Abstract
To evaluate the effect of fosfomycin on proinflammatory cytokines, a bolus of 2 ng of bacterial lipopolysaccharide/kg of body weight was injected intravenously into healthy volunteers. After 2 h, subjects received 8 g of fosfomycin or placebo in a randomized crossover study design. The resulting concentrations of tumor necrosis factor alpha, interleukin-1β (IL-1β), and IL-6 expressed as protein and mRNA levels were almost identical with and without fosfomycin.
Despite the availability of potent antibiotics for the treatment of septic patients, the overwhelming inflammatory response to infection or bacterial endotoxin (lipopolysaccharide [LPS]) remains a major problem due to frequent development of multiorgan failure (1, 20). Tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), IL-6, and IL-8 have been identified as important mediators of endotoxin shock (15, 22). Several therapeutic strategies to reduce the severity of sepsis by decreasing proinflammatory cytokines are under investigation (4, 5).
Fosfomycin (FOF), a broad-spectrum bactericidal antibiotic with favorable penetration properties, is approved in distinct member states of the European Union for the therapy of soft-tissue infections and sepsis (7, 11). Renewed attention has been paid to FOF because of its effects on the acute inflammatory cytokine response in vitro as well as in vivo in mice (8, 15, 18, 19). Since these effects might be beneficial also in septic patients, the present study was carried out to quantify the immunomodulatory properties of FOF in vivo during experimental endotoxemia in humans induced by the administration of bacterial LPS (10, 16).
This prospective, controlled, two-sequence, one-period, randomized, and analyst-blinded study was performed according to a crossover design. The ages of the 12 healthy male subjects ranged between 18 and 40 years. The sample size was estimated, enabling detection of a 20% difference in main outcome values with a power of 80% (21). LPS derived from Escherichia coli (National Reference Endotoxin; United States Pharmacopeia, Rockville, MD) was administered intravenously as a bolus at a dosage of 2 ng/kg of body weight on 2 study days, which were separated by a washout period of 1 week. After injection of the LPS bolus, most volunteers felt sick and developed symptoms of systemic inflammation, such as chills, fever, and headache. Two hours after the administration of LPS, subjects received 8 g of FOF (Sandoz, Kundl, Austria) on one study day (either study day 1 or day 2, randomly allocated) and placebo (Ringer's solution; Mayrhofer Pharma Gesellschaft, Linz, Austria) on the other study day. Blood samples were taken before and 2, 4, 8, 12, and 24 h after LPS administration. No serious adverse events occurred during study days.
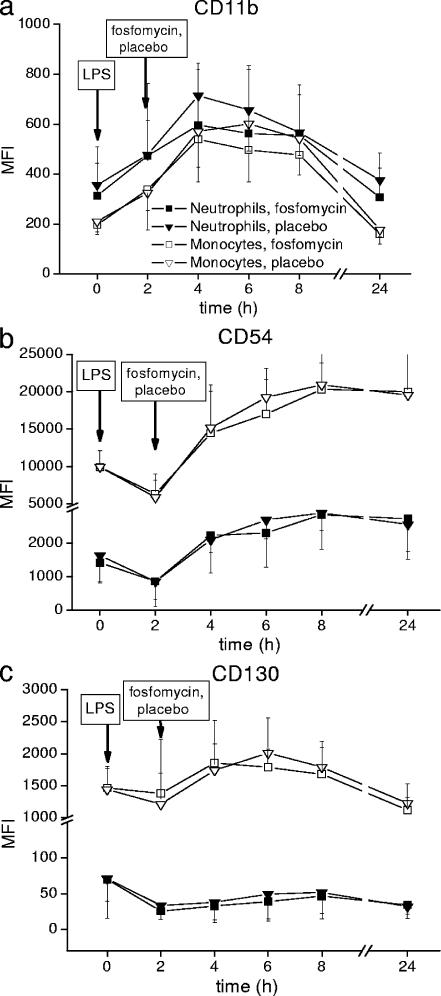
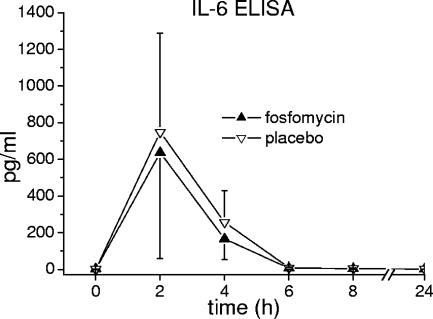
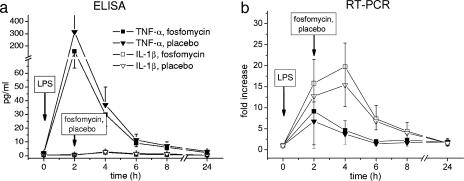
Expression of cell surface activity markers CD11b, CD54, and CD130 on neutrophil granulocytes and monocytes was measured on a FACSCalibur flow cytometer (Becton Dickinson, Vienna, Austria) and expressed as mean fluorescence intensity. Expression of CD11b, CD54, and CD130 over time is shown in Fig. 1. IL-6, IL-1β, and TNF-α were measured as protein levels in plasma by high-sensitivity enzyme immunoassays (R&D Systems, Abingdon, United Kingdom). The concentration-versus-time profiles of IL-6, IL-1β, and TNF-α in plasma are shown in Fig. 2 and 3a. mRNA levels of IL-1β and TNF-α were quantified by real-time PCR using an ABI PRISM 7700 apparatus and commercially available primers and probes (Applied Biosystems, Foster City, CA). Tested mRNA was normalized against the reference gene (18S) according to the 2−ΔΔCT method (13). Changes of TNF-α and IL-1β in relation to mRNA baseline levels over time were expressed as fold increases (Fig. 3b). Evidently, the profiles of the tested markers were almost the same after administration of placebo or FOF (P > 0.05). Also, counts of neutrophils, monocytes, lymphocytes, and platelets over time (determined using a Sysmex [Milton Keynes, United Kingdom] cell counter) were found to be similar to previously observed profiles in human experimental endotoxemia (16), independently of concomitant administration of FOF or placebo (data not shown). Thus, the pronounced immunomodulatory effects of FOF previously observed in vitro and in animals (8, 9, 14, 18, 19) were not reproduced in the present study.
FIG. 1.
Expression of leukocyte surface activity markers CD11b (a), CD54 (b), and CD130 (c) in healthy humans during experimental endotoxemia with and without subsequent administration of FOF. MFI, mean fluorescence intensity.
FIG. 2.
Effect of FOF on levels of IL-6 in plasma during experimental endotoxemia in healthy humans. ELISA, enzyme-linked immunosorbent assay.
FIG. 3.
Effect of FOF on IL-1β and TNF-α expressed as protein level (a) and mRNA level (b) during experimental endotoxemia in healthy humans. ELISA, enzyme-linked immunosorbent assay.
What is the reason for the discrepancy between the striking previous data and our findings? First, the pronounced effects of FOF observed in distinct in vitro studies may be based in part on the significantly higher amounts of LPS applied in vitro or the use of other agents to stimulate leukocytes (8, 19). Second, in experimental models the consecutive order of administration of LPS and FOF exerts a major impact on immunological processes. It is a major limitation of many previous experiments that FOF was administered prior to LPS injection (8, 17, 18), because in clinical practice patients become septic first and are treated subsequently with antibiotics.
Yet, the dose and the order of injection of LPS and FOF are not the only reasons for the difference in findings in the cited studies. In mice, survival rates were significantly improved (80 versus 30%) and levels of TNF-α, IL-1β, and IL-6 were reduced if the antimicrobially inactive enantiomer FOF(+) was administered several days after initiation of gut-derived sepsis (15). FOF(+) was injected into mice intraperitoneally at doses which were approximately 2.5-fold higher than the maximum single dose approved for humans (adjusted for body weight). Actually, it remains unclear to what extent the beneficial immunomodulatory effects of FOF which manifested in mice (15) can be attributed to gut-, rodent-, application-, or dose-specific properties or to the superiority of bacterial inoculation over an endotoxemia model.
The major limitation of the present study is the fact that injection of purified LPS alone cannot completely simulate septic shock because actually both LPS-induced cytokine reactions and bacterial inoculation per se are involved in sepsis and mortality (12). Moreover, in septic patients LPS is released in a delayed manner, depending, e.g., on bacterial killing, species, and inoculum, and not as a single bolus.
In spite of these limitations, the present findings are in line with clinical experience showing that fully activated cytokine, complement, or coagulation cascades cannot be easily down-regulated by a single agent. Hitherto, efforts to decrease sepsis-related mortality by modulation of specific mediators (for example, prostaglandins, immunoglobulins, gamma interferon, or granulocyte colony-stimulating factor) or receptors have rendered only little, if any, clinical success (2, 3, 6).
In summary, there was no evidence that a therapeutic dose of FOF exerts immunomodulatory effects on IL-1β, TNF-α, and IL-6 during experimental endotoxemia in healthy humans.
Acknowledgments
We are indebted to Edith Lackner and to Christa Drucker for their essential contributions to this study.
Footnotes
Published ahead of print on 12 March 2007.
REFERENCES
- 1.Abraham, E. 1997. Therapies for sepsis. Emerging therapies for sepsis and septic shock. West. J. Med. 166195-200. [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham, E., P. F. Laterre, R. Garg, H. Levy, D. Talwar, B. L. Trzaskoma, B. Francois, J. S. Guy, M. Bruckmann, A. Rea-Neto, R. Rossaint, D. Perrotin, A. Sablotzki, N. Arkins, B. G. Utterback, and W. L. Macias. 2005. Drotrecogin alfa (activated) for adults with severe sepsis and a low risk of death. N. Engl. J. Med. 3531332-1341. [DOI] [PubMed] [Google Scholar]
- 3.Abraham, E., K. Reinhart, S. Opal, I. Demeyer, C. Doig, A. L. Rodriguez, R. Beale, P. Svoboda, P. F. Laterre, S. Simon, B. Light, H. Spapen, J. Stone, A. Seibert, C. Peckelsen, C. De Deyne, R. Postier, V. Pettila, A. Artigas, S. R. Percell, V. Shu, C. Zwingelstein, J. Tobias, L. Poole, J. C. Stolzenbach, and A. A. Creasey. 2003. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. JAMA 290238-247. [DOI] [PubMed] [Google Scholar]
- 4.Arndt, P., and E. Abraham. 2001. Immunological therapy of sepsis: experimental therapies. Intensive Care Med. 27(Suppl. 1)S104-S115. [DOI] [PubMed] [Google Scholar]
- 5.Carlet, J. 2001. Immunological therapy in sepsis: currently available. Intensive Care Med. 27(Suppl. 1)S93-S103. [DOI] [PubMed] [Google Scholar]
- 6.Cars, O., S. Molstad, and A. Melander. 2001. Variation in antibiotic use in the European Union. Lancet 3571851-1853. [DOI] [PubMed] [Google Scholar]
- 7.Frossard, M., C. Joukhadar, B. M. Erovic, P. Dittrich, P. E. Mrass, M. Van Houte, H. Burgmann, A. Georgopoulos, and M. Muller. 2000. Distribution and antimicrobial activity of fosfomycin in the interstitial fluid of human soft tissues. Antimicrob. Agents Chemother. 442728-2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honda, J., Y. Okubo, M. Kusaba, M. Kumagai, N. Saruwatari, and K. Oizumi. 1998. Fosfomycin (FOM: 1R-2S-epoxypropylphosphonic acid) suppress [sic] the production of IL-8 from monocytes via the suppression of neutrophil function. Immunopharmacology 39149-155. [DOI] [PubMed] [Google Scholar]
- 9.Ishizaka, S., H. Takeuchi, M. Kimoto, S. Kanda, and S. Saito. 1998. Fosfomycin, an antibiotic, possessed TGF-beta-like immunoregulatory activities. Int. J. Immunopharmacol. 20765-779. [DOI] [PubMed] [Google Scholar]
- 10.Jilma, B. 2005. Safety of endotoxin challenge in healthy volunteers: bradycardia. Intensive Care Med. 31496. [DOI] [PubMed] [Google Scholar]
- 11.Joukhadar, C., N. Klein, P. Dittrich, M. Zeitlinger, A. Geppert, K. Skhirtladze, M. Frossard, G. Heinz, and M. Muller. 2003. Target site penetration of fosfomycin in critically ill patients. J. Antimicrob. Chemother. 511247-1252. [DOI] [PubMed] [Google Scholar]
- 12.Kawaguchi, K., R. Hasunuma, S. Kikuchi, R. Ryll, K. Morikawa, and Y. Kumazawa. 2002. Time- and dose-dependent effect of fosfomycin on suppression of infection-induced endotoxin shock in mice. Biol. Pharm. Bull. 251658-1661. [DOI] [PubMed] [Google Scholar]
- 13.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25402-408. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto, T., K. Tateda, S. Miyazaki, N. Furuya, A. Ohno, Y. Ishii, Y. Hirakata, and K. Yamaguchi. 1999. Fosfomycin alters lipopolysaccharide-induced inflammatory cytokine production in mice. Antimicrob. Agents Chemother. 43697-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumoto, T., K. Tateda, S. Miyazaki, N. Furuya, A. Ohno, Y. Ishii, Y. Hirakata, and K. Yamaguchi. 1997. Immunomodulating effect of fosfomycin on gut-derived sepsis caused by Pseudomonas aeruginosa in mice. Antimicrob. Agents Chemother. 41308-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayr, F. B., A. Spiel, J. Leitner, C. Marsik, P. Germann, R. Ullrich, O. Wagner, and B. Jilma. 2005. Effects of carbon monoxide inhalation during experimental endotoxemia in humans. Am. J. Respir. Crit. Care Med. 171354-360. [DOI] [PubMed] [Google Scholar]
- 17.Morikawa, K., M. Nonaka, I. Torii, and S. Morikawa. 2003. Modulatory effect of fosfomycin on acute inflammation in the rat air pouch model. Int. J. Antimicrob. Agents 21334-339. [DOI] [PubMed] [Google Scholar]
- 18.Morikawa, K., H. Watabe, M. Araake, and S. Morikawa. 1996. Modulatory effect of antibiotics on cytokine production by human monocytes in vitro. Antimicrob. Agents Chemother. 401366-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morikawa, K., J. Zhang, M. Nonaka, and S. Morikawa. 2002. Modulatory effect of macrolide antibiotics on the Th1- and Th2-type cytokine production. Int. J. Antimicrob. Agents 1953-59. [DOI] [PubMed] [Google Scholar]
- 20.Parrillo, J. E., M. M. Parker, C. Natanson, A. F. Suffredini, R. L. Danner, R. E. Cunnion, and F. P. Ognibene. 1990. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann. Intern. Med. 113227-242. [DOI] [PubMed] [Google Scholar]
- 21.Stolley, P. D., and B. L. Strom. 1986. Sample size calculations for clinical pharmacology studies. Clin. Pharmacol. Ther. 39489-490. [DOI] [PubMed] [Google Scholar]
- 22.Takala, A., I. Nupponen, M. L. Kylanpaa-Back, and H. Repo. 2002. Markers of inflammation in sepsis. Ann. Med. 34614-623. [DOI] [PubMed] [Google Scholar]