Abstract
Miltefosine (hexadecylphosphocholine), the first oral drug against visceral leishmaniasis, triggered pneumococcal autolysis at concentrations higher than 2.5 μM. Bactericidal activity was also observed in cultures of other streptococci, although these failed to undergo lysis. The autolysis elicited by miltefosine can be attributed to triggering of the pneumococcal autolysin LytA.
The alarming rise in antibiotic resistance worldwide has fostered the search for new antibacterial drugs. Streptococcus pneumoniae, the main bacterium responsible for community-acquired pneumonia, meningitis, bacteremia, and otitis media, besides showing ever-increasing resistance to penicillin, is also acquiring resistance to other antimicrobial classes such as macrolides, tetracyclines, and sulfonamides. This situation has prompted the development of new anti-infectives for the treatment of pneumococcal infections, particularly those produced by multidrug-resistant pneumococci (19).
Choline is an absolute nutritional requirement for S. pneumoniae (42) and is a component of the teichoic and lipoteichoic acids present, respectively, in the cell wall and membrane of this notorious human pathogen (14). Choline serves as an anchor for a well-known family of surface proteins, the choline-binding proteins (CBPs), which are involved in key physiological functions of the bacterium, e.g., remodeling and lysis of the cell wall (26) or adhesion and evasion of complement pathways (2). In fact, elucidation of the three-dimensional structure of the choline-binding domain of several CBPs (12, 13, 17, 18) has led to the use of these proteins as targets for the design of antipneumococcal drugs (11).
The addition of 2% choline chloride to actively growing pneumococci promotes the release of some CBPs from the cell wall (38, 48) and/or the complete inhibition of CBPs with cell wall hydrolase activity (26). Miltefosine (hexadecylphosphocholine) is one of several alkyllysophospholipid derivatives collectively known as alkylphosphocholines that were originally developed as anticancer agents (6). The biocidal action of miltefosine against Leishmania species was demonstrated in the mid 1980s (3, 7). Since then, trials for its clinical evaluation have led to the licensing of miltefosine for the oral treatment of visceral leishmaniasis in India, Colombia, and Germany (37).
Given that miltefosine features a phosphocholine group (Fig. 1), we speculated that this drug might be able to release and/or inhibit pneumococcal CBPs in a manner similar to that of choline itself. We report here that miltefosine shows noticeable bacteriolytic or bactericidal action when added to pneumococcal cultures and that this effect is extended to other pathogenic streptococcal species.
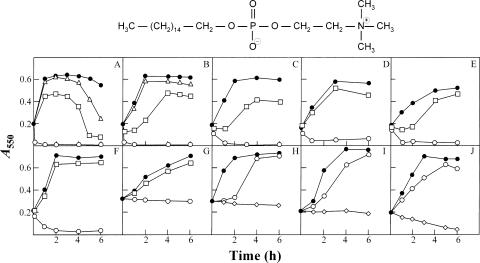
FIG. 1.
Effect of miltefosine on cultures of several streptococcal species. Exponentially growing cultures were incubated in THY broth to an A550 of about 0.2. Miltefosine was then added to a portion of the culture, and incubation was continued without shaking at 37°C. Panels: A, S. pneumoniae R6; B, S. pneumoniae Spain23F-1; C, S. pneumoniae 8249; D, S. pseudopneumoniae type strain; E, Streptococcus sp. strain 789/96; F, Streptococcus sp. strain 11923/96; G, S. pyogenes type strain; H, S. agalactiae type strain; I, S. mutans type strain; J, S. sanguinis type strain. Solid circles represent untreated control cultures. Miltefosine was added at the following concentrations: ⋄, 25 μM; ○, 10 μM; □, 5 μM; ▵, 2.5 μM. The chemical structure of miltefosine is shown at the top.
Miltefosine causes the lysis of pneumococcal cultures.
Pneumococcal strains (Table 1) were grown in C medium (22) supplemented with yeast extract (0.08%) or Todd-Hewitt broth (Difco) containing 0.5% yeast extract (THY) at 37°C with no shaking until the early exponential growth phase. In preliminary experiments, miltefosine (a generous gift from Zentaris GmbH, Frankfurt, Germany) was added to cultures of unencapsulated, antibiotic-susceptible laboratory strain R6 at a final concentration of 100 μM (about 41 μg/ml). Surprisingly, all pneumococcal cultures were lysed within 1 min (data not shown). Several miltefosine concentrations were then tested, and similar lysis was observed at 10 μM, whereas lower concentrations (2.5 and 5 μM) clearly expedited the characteristic autolysis of the cultures in the stationary phase of growth (Fig. 1A). Interestingly, encapsulated, multiply antibiotic-resistant S. pneumoniae strains (8249 and Spain23F-1) also underwent rapid lysis upon addition of the drug, although at slightly higher concentrations (Fig. 1B and C). It should be emphasized that South African strain 8249, in addition to being highly penicillin resistant, is tolerant to penicillin and other β-lactam antibiotics yet is lysed by other cell wall inhibitors (cycloserine and vancomycin) and detergents and also undergoes autolysis in the stationary phase of growth (24). As expected, immediate lysis also took place when an encapsulated, antibiotic-susceptible pneumococcal strain (ATCC 6303) was treated with miltefosine (data not shown).
TABLE 1.
Bacterial strains used in the present work and calculated MICs
| Bacterial species or strain | Relevant characteristic(s) (drug, MIC [μg/ml]) | Miltefosine susceptibility MIC (μM)a | Source or referenceb |
|---|---|---|---|
| S. pneumoniae | |||
| R6 | Unencapsulated laboratory strain; lytA+ | 5 | 33 |
| M32 | Unencapsulated laboratory strain; ΔlytA32 | 5 | 28 |
| 8249 | Serotype 19A, clinical isolate; multiresistant; lytA+ (penicillin, 6) | 6.25 | 24 |
| Spain23F-1 | Serotype 23F; lytA+ (penicillin, 1-2; tetracycline, 8; chloramphenicol, >8) | 6.25 | 30 |
| ATCC 6303 | Serotype 3; lytA+; Preceptrol strain | 6.25 | ATCC |
| S. pseudopneumoniae CCUG 49455T | Type strain; lytA+ | ND | 1; CCUG |
| Streptococcus sp. strain 782/96 | lytA+ (penicillin, 0.25; tetracycline, 128; erythromycin, >128) | ND | 32 |
| Streptococcus sp. strain 11923/96 | lytA+ (penicillin, 8; tetracycline, 0.5; erythromycin, 4) | ND | 32 |
| S. mitis NCTC 12161T | Type strain | 10 | NCTC |
| S. oralis NCTC 11427T | Type strain | 10 | NCTC |
| S. pyogenes CECT 985T | Type strain | 10 | CECT |
| S. agalactiae CECT 183T | Type strain | 20 | CECT |
| S. mutans CECT 479T | Type strain | 20 | CECT |
| S. sanguinis CECT 480T | Type strain | ND | CECT |
| S. uberis CECT 994T | Type strain | ND | CECT |
| E. faecalis | Vancomycin resistant (vanA) | 20 | F. Baquero, Hospital Ramón y Cajal, Madrid, Spain |
MICs are the means of three independent determinations; ND, not determined.
ATCC, American Type Culture Collection; CCUG, Culture Collection, University of Göteborg; CECT, Colección Española de Cultivos Tipo; NCTC, National Collection of Type Cultures.
The MIC of miltefosine for pneumococcal strains was calculated according to a standard procedure (Clinical and Laboratory Standards Institute, formerly the National Committee for Clinical Laboratory Standards) (31) in which Mueller-Hinton broth is supplemented with 4% lysed horse blood. This protocol gave rise to MICs of about 60 μM (data not shown). However, it has been consistently found that the hydrophobic moiety of miltefosine binds to serum components, reducing the effective concentration of the drug (16). To minimize this possibility, the same test was performed with THY broth. Under these conditions, MICs ranged from 5 to 6.25 μM for S. pneumoniae strains and from 10 to 20 μM for other streptococci (Table 1). On the other hand, miltefosine showed no effect on the growth of two gram-negative species tested, i.e., Escherichia coli and Pseudomonas aeruginosa (unpublished observations), suggesting that the outer membrane prevents access of the drug to the cytoplasmic membrane.
Bactericidal effects of miltefosine on other pathogenic streptococci.
To test whether the lytic effect of miltefosine was restricted to pneumococci, cultures of several pathogenic streptococci were incubated with the drug at concentrations equal to or slightly higher than the MIC. Interestingly, the Streptococcus pseudopneumoniae type strain and two streptococci of the mitis group (strains 782/96 and 11923/96) were also lysed rapidly by 10 μM miltefosine (Fig. 1D, E, and F). Remarkably, all of these strains synthesize a partly active LytA-like autolysin, albeit one different from the LytA enzyme characteristic of pneumococci (25, 32). With the possible exception of the Streptococcus sanguinis type strain, in which lysis occurred to some extent following the addition of 25 μM miltefosine (Fig. 1J), none of the remaining streptococci, namely, the Streptococcus pyogenes type strain, the Streptococcus agalactiae type strain, or the Streptococcus mutans type strain, showed clear autolytic behavior after exposure to the drug. Moreover, while S. pyogenes exhibited a tolerant response when treated with 10 μM miltefosine, S. agalactiae and S. mutans only stopped growing when at least 25 μM miltefosine was added to the cultures (Fig. 1G, H, and I). The other streptococcal species tested (including a vancomycin-resistant Enterococcus faecalis strain) (Table 1) exhibited similar tolerance to miltefosine (unpublished observations). Interestingly, a rapid loss of viability was observed (between 1.5 and 3 log units in 3 h, depending on the species) in experiments performed with streptococcal species that are not lysed by exposure to miltefosine (unpublished data). This behavior resembles the response shown by the autolytically defective strain S. pneumoniae M32 (see below).
Miltefosine promotes the uncontrolled action of LytA autolysin.
As illustrated above, rapid and complete lysis of bacterial cultures in response to miltefosine only occurred when the bacteria (either pneumococci or closely related streptococcal isolates) produced a LytA-like autolysin. To verify that LytA was involved in the lytic effect, a pneumococcal ΔlytA mutant (strain M32) (Table 1) was incubated in the presence of the drug and a tolerant response was noted (Fig. 2A). Early experiments had shown that the LytA-deficient mutants could be phenotypically “cured” by the addition of exogenous autolysin (44). Thus, curing of the M32 strain with electrophoretically pure LytA amidase (10 μg/ml) (15) prior to the addition of miltefosine was sufficient to restore the characteristic lytic effect of the drug (Fig. 2A). Most interestingly, even in the absence of autolysin, 25 μM miltefosine produced a 2-log drop in viability after only 15 min and a 4-log decrease after 3 h of treatment (Fig. 2A). This indicates that even when a tolerant response is observed, miltefosine is very efficient at killing pneumococcal cells. Although the term tolerance is frequently used as meaning inhibition of growth without cell death, it must be kept in mind that tolerant cells are also killed by the corresponding drugs, but in these cultures the loss of viability occurs at a substantially slower rate than in the case of wild-type cells (43). Experimental evidence showing that the autolysin LytA is responsible for as much as 90% (1 log unit) of the bactericidal action of penicillin against S. pneumoniae has been previously reported (27).
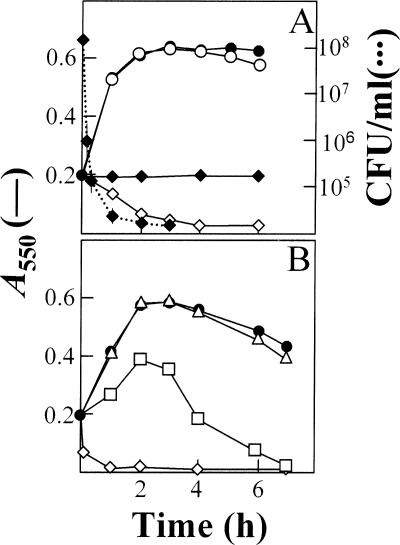
FIG. 2.
Triggering of the autolysin LytA by miltefosine and effect of hydrophobic tail length on pneumococcal lysis. (A) An exponentially growing culture of S. pneumoniae strain M32 (ΔlytA32) was incubated in C medium-0.08% yeast extract with (open symbols) or without (solid symbols) the pure LytA enzyme (10 μg/ml) for 1 h at 37°C. The culture was then diluted to an A550 of 0.2 and divided into two portions. Miltefosine was added at 25 μM (⋄, ♦) to one portion, and the other portion was left untreated (○, •). Incubation was continued at 37°C. Survival of the culture treated only with miltefosine was determined by plating at different incubation times (dotted lines). (B) Growth (and lysis) curves of the pneumococcal R6 strain treated with miltefosine (⋄), tetradecylphosphocholine (□), or dodecylphosphocholine (▵); each drug was added at a final concentration of 25 μM. The growth curve of an untreated R6 culture is indicated by solid circles.
Effects of miltefosine-related compounds on triggering of LytA.
The rapid pneumococcal lysis triggered by miltefosine is reminiscent of the bile solubility test used to identify S. pneumoniae in most clinical laboratories (29). It is well known that bile (i.e., deoxycholate) causes the release of lipoteichoic acid, the natural inhibitor of LytA autolysin (20), from the pneumococcal membrane, allowing this enzyme to rapidly degrade cell wall peptidoglycan. In fact, miltefosine and its related compounds have a detergent effect and dodecylphosphocholine has been used to solubilize membrane proteins (23). Collectively, these results suggest that the primary effect of miltefosine on S. pneumoniae and other streptococci may involve its action as a detergent. To establish the influence of the length of the alkyl tail on bactericidal activity, two additional shortened alkylphosphocholines were tested, i.e., dodecylphosphocholine and tetradecylphosphocholine (both purchased from Anatrace, Inc., Maumee, OH). As shown in Fig. 2B, the length of the alkyl chain was found to be directly proportional to the lysis-promoting capacity of pneumococcal strain R6. In fact, dodecylphosphocholine was practically devoid of any lytic activity even when applied at a concentration of 25 μM. These observations are consistent with the critical micellar concentration reported for these compounds (Anatrace, Inc., catalog, 2006 ed. [http://www.anatrace.com/Literature/Catalog%20Sept%202006.pdf]).
In addition to its antitumor and antileishmanial activities, miltefosine is also active against a variety of protozoa such as Trypanosoma (8), Trichomonas (5), and several amoebas (39, 40, 46). Indeed, in 2005 miltefosine was designated an orphan medicinal product for the treatment of Acanthamoeba keratitis by the European Medicines Agency (http://www.emea.eu.int/pdfs/human/comp/20357405en.pdf). The antifungal activity of miltefosine has also been recently recognized (47). To the best of our knowledge, however, there are no previous reports of the antibacterial effect of miltefosine. Interestingly, the MICs of the drug found here to be effective against pathogenic streptococci (5 to 20 μM, corresponding to 2 to 8 μg/ml) match those described for several pathogenic fungi (47) and, most importantly, are very close to those quoted for several Leishmania species (9). This does not, however, necessarily suggest similar mechanisms of action. Thus, the killing action of miltefosine in Leishmania is induced by a far more complex system than merely detergent-induced lysis. Parasite killing requires drug uptake by the specific transporter LdMT, an aminophospholipid translocase (36), and induction of an apoptosis-like process (35), which would be fairly unlikely if produced solely through a detergent-like effect. The MICs determined here for streptococci are lower than the steady-state miltefosine concentrations (120 μM and >500 nmol/g, respectively) attained in the serum and lungs of rats after 11 days of daily oral treatment (21). Many pharmacological and pharmacokinetic studies have been done (see reference 41 for a recent review), and oral administration of miltefosine is effective and well tolerated in children (4). Accordingly, this drug could be useful in vivo against streptococcal and, particularly, pneumococcal infections. As a possible shortcoming, it should be considered that these infections develop quite rapidly, sometimes in a few days or even hours, such that orally administrated miltefosine may not achieve sufficiently high concentrations in plasma to kill rapidly multiplying bacteria. It is conceivable, however, that the new miltefosine liposomal formulations (34), nasal instillations complexed with an artificial lung surfactant (45), and/or alternative alkylphospholipid derivatives such as erucylphosphocholine, which can be administered intravenously with no detectable hemolytic effects (10), may prove useful for the in vivo treatment of streptococcal infections in the near future.
Acknowledgments
This work was supported by grants (BMC2003-00074 and SAF2006-00390) from the Ministerio de Educación y Ciencia awarded to E. García and grants from the EU (QLK2-CT-2001-01404) and the Fondo de Investigaciones Sanitarias (061125) awarded to L. Rivas. Additional financial support was also provided by the COMBACT program (S-BIO-0260/2006) from the Comunidad de Madrid.
We thank M. Moscoso and R. López for helpful discussions and critical reading of the manuscript, A. Burton for correcting the English version, and E. Cano for skillful technical assistance.
Footnotes
Published ahead of print on 12 March 2007.
REFERENCES
- 1.Arbique, J. C., C. Poyart, P. Trieu-Cuot, G. Quesne, M. D. S. Carvalho, A. G. Steigerwalt, R. E. Morey, D. Jackson, R. J. Davidson, and R. R. Facklam. 2004. Accuracy of phenotypic and genotypic testing for identification of Streptococcus pneumoniae and description of Streptococcus pseudopneumoniae sp. nov. J. Clin. Microbiol. 424686-4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergmann, S., and S. Hammerschmidt. 2006. Versatility of pneumococcal surface proteins. Microbiology 152295-303. [DOI] [PubMed] [Google Scholar]
- 3.Berman, J. D. 2006. Development of miltefosine for the leishmaniases. Mini Rev. Med. Chem. 6145-151. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharya, S. K., T. K. Jha, S. Sundar, C. P. Thakur, J. Engel, H. Sindermann, K. Junge, J. Karbwang, A. D. M. Bryceson, and J. D. Berman. 2004. Efficacy and tolerability of miltefosine for childhood visceral leishmaniasis in India. Clin. Infect. Dis. 38217-221. [DOI] [PubMed] [Google Scholar]
- 5.Blaha, C., M. Duchene, H. Aspöck, and J. Walochnik. 2006. In vitro activity of hexadecylphosphocholine (miltefosine) against metronidazole-resistant and -susceptible strains of Trichomonas vaginalis. J. Antimicrob. Chemother. 57273-278. [DOI] [PubMed] [Google Scholar]
- 6.Brachwitz, H., and C. Vollgraf. 1995. Analogs of alkyllysophospholipids: chemistry, effects on the molecular level and their consequences for normal and malignant cells. Pharmacol. Ther. 6639-82. [DOI] [PubMed] [Google Scholar]
- 7.Croft, S. L., and J. Engel. 2006. Miltefosine—discovery of the antileishmanial activity of phospholipid derivatives. Trans. R. Soc. Trop. Med. Hyg. 100S4-S8. [DOI] [PubMed] [Google Scholar]
- 8.Croft, S. L., D. Snowdon, and V. Yardley. 1996. The activities of four anticancer alkyllysophospholipids against Leishmania donovani, Trypanosoma cruzi and Trypanosoma brucei. J. Antimicrob. Chemother. 381041-1047. [DOI] [PubMed] [Google Scholar]
- 9.Croft, S. L., S. Sundar, and A. H. Fairlamb. 2006. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 19111-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erdlenbruch, B., V. Jendrossek, A. Gerriets, F. Vetterlein, H. Eibl, and M. Lakomek. 1999. Erucylphosphocholine: pharmacokinetics, biodistribution and CNS-accumulation in the rat after intravenous administration. Cancer Chemother. Pharmacol. 44484-490. [DOI] [PubMed] [Google Scholar]
- 11.Fernández-Tornero, C., E. García, B. de Pascual-Teresa, R. López, G. Giménez-Gallego, and A. Romero. 2005. Ofloxacin-like antibiotics inhibit pneumococcal cell wall degrading virulence factors. J. Biol. Chem. 28019948-19957. [DOI] [PubMed] [Google Scholar]
- 12.Fernández-Tornero, C., E. García, R. López, G. Giménez-Gallego, and A. Romero. 2002. Two new crystal forms of the choline-binding domain of the major pneumococcal autolysin: insights into the dynamics of the active homodimer. J. Mol. Biol. 321163-173. [DOI] [PubMed] [Google Scholar]
- 13.Fernández-Tornero, C., R. López, E. García, G. Giménez-Gallego, and A. Romero. 2001. A novel solenoid fold in the cell wall anchoring domain of the pneumococcal virulence factor LytA. Nat. Struct. Biol. 81020-1024. [DOI] [PubMed] [Google Scholar]
- 14.Fischer, W. 2000. Pneumococcal lipoteichoic and teichoic acid, p. 155-177. In A. Tomasz (ed.), Streptococcus pneumoniae—molecular biology and mechanisms of disease. Mary Ann Liebert, Inc., Larchmont, NY.
- 15.García, J. L., E. García, and R. López. 1987. Overproduction and rapid purification of the amidase of Streptococcus pneumoniae. Arch. Microbiol. 14952-56. [DOI] [PubMed] [Google Scholar]
- 16.Goppelt-Struebe, M., and I. Winter. 1995. Effects of hexadecylphosphocholine on fatty acid metabolism: relation to cytotoxicity. Cancer Chemother. Pharmacol. 35519-526. [DOI] [PubMed] [Google Scholar]
- 17.Hermoso, J. A., L. Lagartera, A. González, M. Stelter, P. García, M. Martínez-Ripoll, J. L. García, and M. Menéndez. 2005. Insights into pneumococcal pathogenesis from crystal structure of the modular teichoic acid phosphorylcholine esterase Pce. Nat. Struct. Mol. Biol. 12533-538. [DOI] [PubMed] [Google Scholar]
- 18.Hermoso, J. A., B. Monterroso, A. Albert, B. Galán, O. Ahrazem, P. García, M. Martínez-Ripoll, J. L. García, and M. Menéndez. 2003. Structural basis for selective recognition of pneumococcal cell wall by modular endolysin from phage Cp-1. Structure 111239-1249. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman-Roberts, H. L., E. C Babcock, and I. F. Mitropoulos. 2005. Investigational new drugs for the treatment of resistant pneumococcal infections. Expert Opin. Investig. Drugs 14973-995. [DOI] [PubMed] [Google Scholar]
- 20.Höltje, J.-V., and A. Tomasz. 1975. Lipoteichoic acid: a specific inhibitor of autolysin activity in pneumococcus. Proc. Natl. Acad. Sci. USA 721690-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kötting, J., M. Berger, C. Unger, and H. Eibl. 1992. Alkylphosphocholines: influence of structural variation on biodistribution at antineoplastically active concentrations. Cancer Chemother. Pharmacol. 30105-112. [DOI] [PubMed] [Google Scholar]
- 22.Lacks, S., and R. D. Hotchkiss. 1960. A study of the genetic material determining an enzyme activity in Pneumococcus. Biochim. Biophys. Acta 39508-517. [DOI] [PubMed] [Google Scholar]
- 23.le Maire, M., P. Champeil, and J. V. Møller. 2000. Interaction of membrane proteins and lipids with solubilizing detergents. Biochim. Biophys. Acta 150886-111. [DOI] [PubMed] [Google Scholar]
- 24.Liu, H. H., and A. Tomasz. 1985. Penicillin tolerance in multiply drug-resistant natural isolates of Streptococcus pneumoniae. J. Infect. Dis. 152365-372. [DOI] [PubMed] [Google Scholar]
- 25.Llull, D., R. López, and E. García. 2006. Characteristic signatures of the lytA gene provide a rapid and reliable diagnosis of Streptococcus pneumoniae infections. J. Clin. Microbiol. 441250-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.López, R., E. García, P. García, and J. L. García. 2004. Cell wall hydrolases, p. 75-88. In E. I. Tuomanen, T. J. Mitchell, D. A. Morrison, and B. G. Spratt (ed.), The pneumococcus. ASM Press, Washington, DC.
- 27.López, R., C. Ronda, and E. García. 1990. Autolysins are direct involved in the bactericidal effect caused by penicillin in wild type and in tolerant pneumococci. FEMS Microbiol. Lett. 54317-322. [DOI] [PubMed] [Google Scholar]
- 28.López, R., J. M. Sánchez-Puelles, E. García, J. L. García, C. Ronda, and P. García. 1986. Isolation, characterization and physiological properties of an autolytic-deficient mutant of Streptococcus pneumoniae. Mol. Gen. Genet. 204237-242. [DOI] [PubMed] [Google Scholar]
- 29.Lund, E., and J. Henrichsen. 1978. Laboratory diagnosis, serology and epidemiology of Streptococcus pneumoniae. Methods Microbiol. 12241-262. [Google Scholar]
- 30.Muñoz, R., T. J. Coffey, M. Daniels, C. G. Dowson, G. Laible, J. Casal, R. Hakenbeck, M. Jacobs, J. M. Musser, B. G. Spratt, and A. Tomasz. 1991. Intercontinental spread of a multiresistant clone of serotype 23F Streptococcus pneumoniae. J. Infect. Dis. 164302-306. [DOI] [PubMed] [Google Scholar]
- 31.National Committee for Clinical Laboratory Standards. 2004. Performance standards for antimicrobial susceptibility testing. Approved standard M100-S14. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 32.Obregón, V., P. García, E. García, A. Fenoll, R. López, and J. L. García. 2002. Molecular peculiarities of the lytA gene isolated from clinical pneumococcal strains that are bile insoluble. J. Clin. Microbiol. 402545-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ottolenghi, E., and R. D. Hotchkiss. 1962. Release of genetic transforming agent from pneumococcal cultures during growth and disintegration. J. Exp. Med. 116491-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papagiannaros, A., C. Bories, C. Demetzos, and P. M. Loiseau. 2005. Antileishmanial and trypanocidal activities of new miltefosine liposomal formulations. Biomed. Pharmacother. 59545-550. [DOI] [PubMed] [Google Scholar]
- 35.Paris, C., P. M. Loiseau, C. Bories, and J. Bréard. 2004. Miltefosine induces apoptosis-like death in Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 48852-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pérez-Victoria, F. J., F. Gamarro, M. Ouellette, and S. Castanys. 2003. Functional cloning of the miltefosine transporter: a novel P-type phospholipid translocase from Leishmania involved in drug resistance. J. Biol. Chem. 27849965-49971. [DOI] [PubMed] [Google Scholar]
- 37.Pérez-Victoria, F. J., M. P. Sánchez-Cañete, K. Seifert, S. L. Croft, S. Sundar, S. Castanys, and F. Gamarro. 2006. Mechanisms of experimental resistance of Leishmania to miltefosine: implications for clinical use. Drug Resist. Updates 926-39. [DOI] [PubMed] [Google Scholar]
- 38.Rosenow, C., P. Ryan, J. N. Weiser, S. Johnson, P. Fontan, A. Ortqvist, and H. R. Masure. 1997. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol. Microbiol. 25819-829. [DOI] [PubMed] [Google Scholar]
- 39.Schuster, F. L., B. J. Guglielmo, and G. S. Visvesvara. 2006. In-vitro activity of miltefosine and voriconazole on clinical isolates of free-living amebas: Balamuthia mandrillaris, Acanthamoeba spp., and Naegleria fowleri. J. Eukaryot. Microbiol. 53121-126. [DOI] [PubMed] [Google Scholar]
- 40.Seifert, K., M. Duchêne, W. H. Wernsdorfer, H. Kollaritsch, O. Scheiner, G. Wiedermann, T. Hottkowitz, and H. Eibl. 2001. Effects of miltefosine and other alkylphosphocholines on human intestinal parasite Entamoeba histolytica. Antimicrob. Agents Chemother. 451505-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sindermann, H., and J. Engel. 2006. Development of miltefosine as an oral treatment for leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 100S17-S20. [DOI] [PubMed] [Google Scholar]
- 42.Tomasz, A. 1967. Choline in the cell wall of a bacterium: novel type of polymer-linked choline in Pneumococcus. Science 157694-697. [DOI] [PubMed] [Google Scholar]
- 43.Tomasz, A., A. Albino, and E. Zanati. 1970. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature 227138-140. [DOI] [PubMed] [Google Scholar]
- 44.Tomasz, A., and S. Waks. 1975. Enzyme replacement in a bacterium: phenotypic correction by the experimental introduction of the wild type enzyme into a live enzyme defective mutant pneumococcus. Biochem. Biophys. Res. Commun. 651311-1319. [DOI] [PubMed] [Google Scholar]
- 45.Vermehren, C., S. Frokjaer, T. Aurstad, and J. Hansen. 2006. Lung surfactant as a drug delivery system. Int. J. Pharm. 30789-92. [DOI] [PubMed] [Google Scholar]
- 46.Walochnik, J., M. Duchêne, K. Seifert, A. Obwaller, T. Hottkowitz, G. Wiedermann, H. Eibl, and H. Aspöck. 2002. Cytotoxic activities of alkylphosphocholines against clinical isolates of Acanthamoeba spp. Antimicrob. Agents Chemother. 46695-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Widmer, F., L. C. Wright, D. Obando, R. Handke, R. Ganendren, D. H. Ellis, and T. C. Sorrell. 2006. Hexadecylphosphocholine (miltefosine) has broad-spectrum fungicidal activity and is efficacious in a mouse model of cryptococcosis. Antimicrob. Agents Chemother. 50414-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yother, J., and J. M. White. 1994. Novel surface attachment mechanism of the Streptococcus pneumoniae protein PspA. J. Bacteriol. 1762976-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]