nonmucoid strain of P. aeruginosa can form a biofilm that tolerates ciprofloxacin at clinically relevant concentrations. Neither asiatic acid nor corosolic acid reduced the viable cell density of P. aeruginosa biofilms. However, both compounds increased the susceptibility of biofilm bacteria to subsequent treatment with tobramycin, suggesting asiatic acid and corosolic acid to be compounds that potentiate the activity of antibiotics. A similar statistical interaction was observed between ciprofloxacin and subsequent treatment with tobramycin.
Pseudomonas aeruginosa airway infections are the leading cause of death in the cystic fibrosis (CF) patient population (17). The versatile gram-negative bacterium colonizes the airways of CF children soon after birth (6, 27), initiating an infection and massive immune response from the host that in turn will cause severe damage to the lung tissues (9, 21). Initial acute CF lung infections can be treated and eradicated by antibiotics. However, these infections are reoccurring and develop by 10 years of age into a serious chronic infection that can resist antibiotic therapies. The persistence of P. aeruginosa infections appears to be due essentially to the selection of strains resistant to antimicrobial therapy (14) and the presence of bacterial biofilms (8, 10, 30).
Standard susceptibility test methods for determining MICs using planktonic bacteria have been used to select for the most appropriate antibiotic combinations to treat CF airway infections (29). However, this approach has limited relevance as these methods do not consider the challenges posed by biofilms. An antibiofilm strategy is needed to manage the patient's care and to develop new drug leads.
Recently, different methods of determining biofilm susceptibility have been developed to address this problem (7, 12, 20). Biofilm inhibitory concentrations or minimum biofilm eradication concentrations appear in the literature for different antibiotics, which are as expected higher than the corresponding MICs determined by standard methods. In particular, penicillins and cephalosporins are generally ineffective against biofilms produced by these methods (7). However, biofilm inhibitory concentration and minimum biofilm eradication concentration values vary greatly among the different biofilm susceptibility test methods, suggesting that the characteristics of the biofilms are strongly related to the laboratory system used to grow them (20). The discrepancy between these results underscores the difficulty of selecting and developing true biofilm inhibitors as well as compounds that potentiate the activity of antibiotics against biofilms (22). Obviously, not only is it important to grow a bacterial biofilm, but it is also essential to make sure that it is developed in a system that produces antibiotic tolerances similar to those encountered in the clinic.
The present study was performed first to examine the susceptibility of P. aeruginosa biofilm bacteria to ciprofloxacin and tobramycin in the rotating disk reactor (RDR) (4, 14, 34). The susceptibility of biofilm bacteria to ciprofloxacin was of interest because it has been difficult to grow P. aeruginosa biofilms that can tolerate ciprofloxacin at concentrations higher than 1 μg/ml (5), which is the MIC of ciprofloxacin on planktonic P. aeruginosa PAO1 (data not shown). Maximum concentrations of ciprofloxacin in serum and epithelial lining fluid of adults have been shown to be approximately 2 μg/ml (13). This suggests that this concentration of ciprofloxacin is unable to eradicate a chronic biofilm infection. Therefore, a need exists to find a biofilm model that can tolerate concentrations of ciprofloxacin greater than 2 μg/ml.
The RDR was also used to determine the susceptibility of P. aeruginosa biofilms to asiatic acid and corosolic acid (Fig. 1), two compounds isolated from a library of natural products (11). These compounds were identified as biofilm inhibitors during the screening of the library in a high-throughput biofilm assay using 96-well microtiter plates. The biofilm inhibition ability of asiatic acid and corosolic acid analogs has been previously reported by our groups (16, 25). The microtiter plate assay selects for compounds that reduce the formation of biofilms but does not test for potential effects on established biofilms. The RDR assay was chosen as a basis for a secondary screen that can assess the efficacy of a compound to either reduce mature biofilms alone or potentiate the activities of antibiotics. An important goal of this project was to evaluate asiatic acid and corosolic acid for their potential to enhance the susceptibility of established P. aeruginosa biofilm bacteria to tobramycin treatment.
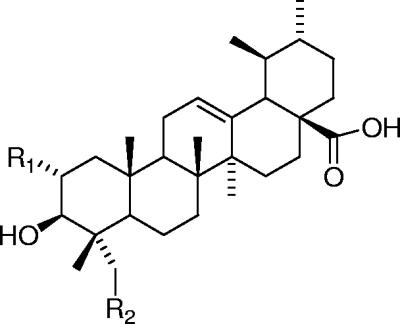
FIG. 1.
Structures of asiatic acid (R1 = R2 = OH), corosolic acid (R1 = OH; R2 = H), and ursolic acid (R1 = R2 = H).
The RDR was originally developed by the Center for Biofilm Engineering as a laboratory model system to evaluate the efficacy of biocides against toilet bowl biofilms (23, 34). This system was further developed by the Center for Biofilm Engineering as a standardized biofilm test method (35) and accepted by the American Society of Testing and Materials (ASTM) as a standard test method for growing repeatable P. aeruginosa biofilms in 2002 (designation E-2196-02). Other than the original toilet studies and method development work, there have been few reports in the literature of research conducted using the RDR system. Thus, it was of interest to examine the repeatability of the method in this application.
MATERIALS AND METHODS
Chemicals.
Corosolic acid was identified as previously described from Diospyros dendo, commonly called Gabon ebony (16). Additional quantities of corosolic acid were purchased from Chromadex (Santa Ana, CA). Asiatic acid was purchased from LKT Laboratories (St Paul, MN).
RDR biofilm experiments.
The RDR (Biosurface Technologies Corporation, Bozeman, MT) consists of a 1-liter glass beaker fitted with a drain spout. The bottom of the vessel contains a magnetically driven rotor with six 1.27-cm-diameter coupons. The coupons can be made of various materials determined by system requirements such as stainless steel or polyurethane. For the purposes of these experiments, the coupons were constructed from polystyrene. This surface was chosen to be consistent with initial high-throughput screening tests, which utilized 96-well polystyrene microtiter plates. The rotor consisted of a star-head magnetic stir bar upon which a disk was affixed to hold the coupons. The vessel with the stir bar was placed on a stir plate and rotated at approximately 200 rpm. A nutrient solution (AB trace medium with 0.3 mM glucose [18]) was added through a stopper in the top of the reactor at a flow rate of 3 ml/min. The reactor volume was approximately 180 ml, varying slightly between reactors depending on the placement of the drain spout and the rotational speed of the rotor. At a volume of 180 ml, the hydraulic residence time of nutrient solutions in the reactors was 60 min. The reactors were operated at room temperature (ca. 26°C).
For each test, two RDRs were operated in parallel, with one receiving the test compound and the other serving as an untreated control. The RDRs were sterilized by autoclaving and then filled with sterile medium and inoculated with P. aeruginosa strain PAO1 per the ASTM method (designation E-2196-02). The reactors were then incubated at room temperature in batch mode (no medium flow) for a period of 24 h, after which flow was initiated for an additional 24-h incubation. Fluid shear was maintained throughout the experiments including batch incubation, flow incubation, and treatment by rotating the stir bar, as described above. Test compounds were dissolved in 10 ml ethanol or 1.0 ml dimethyl sulfoxide to achieve a concentration of 1.8 mg/ml or 18 mg/ml, respectively. After the 48 h of biofilm development described above, the test compound was added to the reactor to achieve a final concentration of approximately 100 μg/ml. Control reactors received 10 ml ethanol or 1.0 ml dimethyl sulfoxide without test compounds. The reactors were then incubated for an additional 24 h in batch (no flow) mode. After this incubation period, the six coupons were removed from each reactor and placed in sterile 12-well polystyrene tissue culture plates with wells containing either 2 ml of a 100-μg/ml tobramycin solution or 2 ml of phosphate-buffered saline (PBS). These plates were incubated at room temperature for 2 hours. The coupons were then rinsed three times by transferring them to plates containing 2 ml of fresh PBS.
For each pair of RDRs run in parallel, four sets of three coupons were obtained: (i) the test compound and tobramycin combined, (ii) the test compound alone, (iii) tobramycin alone (positive control), and (iv) no treatment (negative control). The coupons were placed in 10 ml of PBS and sonicated for 5 minutes to dislodge and disperse biofilm cells. The resulting bacterial suspensions were then serially diluted in PBS and plated on tryptic soy agar plates for enumeration of culturable bacteria. The plates were incubated for 24 h at 37°C before CFU were determined. The results were expressed as the viable cell density per coupon surface area (CFU/cm2).
Experiments were also done with ciprofloxacin at 10 μg/ml applied simultaneously with asiatic acid at either 10 μg/ml, 50 μg/ml, or 100 μg/ml. After 2 hours, the coupons were rinsed and treated as described above.
LD and LR.
Viable cell density for each coupon was measured for each set of experiments including treatment and control. For purposes of statistical analysis, each density was log10 transformed to create a log density (LD) value. The LD values for each treatment were averaged across coupons, resulting in a mean LD. For the active treatments, the log reduction (LR) was calculated by subtracting the mean LD for the active treatment from the mean LD for the negative control.
Positive and negative controls.
To monitor the antibiotic resistance of biofilms from experiments performed weeks and months apart, each experiment included a positive control, 100 μg/ml of tobramycin, applied for 2 hours. This tobramycin regimen was established for two reasons. The bactericidal activities of aminoglycoside antibiotics are concentration dependent, so we selected a concentration of tobramycin that was expected to produce a positive but small LR (≤2). Secondly, data suggest that 90% of tobramycin concentrations in the lung tissue of CF patients are cleared within 2 hours, so we selected a similar exposure period (19).
The RDR protocol required that the mean LD for negative, untreated control coupons be at least 7.0. The protocol also required that the positive control, tobramycin, produce an LR no greater than 2.0 to ensure a consistent level of tobramycin resistance among the experiments.
Statistical interaction.
It was of interest to determine whether the tobramycin effect was enhanced when tobramycin was applied in combination with the test compound: that is, to determine whether there was a positive interaction between the test compound and tobramycin. The quantitative measure of interaction was the negative of the statistical interaction effect conventionally used when conducting an analysis of variance (24): interaction = −(mean LD for the test compound and tobramycin) + (mean LD for the test compound alone) + (mean LD for tobramycin alone) − (mean LD for negative control). An alternative, mathematically equivalent formula for interaction can be expressed in terms of the LR values: interaction = (LR for the test compound and tobramycin) − (LR for the test compound alone) − (LR for tobramycin alone).
Many different formulas for quantifying the concept of “synergism” between two treatments appear in the literature. Among those formulas, statistical interaction is a commonly used “effect additive” definition. In the context of evaluating synergism, a positive statistical interaction indicates synergism, a negative interaction indicates antagonism, and a zero interaction, which is the null value for significance testing, indicates absence of either synergism or antagonism. An important special case of synergism, known as potentiation, occurs when the test compound has no effect by itself (LR = 0) and it produces a positive statistical interaction with tobramycin. After the statistical interaction was calculated, a t test was performed as described below to determine if statistical significance was achieved.
Repeatability and statistics.
Upper one-tailed t tests were used to test for a statistically significant effect, LR or interaction. The key experiments were repeated two or three times. For those experiments, it was possible to calculate the repeatability standard deviation (Sr) for an effect, where the effect is either LR or interaction. The Sr is the typical difference, sign neglected, between the effect for a randomly chosen experiment and the mean effect over all experiments. A small Sr indicates good repeatability. In some cases, it was possible to use a random-effect analysis of variance to partition the repeatability into within-experiment and between-experiment variances. In testing interactions, the pooled Sr was used. All statistical calculations were performed in either R (http://cran.r-project.org/), Minitab, or MS Excel.
CDC biofilm reactor system.
CDC biofilm reactors (Biosurface Technologies Corporation, Bozeman, MT) have been demonstrated to be a repeatable and rugged system for growing bacterial biofilms (12). The CDC biofilm reactor consists of a 1-liter glass vessel with eight polypropylene coupon holders suspended from ported lids. Each vertical rod holds three coupons for a total of 24 coupons per reactor. The operational fluid capacity of the reactor was approximately 350 ml. A fluid shear force of approximately 0.02 N/m2 was generated in the reactor by rotating the stir bar at approximately 125 rpm. Reactor operation for biofilm growth (24-h batch culture/24-h continuous flow) and coupon treatments were identical to those described above for the RDR experiments. During continuous flow biofilm growth, medium was supplied at a rate of 13 ml/min to provide a hydraulic residence time of approximately 27 min. Other experimental conditions (coupons, inoculum, and medium composition) as well as coupon analyses were identical to those described for the RDR experiments.
RESULTS
Biofilms grown in the RDR system.
Ciprofloxacin at 10 μg/ml was tested in three RDR experiments, producing negligible and small negative LR values of −0.71, −0.13, and −0.02. This is the first reported demonstration of the complete tolerance of a P. aeruginosa laboratory biofilm to 10 μg/ml of ciprofloxacin. The positive-control tobramycin for these experiments produced a mean LR of 0.4, achieving our stated criteria (Table 1). Coupons treated sequentially with 10 μg/ml of ciprofloxacin and tobramycin produced a mean LR of 1.6, higher than the LR for tobramycin treatment alone (Table 1). The mean statistical interaction between 10 μg/ml of ciprofloxacin and tobramycin was 1.5 (P < 0.01).
TABLE 1.
Statistical summary (estimate ± standard error of the mean) for LR and interaction estimates obtained in RDR experiments with 10-μg/ml ciprofloxacin
| Effect and drug(s) | Value for ciprofloxacin (3 expts) |
|---|---|
| LR | |
| Tobramycin and ciprofloxacin | 1.60 ± 0.36 |
| Ciprofloxacin | −0.29 ± 0.44 |
| Tobramycin | 0.40 ± 0.25 |
| Interaction of tobramycin and ciprofloxacin (Pa) | 1.48 ± 0.34 (<0.01) |
One-tailed t test of the null hypothesis that the true interaction is ≤0.
When applied alone, each of the asiatic acid and corosolic acid treatments produced negligibly small LR values (Table 2). However, 100 μg/ml of asiatic acid and corosolic acid each demonstrated significant statistical interactions with tobramycin. Although 10-μg/ml and 50-μg/ml concentrations of asiatic acid in combination with tobramcyin produced a greater LR than tobramycin alone did, the interaction effects were not statistically significant. The MICs of corosolic acid and asiatic acid for P. aeruginosa were determined to be greater than 128 μg/ml (data not shown).
TABLE 2.
Statistical summary (mean ± standard error of the mean) for LR and interaction estimates obtained in RDR experiments with each concentration of the two test compounds asiatic acid and corosolic acida
| Effect and drug(s) | Value for concn (μg/ml) of drug (no. of expts)
|
||||
|---|---|---|---|---|---|
| Asiatic acid
|
Corosolic acid
|
||||
| 10 (n = 3) | 50 (n = 2) | 100 (n = 3) | 50 (n = 1) | 100 (n = 3) | |
| LR | |||||
| Tobramycin and test compound | 2.24 ± 0.17 | 1.95 ± 0.09 | 2.76 ± 0.55 | 1.11 ± 0.27 | 2.21 ± 0.25 |
| Test compound | 0.14 ± 0.17 | 0.12 ± 0.16 | 0.19 ± 0.31 | −0.04 ± 0.07 | 0.02 ± 0.22 |
| Tobramycin | 1.51 ± 0.33 | 1.43 ± 0.09 | 1.52 ± 0.39 | 0.78 ± 0.07 | 1.36 ± 0.31 |
| Interaction of tobramycin and test compound (Pb) | 0.59 ± 0.34 (0.052) | 0.40 ± 0.41 (0.17) | 1.05 ± 0.34 (<0.01) | 0.37 ± 0.58 (0.27) | 0.83 ± 0.34 (0.015) |
The standard error of the mean is based on the Sr.
One-tailed t test of the null hypothesis that the true interaction is ≤0.
Asiatic acid in combination with ciprofloxacin.
When asiatic acid at concentrations of 50 or 100 μg/ml was applied in combination with 10 μg/ml of ciprofloxacin, the observed mean LR values were 1.9 and 1.4, respectively (Table 3). Comparing these LR values to the 10-μg/ml ciprofloxacin LR value in Table 1 and the asiatic acid LR values in Table 2, there is an apparent interaction between ciprofloxacin and asiatic acid at both concentrations.
TABLE 3.
Statistical summary (estimate ± standard error of the mean) for LR and interaction estimates obtained in RDR experiments with each concentration of asiatic acid plus 10-μg/ml ciprofloxacin
| Effect and drug(s) | Value for concn (μg/ml) of asiatic acid (no. of expts)
|
|
|---|---|---|
| 50 (n = 2) | 100 (n = 3) | |
| LR | ||
| Tobramycin, test compound, and ciprofloxacin | 3.07 ± 0.55 | 2.74 ± 0.62 |
| Test compound and ciprofloxacin | 1.88 ± 0.34 | 1.36 ± 0.41 |
| Tobramycin | 0.96 ± 0.38 | 1.41 ± 0.37 |
| Interaction of tobramycin, test compound, and ciprofloxacin (Pa) | 0.24 ± 0.41 (0.29) | −0.03 ± 0.34 (0.53) |
One-tailed t test of the null hypothesis that the true interaction is ≤0.
Repeatability.
The mean LD of the untreated control coupons (n = 54) in these experiments was 7.7, similar to previously reported RDR experiments with P. aeruginosa (34), even though a different growth medium and test surface were used. The Sr was 0.53, which is higher than the value of 0.27 reported by Zelver et al. (34) but near that stated in the standard method (0.5, ASTM, E-2196-02). In the current study, the total variance was 73% attributable to variation between experiments and 26% attributable to variance within an experiment.
In this study, biofilms treated with tobramycin only were subjected to a 100-μg/ml concentration for 2 hours. Across 20 experiments, the LR ranged from 0.4 to 1.9 with a mean (± standard error of the mean) of 1.2 (±0.09). The Sr of the tobramycin LR values was 0.46, which is lower than that reported from previous antibiofilm tests with the RDR (35) and well within the range (0.3 to 1.5; median, 0.9) of established standard antimicrobial tests (32). In the current study, interaction estimates were also determined for coupons receiving multiple treatments. The pooled Sr for interaction values was 0.58.
Biofilms grown in the CDC reactor system.
For biofilms grown in the CDC reactor system, LR values for asiatic acid and corosolic acid were calculated and compared to those for biofilms grown in the RDR system. Asiatic acid and corosolic acid were added to CDC reactors at the same concentrations as in the RDR, at 50 μg/ml and 100 μg/ml, respectively. Asiatic acid and corosolic acid each produced small LR values of 0.8 and 0.7, respectively (data not shown). When combined with tobramycin, asiatic acid at 50 μg/ml produced an LR of 2.9 and corosolic acid at 100 μg/ml produced an LR of 3.7. Although the LR and interaction values are slightly higher for the CDC biofilm than for the RDR biofilm, the CDC results are consistent with the RDR results.
DISCUSSION
Using the RDR technique as a model system, we were able for the first time to produce a nonmucoid P. aeruginosa PAO1 biofilm that displayed resistance to 10-μg/ml ciprofloxacin, which corresponds to 10 times its planktonic MIC. Our data suggest that the RDR system provides a relevant system to study the susceptibility of biofilms to antibiotics and other novel test compounds. In this regard some parameters of the RDR model, namely, shear forces and nutrient limitation (26), appear to be essential for the production of a rugged biofilm (1, 12). These results further suggest that mucoidy is not required to obtain resistance to ciprofloxacin at clinically significant concentrations. A previous report demonstrated that P. aeruginosa PAO1 produces alginate when treated with imipenem, and so alginate production may have occurred during our experiments (2).
Asiatic acid and corosolic acid exhibited positive interactions with tobramycin, indicating that these two natural products reduce the tolerance of P. aeruginosa biofilm bacteria to antibiotics. Moreover, when P. aeruginosa biofilms were grown in the presence of 10 μg/ml of asiatic acid, they became more susceptible to 10 μg/ml of ciprofloxacin. These data suggest that asiatic acid and its analogs are compounds that potentiate the activity of antibiotics. These results are very encouraging and suggest further study with asiatic acid and its analogs to establish its activity using a mucoid strain of P. aeruginosa in the RDR system and to determine as well the exact mechanism of action of these compounds.
Ursolic acid, a triterpene closely related to asiatic acid, demonstrated biofilm inhibition in our 96-well plate assay but did not demonstrate a statistical interaction with tobramycin in the RDR model (data not shown). Ursolic acid has been shown to modulate the expression of the cysB gene in Escherichia coli (25). In these studies, a cysB isogenic mutant produced different biofilms than the wild type did. Asiatic acid, the most potent triterpene tested in this study, was also shown to modulate the expression of the cysB gene in microarray experiments (data not shown). CysB is a LysR transcriptional regulator that controls the expression of genes involved in the biosynthesis of cysteine (33). In addition, cysB has been demonstrated to be involved in the control of acid habituation in E. coli and responses to O-acetyl-l-serine and an unknown signal in conditioned medium (28, 31). Thus, the CysB pathway is an interesting potential target pathway of asiatic acid and its analogs in these biofilm assays. Future studies will investigate the mechanism of action of theses compounds by looking at this and other related pathways. It should be noted that these compounds appear to possess a different mechanism of action than that of furanone 56 (15) or garlic extract (3), either of which is known to modulate quorum sensing.
It is interesting that the combination of asiatic acid at 10 μg/ml with tobramycin produced an LR similar to that for the combination of ciprofloxacin at 10 μg/ml with tobramycin. The MICs of asiatic acid and ciprofloxacin are 128 μg/ml and 1 μg/ml, respectively. Since ciprofloxacin at 10 μg/ml was ineffective by itself, its MIC against planktonic bacteria alone did not predict its effectiveness against biofilm bacteria. This further suggests that in addition to its antibacterial properties ciprofloxacin may potentiate the activity of biofilm inhibitors.
The CDC reactor experiments confirmed the interaction between asiatic acid and tobramycin that was observed in the RDR experiments. The CDC reactor and the RDR systems produced biofilms with similar antibiotic tolerances.
Overall, these results were as repeatable as previously reported tests using the RDR system. Even though slightly different conditions were used, these experiments helped to validate the ASTM standard method (E-2196-02) for developing repeatable P. aeruginosa biofilms and the utility of these biofilms for evaluating antimicrobial efficacy. Finally, these data also suggest that our strategy for the identification of new biofilm inhibitors and potentiators appears successful, as the most and least potent compounds in the 96-well plate screen were also the most and least potent compounds tested in the RDR model. Screening in a 96-well plate format and then confirming the activity in the RDR model is a good approach for the discovery and development of biofilm inhibitors and potentiators.
Acknowledgments
Sequoia Sciences gratefully acknowledges the government of Gabon; L. Nze at IPHAMETRA/CENAREST for permission to collect the plants that were the sources of the natural products presented in this report; and J. Stone, A. Bradley, G. Walters, and J. Miller from the Missouri Botanical Garden for the collection and identification of the plants. We thank C. Hung, S. Martin, and M. O'Neil-Johnson from Sequoia Sciences for their valuable comments on the manuscript and J.-F. Hu for confirming the structure of corosolic acid from D. dendo.
This project was partially supported by the NIH under the STTR grant R42RR016363-02.
Footnotes
Published ahead of print on 12 March 2007.
REFERENCES
- 1.Anderl, J. N., J. Zahller, F. Roe, and P. S. Stewart. 2003. Role of nutrient limitation and stationary-phase existence in Kleibsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chemother. 471251-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagge, N., M. Schuster, M. Hentzer, O. Ciofu, M. Givskov, E. P. Greenberg, and N. Hoiby. 2004. Pseudomonas aeruginosa biofilms exposed to imipenem exhibit changes in global gene expression and beta-lactamase and alginate production. Antimicrob. Agents Chemother. 481175-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjarnsholt, T., P. O. Jensen, T. B. Rasmussen, L. Christophersen, H. Calum, M. Hentzer, H.-P. Hougen, J. Rygaard, C. Moser, L. Eberl, N. Hoiby, and M. Givskov. 2005. Garlic blocks quorum sensing and promotes rapid clearing of pulmonary Pseudomonas aeruginosa infections. Microbiology 1513873-3880. [DOI] [PubMed] [Google Scholar]
- 4.Boles, B. R., M. Thoendel, and P. K. Singh. 2004. Self-generated diversity produces “insurance effects” in biofilm communities. Proc. Natl. Acad. Sci. USA 10116630-16635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borriello, G., L. Richards, G. D. Ehrlich, and P. S. Stewart. 2006. Arginine or nitrate enhances antibiotic susceptibility of Pseudomonas aeruginosa in biofilms. Antimicrob. Agents Chemother. 50382-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burns, J. L., R. L. Gibson, S. McNamara, D. Yim, J. Emerson, M. Rosenfeld, P. Hiatt, K. McCoy, R. Castile, A. L. Smith, and B. W. Ramsey. 2001. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J. Infect. Dis. 183444-452. [DOI] [PubMed] [Google Scholar]
- 7.Ceri, H., M. E. Olson, C. Stremick, R. R. Read, D. Morck, and A. Buret. 1999. The Calgary biofilm device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 371771-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 2841318-1322. [DOI] [PubMed] [Google Scholar]
- 9.Dakin, C. J., A. H. Numa, H. E. Wang, J. R. Morton, C. C. Vertzyas, and R. L. Henry. 2002. Inflammation, infection, and pulmonary function in infants and young children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 165904-910. [DOI] [PubMed] [Google Scholar]
- 10.Drenkard, E., and F. M. Ausubel. 2002. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature 416740-743. [DOI] [PubMed] [Google Scholar]
- 11.Eldridge, G., H. C. Vervoort, C. M. Lee, P. A. Cremin, C. T. Williams, S. M. Hart, M. G. Goering, M. O'Neil-Johnson, and L. Zeng. 2002. High-throughput method for the production and analysis of large natural product libraries for drug discovery. Anal. Chem. 743963-3971. [DOI] [PubMed] [Google Scholar]
- 12.Goeres, D. M., L. R. Loetterle, M. A. Hamilton, R. Murga, D. W. Kirby, and R. M. Donlan. 2005. Statistical assessment of a laboratory method for growing biofilms. Microbiology 151757-762. [DOI] [PubMed] [Google Scholar]
- 13.Gotfried, M. H., L. H. Danziger, and K. A. Rodvold. 2001. Steady-state plasma and intrapulmonary concentrations of levofloxacin and ciprofloxacin in healthy adult subjects. Chest 1191114-1122. [DOI] [PubMed] [Google Scholar]
- 14.Hentzer, M., G. M. Teitzel, G. J. Balzer, A. Heydorn, S. Molin, M. Givskov, and M. R. Parsek. 2001. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J. Bacteriol. 1835395-5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hentzer, M., K. Riedel, T. B. Rasmussen, A. Heydorn, J. B. Andersen, M. R. Parsek, S. A. Rice, L. Eberl, S. Molin, N. Hoiby, S. Kjelleberg, and M. Givskov. 2002. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by halogenated furanone compound. Microbiology 14887-102. [DOI] [PubMed] [Google Scholar]
- 16.Hu, J.-F., E. Garo, M. G. Goering, M. Pasmore, H.-D. Yoo, T. Esser, J. Sestrich, P. A. Cremin, G. W. Hough, P. Perrone, Y.-S. Lee, N.-T. Le, M. O'Neil-Johnson, J. W. Costerton, and G. R. Eldridge. 2006. Bacterial biofilm inhibitors from Diospyros dendo. J. Nat. Prod. 69118-120. [DOI] [PubMed] [Google Scholar]
- 17.Hudson, V. L., C. L. Wielinski, and W. E. Regelmann. 1993. Prognostic implications of initial oropharyngeal bacterial flora in patients with cystic fibrosis diagnosed before the age of two years. J. Pediatr. 122854-860. [DOI] [PubMed] [Google Scholar]
- 18.Mathee, K., O. Ciofu, C. Sternberg, P. W. Lindum, J. I. A. Campbell, P. Jensen, A. H. Johnsen, M. Givskov, D. E. Ohman, S. Molin, N. Hoiby, and A. Kharazmi. 1999. Mucoid conversion of Pseudomonas aeruginosa by hydrogen peroxide: a mechanism for virulence activation in the cystic fibrosis lung. Microbiology 1451349-1357. [DOI] [PubMed] [Google Scholar]
- 19.Medical Economics Staff. 2001. Physicians' desk reference, p. 2463. Thomson Healthcare, Montvale, NJ.
- 20.Moskowitz, S. M., J. M. Foster, J. Emerson, and J. L. Burns. 2004. Clinically feasible biofilm susceptibility assay for isolates of Pseudomonas aeruginosa from patients with cystic fibrosis. J. Clin. Microbiol. 421915-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muhlebach, M. S., P. W. Stewart, M. W. Leigh, and T. L. Noah. 1999. Quantitation of inflammatory responses to bacteria in young cystic fibrosis and control patients. Am. J. Respir. Crit. Care Med. 160186-191. [DOI] [PubMed] [Google Scholar]
- 22.Parsek, M. R., and C. Fuqua. 2004. Biofilms 2003: emerging themes and challenges in studies of surface-associated microbial life. J. Bacteriol. 1864427-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pitts, B., A. Willse, G. A. McFeters, M. A. Hamilton, N. Zelver, and P. S. Stewart. 2001. A repeatable laboratory method for testing the efficacy of biocides against toilet bowl biofilms. J. Appl. Microbiol. 91110-117. [DOI] [PubMed] [Google Scholar]
- 24.Rao, P. V. 1998. Statistical research methods in the life sciences. Duxbury Press, Pacific Grove, CA.
- 25.Ren, D., R. Zuo, A. F. González Barrios, L. A. Bedzyk, G. R. Eldridge, M. E. Pasmore, and T. K. Wood. 2005. Differential gene expression for investigation of Escherichia coli biofilm inhibition by plant extract ursolic acid. Appl. Environ. Microbiol. 714022-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts, M. E., and P. S. Stewart. 2004. Modeling antibiotic tolerance in biofilm by accounting for nutrient limitation. Antimicrob. Agents Chemother. 4848-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenfeld, M., R. L. Gibson, S. McNamara, J. Emerson, J. Burns, R. Castile, P. Hiatt, K. McCoy, C. B. Wilson, A. Inglis, A. Smith, T. R. Martin, and B. W. Ramsey. 2001. Early pulmonary infection, inflammation, and clinical outcomes in infants with cystic fibrosis. Pediatr. Pulmon. 32356-366. [DOI] [PubMed] [Google Scholar]
- 28.Rowbury, R. L. 2001. Cross-talk involving extracellular sensors and extracellular alarmones gives early warning to unstressed Escherichia coli of impending lethal chemical stress and leads to induction of tolerance responses. J. Appl. Microbiol. 90677-695. [DOI] [PubMed] [Google Scholar]
- 29.Saiman, L., J. L. Burns, S. Whittier, J. Krzewinski, S. A. Marshall, and R. N. Jones. 1999. Evaluation of reference dilution test methods for antimicrobial susceptibility testing of Pseudomonas aeruginosa strains isolated from patients with cystic fibrosis. J. Clin. Microbiol. 372987-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh, P. K., A. L. Schaefer, M. R. Parsek, T. O. Moninger, M. J. Welsch, and E. P. Greenberg. 2000. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407762-764. [DOI] [PubMed] [Google Scholar]
- 31.Sturgill, G., C. M. Toutain, J. Komperda, G. A. O'Toole, and P. N. Rather. 2004. Role of CysE in production of an extracellular signaling molecule in Providencia stuartii and Escherichia coli: loss of cysE enhances biofilm formation in Escherichia coli. J. Bacteriol. 1867610-7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tilt, N., and M. A. Hamilton. 1999. Repeatability and reproducibility of germicide tests: a literature review. J. AOAC Int. 82384-389. [PubMed] [Google Scholar]
- 33.Verschueren, K. H. G., C. Addy, E. J. Dodson, and A. J. Wilkinson. 2001. Crystallization of full-length CysB of Klebsiella aerogenes, a LysR-type transcriptional regulator. Acta Crystallogr. 57260-262. [DOI] [PubMed] [Google Scholar]
- 34.Zelver, N., M. Hamilton, B. Pitts, D. Goeres, D. Walker, P. Sturman, and J. Heersink. 1999. Measuring antimicrobial effects on biofilm bacteria: from laboratory to field. Methods Enzymol. 310608-628. [DOI] [PubMed] [Google Scholar]
- 35.Zelver, N., M. Hamilton, B. Pitts, D. Goeres, D. Walker, P. Sturman, and J. Heersink. 2001. Development of a standardized antibiofilm test. Methods Enzymol. 337363-376. [DOI] [PubMed] [Google Scholar]