Abstract
Previous studies have shown that peptides containing the protein transduction domain (PTD) of the human immunodeficiency virus tat protein (GRKKRRQRRR) were effective inhibitors of herpes simplex virus type 1 (HSV-1) entry (H. Bultmann and C. R. Brandt, J. Biol. Chem. 277:36018-36023, 2002). We now show that the addition of a single cysteine residue to the C terminus of the TAT PTD (TAT-C peptide) improves the antiviral activity against HSV-1 and HSV-2. The principle effect of adding the cysteine was to enable the peptide to inactivate virions and to induce a state of resistance to infection in cells pretreated with peptide. The TAT-C peptide acted extracellularly, immediately blocked entry of adsorbed virus, prevented VP16 translocation to the nucleus, and blocked syncytium formation and cell-cell spread. Thus, TAT-C peptides are fusion inhibitors. The induction of the resistance of cells to infection was rapid, recovered with a half-life of 5 to 6 h, and could be reinduced by peptide treatment. TAT-C bound to heparan sulfate but was a poor competitor for viral attachment. The antiviral activity depended on the net positive charge of the peptide but not on chirality, and a free sulfhydryl group was not essential for antiviral activity because TAT-C dimers were at least as effective as monomers. The unique combination of antiviral activities and low toxicity combine to make TAT-C a strong candidate for further development as a drug to block HSV infection.
Herpes simplex virus types 1 and 2 (HSV-1 and HSV-2) are widespread human pathogens. Between 70 and 90% of adults in the United States are infected with HSV-1, and 22% of individuals over the age of 12 are seropositive for HSV-2 (7, 61). Both viruses infect mucous membranes causing ulcerative lesions that are usually self-limited in immunocompetent individuals. However, serious infections can occur, including lethal neonatal HSV, encephalitis, and blinding keratitis (34, 61). A number of antivirals are approved for HSV treatment, and the use of these drugs has had a significant impact on the disease. These antivirals reduce the frequency of recurrent viral shedding and disease and reduce transmission of genital herpes between discordant partners (12, 42, 46). Although these treatment regimens significantly reduce disease, they are not completely effective and cannot clear latent infections. One significant problem in dealing with HSV infections is the ability of the virus to persist in the host in the form of latent infection (45). Preventing the establishment of a persistent infection, which could be accomplished either by blocking the initial infection or preventing the establishment of latency, would be ideal for dealing with this virus.
One strategy for preventing infection is to block the attachment of virus. Attachment of HSV can be inhibited by heparin, which competes directly with the binding of gC and gB to cell surface heparan sulfate (HS) on cells (23, 24, 26-28). Other polymeric anions and polyoxotungstate compounds have been shown to inhibit attachment (13, 19, 25, 26, 35, 40). Preventing entry is also a viable strategy for blocking initial HSV infection. Four HSV glycoproteins, gB, gD, and gH/gL are required for fusion and entry of HSV (50, 51). Antibodies to gB, gD, or gH prevent entry (50), and the administration of monoclonal antibodies to these proteins reduces the severity of HSV infection (6, 14, 16, 47, 60). However, vaccination protocols that induce antibodies to these proteins have not shown sufficient efficacy in clinical trials (11, 37, 52). The HSV gD protein binds to one of four types of cellular coreceptors, and the soluble ectodomain of gD inhibits fusion by competing for binding to these receptors (20, 37). The soluble ectodomains of the cellular receptors have also been reported to prevent entry (19, 38). Although these soluble ectodomains inhibit fusion in cell culture, they have not been tested in vivo.
A number of HSV entry inhibitors have been described, including a 23-amino-acid structural analog of melittin (hecate), a 33-amino-acid peptide homologous to a heptad-like repeat structure in bovine herpes virus type 1, n-docosanol, and cobalt chelates (3, 4, 22, 40, 41, 43, 48, 53). Some of these agents may act by disrupting the membrane or envelope structure, but little is known about the actual mechanisms involved.
Several cationic peptides or proteins including lactoferrin analogues and peptides derived from it, rabbit α-defensin NP-1, dermaseptins, magainin derivatives, and a synthetic θ-defensin (retrocyclin 2 [RC2]), inhibit HSV infection (2, 5, 15, 25, 29, 30, 31, 35, 36, 49, 58, 63) by blocking attachment and/or entry. RC2 binds to N- and O-linked carbohydrates on viruses and may be acting as a “mini-lectin” (33, 57, 63).
We previously reported (8, 9) that various peptides containing a protein transduction domain (PTD), such as TAT, EB, and penetratin (59), inhibited HSV-1 infection with 50% effective concentration (EC50) values in the low micromolar range. One of these peptides, bTAT-9, consisted of the PTD sequence of the HIV-1 Tat protein coupled to the carboxy-terminal 9 amino acids of the HSV-1 ribonucleotide reductase small subunit, and its antiviral activity appeared to be associated with the PTD sequence rather than the coupled peptide (9). Subsequently, we found that the antiviral activity of the TAT PTD could be improved by the addition of novel peptide extensions. To facilitate further studies, we added a cysteine residue to the C terminus of the TAT PTD (TAT-C peptide) as a potential coupling site for various peptide extensions.
The goal of the present work was to characterize the antiviral activity of the TAT-C peptide. Our data show that the addition of the cysteine residue improved the antiviral activity of TAT against HSV. The TAT-C peptide inactivated virions in solution in an essentially irreversible manner, blocked entry, and also induced resistance to infection when cells were pretreated with the peptide. The activity depended on the net positive charge of the peptide but not on chirality. A free sulfhydryl group was not essential for antiviral activity, but dimerization of TAT-C through disulfide bonding improved the antiviral activity primarily by enhancing viral inactivation in solution. Finally, we show that TAT-C is a fusion inhibitor. When these facts are considered together with the low toxicity of these peptides in vitro and in vivo (1), TAT-C seems a strong candidate for further development as a drug to prevent HSV infection.
MATERIALS AND METHODS
Cell culture and viruses.
Most studies were carried out with Vero cells (African green monkey kidney; ATCC CLL-81) cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% calf serum, 5% fetal bovine serum (FBS), 0.06 g/liter penicillin G, and 0.08 g/liter streptomycin sulfate and grown to confluence in 96-well plates. For most experiments, cultures were used at densities of 1.2 × 105 cells per well. Whenever extensive manipulations or prolonged incubations at 4°C were required, the cells were plated in wells coated with poly-l-lysine (Sigma-Aldrich, Inc., St. Louis, MO; catalog no. P4707) and grown to densities of 2 × 105 to 3 × 105 cells/well. Microscopic examination insured that stable normal cell layers were maintained in each well throughout every experiment. For the duration of each experiment, the medium was buffered with 25 mM HEPES (pH 7.3) instead of carbonate buffer and no antibiotics were present. Primary human foreskin fibroblasts and HeLa cells were cultured like Vero cells except that 10% FBS was used. The HSV-1 KOS nonsyncytial mutant hrR3, which expresses Escherichia coli β-galactosidase from the ICP6 promoter (21), was used for all studies with three exceptions. Wild-type HSV-1 KOS was used for electron microscopy and the study of cell-cell spread, and a spontaneous syncytial variant of hrR3 (hrR3S) was used for syncytium formation studies. HSV-2 strain 333 was used to determine if TAT-C was active against HSV-2.
Synthetic peptides.
Synthesis, purification, and analysis of peptides were done at the Biotechnology Center at the University of Wisconsin—Madison as described previously (9). Table 1 shows the sequences of the peptides used in these studies. A peptide with a superscript minus indicates a carboxyl C terminus, and a peptide with a superscript zero indicates an amide C terminus. The purities (full length) of all peptides exceeded 90% as determined by analytical high-performance liquid chromatography (HPLC). Peptide concentrations were determined from absorbance readings at 215 and 225 nm and were corrected for purity. Except where indicated (see Fig. 11), the concentrations of cysteine-containing peptides refer to concentrations of the monomeric forms even if the peptides were partially dimerized.
TABLE 1.
Names, sequences, and antiviral activities of TAT peptides
| Peptide (charge)a | Sequenceb | Activityc
|
||
|---|---|---|---|---|
| Entry inhibitiond | Cellular resistancee | Virus inactivationf | ||
| TAT0 (+9) | NH2GRKKRRQRRRCONH2 | 1.0 | >1,200* | >1,200 |
| TAT-Pd0 (+9) | NH2GRKKRRQRRRPCONH2 | 0.7 | >1,200* | 1,050 |
| TAT-Cd0 (+9) | NH2GRKKRRQRRRCCONH2 | 0.9 (SH), 0.6 (SS) | 1.1-2.4 (SH), 0.4 (SS) | 200-330 (SH), 34 (SS) |
| TAT-Cd− (+8) | NH2GRKKRRQRRRCCOOH | 2.5 | 5.4-10 | 150-290 |
| bTAT-Cd− (+7) | b-GRKKRRQRRRCCOOH | 2.1 | ND | ND |
| TAT-C0 (+9) | NH2GRKKRRQRRRCCONH2 | 0.9 (SH) | 3.9-6.7 (SH) | 390 (SH) |
| bTAT-C0 (+8) | b-GRKKRRQRRRCCONH2 | 5.7 | ND | ND |
| n50,51TAT-C0 (+7) | NH2GRnnRRQRRRCCONH2 | 9.6 | 92 | 115 (SH) |
| TAT-C− (+8) | NH2GRKKRRQRRRCCOOH | 3.1 | 3.3-4.5 | 96-110 |
| n50,51TAT-C− (+6) | NH2GRnnRRQRRRCCOOH | 34-48 | >1,200* | ∼900 |
| n55,56TAT-C− (+6) | NH2GRKKRRQnnRCCOOH | 48 | 230 | ∼900 |
| bn55,56TAT-C− (+5) | b-GRKKRRQnnRCCOOH | 37 | ND | ND |
Names have superscripts indicating whether the C terminus bears a carboxyl group (−) or an amide (0). Numbers in parentheses refer to the nominal net charge of the peptides at neutral pH.
Amino acid sequences are given in single-letter code. Norleucine is represented by “n.” Boldface type indicates d-enantiomers. In some peptides, the N-terminal amino group is replaced by biotin-aminohexanoyl (b).
All activities are given as micromolar EC50s obtained from single dose-response curves based on triplicate determinations at each peptide concentration (cf. Fig. 1, 4, and 6). In some cases, the range of EC50s obtained from multiple response curves is indicated. Activities of cysteine-substituted peptides refer to the monomeric (SH) or dimeric (SS) forms of peptides or to mixtures of the two (unmarked, in monomeric concentrations). Asterisks indicate that cellular susceptibility to infection is enhanced rather than inhibited at or below a peptide concentration of 1,200 μM (see Fig. 6). ND, not determined.
Virus is attached to cells for 1 h at 4°C, peptide is added, and cultures are infected for 1 h at 37°C.
Cells are exposed to peptide for 1 h at 37°C, rinsed, and infected for 1 h at 37°C.
Virions are exposed to peptides for 1 h at 37°C and then diluted or pelleted to remove free peptide prior to infection for 1 h at 37°C.
FIG. 11.
Effects of TAT-Cd0 dimerization on induction of cellular resistance to infection and on virus inactivation in solution. Panel A: induction of cell resistance. Vero cell cultures were exposed for 1 h at 37°C to TAT-Cd0 monomers in the presence of DTT (•; EC50, 1.1 μM), TAT-C0 dimers in the absence of DTT (○; EC50, 0.4 μM), or DTT alone (□). Cultures were then infected in the absence of peptide and DTT and scored for infectivity by measuring β-galactosidase activity in lysates 6 h later. DTT was added to freshly prepared peptide solutions in 10-fold molar excess. Panel B: inactivation of virions in solution. Virions in serum-free medium were exposed for 1 h at 37°C to TAT-Cd0 dimers (○; EC50, 34 μM) or monomers (•; EC50, 200 μM) or to DTT (□; EC50, 90 μM). Free peptide and DTT were removed by pelleting the virus, and infectivity was assayed by measuring β-galactosidase activity in lysates. Exposure to monomer was under strictly anaerobic conditions (argon atmosphere) in the absence of DTT. The data points represent the means of triplicate determinations with standard errors of the means.
Peptide dimerization.
The cysteine-substituted TAT-Cdo peptide was dimerized by cysteine oxidation with dimethyl sulfoxide (54). The peptide was dissolved in 5% acetic acid (5 mg/ml), the pH was adjusted to 6 with 1 M (NH4)2CO3, and dimethyl sulfoxide was added to 20% of the final volume. After 4 h at room temperature, the solute was removed by Speed-Vac concentration, and the peptide was dissolved in trifluoroacetic acid and precipitated with cold ether. HPLC chromatography showed that the peptide was quantitatively converted to the dimeric form. HPLC analysis also showed that dimerization was quantitatively reversed by reducing the TAT-Cd0 dimer for 1 h at 37°C with a 10-fold molar excess of dichlorodiphenyltrichloroethane in serum-free DMEM.
Inhibition of entry.
Virus was adsorbed to precooled cultures for 1 h at 4°C (multiplicity of infection [MOI], 0.02 to 0.03). Free virus was rinsed off, peptide was added at 4°C, and the cultures were switched to 30 to 37°C to initiate entry. One hour later, free peptide was rinsed off and extracellular virus was inactivated by exposure to pH 3.0 citrate buffer for 60 seconds (29). The cultures were fixed 8 h after the temperature shift with 0.5% glutaraldehyde in 5× phosphate-buffered saline (PBS) for 30 min and stained overnight with X-Gal (5-bromo-4-chloro-3-indolyl-β-galactopyranoside; Eppendorf AG, Hamburg, Germany) in PBS containing 2 μM MgCl2, 1.3 mM K4Fe(CN)6, and 1.3 mM K3Fe(CN)6, and blue cells were counted. Alternatively, the cells were lysed after 6 h with 0.5% NP-40 in PBS containing 2 mM MgCl2 and CPR-Gal (5 mM; chlorophenol red-β-galactopyranoside; Roche Diagnostics, Indianapolis, IN), and the initial rate of β-galactosidase activity was measured in an enzyme-linked immunosorbent assay (ELISA) reader at 570 nm. Background activity was measured in cultures treated with 20 μM cycloheximide after infection and subtracted from the data.
Cellular resistance.
Cultures were exposed to peptide for various times (usually 1 h) at temperatures ranging from 4 to 37°C and rinsed three times with medium over a period of 5 min at 23°C to remove any free peptide. The cells were then infected for 1 h at 37°C, external virus was inactivated, and infectivity was measured by X-Gal staining or ELISA as described above. To reverse the induction of cell resistance, peptide-treated cells were rinsed three times for 10 min at 23°C with HEPES-buffered, serum-supplemented DMEM with the addition of 0.5 M NaCl or 50 μM heparin (catalog no. H9399; Sigma-Aldrich, St. Louis, MO; assumed molecular weight, 10,000).
Viral inactivation in solution.
Virus (1 × 106 to 6 × 106 PFU/ml) was mixed on ice with peptide in serum-free DMEM and incubated for 1 h at various temperatures between 4 and 37°C. Aliquots (10 μl) were diluted 1,000-fold into peptide-free, serum-supplemented DMEM at 23°C, and 50-μl aliquots were assayed in triplicate for infectivity in a 96-well plate. Additionally or alternatively, 10-μl aliquots were diluted into 1 ml of ice-cold, peptide-free, serum-supplemented DMEM, and the virus was pelleted at 14,000 × g in an Eppendorf 5415 centrifuge for 99 min at 4°C. The pelleted virions were resuspended in 250 μl of serum-supplemented DMEM by repeated vortexing, sonication, or both before infectivity was measured. Sonication was done with 25 pulses from a microtip at 30% duty cycle with an output setting of 3 in a Branson Sonifier cell disruptor 200. High-salt extractions of peptide-treated virus were done between centrifugations by incubating resuspended virus for 30 min at 4°C in medium adjusted to 0.5 M NaCl. The infectivity of resuspended virions was assayed in triplicate by measuring β-galactosidase activity in lysates as described above.
Trypsinization of virions and peptides.
TAT-C−-inactivated pelleted virions were resuspended in 250 μl of serum-free DMEM and exposed to 0.01% trypsin (BioWhittaker, Walkersville, MD; catalog no. 17-16E) for 1 h at 37°C. Trypsin was inactivated by adding a 1.3-fold molar excess of soybean trypsin inhibitor (Sigma; catalog no. T9003) for 10 min at 37°C, and aliquots were assayed for infectivity as described above. Exposure of free TAT-C− to trypsin under the same conditions completely abolished the antiviral activity of the peptide, as measured by viral inactivation. In controls, trypsin was inactivated with soybean trypsin inhibitor before it was added to virions or peptides.
Electron microscopy.
Virus (5 μl of 1.0 × 1010 PFU/ml HSV-1 KOS) was adsorbed to ionized Pioloform-coated grids for 5 min at room temperature. The grids were then rinsed with serum-free DMEM and treated with 11.4 mM TAT-C− or n50,51TAT-C− in serum-free DMEM or with peptide-free medium for 30 min at 37°C. The grids were rinsed again with serum-free DMEM, exposed to 1% OsO4 in tap water, rinsed twice with distilled water, and then negatively stained with Nano-W (Nanoprobes, Yaphank, NY; catalog no. 2018). Excess stain was removed, and grids were air dried. Some grids with mock-treated control virus were exposed for 1 to 2 min at room temperature to 2% Triton X-100 in 250 mM NaCl and 20 mM Tris-HCl (pH 8.0) (39). Images were taken at a magnification of ×100,000 in a JEOL 100CX electron microscope.
Virus adsorption.
Cells were fixed with 4% paraformaldehyde in PBS (pH 7.0) for 10 min at 4°C and stored in HEPES-buffered, serum-supplemented DMEM at 4°C for at least 1 h before they were exposed to virus (40 μl of 1 × 107 PFU/ml, hrR3) for 1 h at 37°C. Alternatively, the virus was adsorbed to precooled live cells for 2 h at 4°C prior to fixation. Bound virus was detected with polyclonal rabbit anti-HSV-1 antibody (DAKO A/S, Denmark) and alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G (IgG) (Sigma; catalog no. A3687) by measuring the initial rates of phosphatase activity in an ELISA reader at 405 nm. Background readings using cells not exposed to virus were subtracted from the data.
Peptide binding.
Fixed cells were exposed to biotinylated peptides for 30 to 60 min at 37°C. For some studies, a 100-fold molar excess of nonbiotinylated competitor peptide was included. Bound biotin was detected with alkaline phosphatase-conjugated streptavidin (Jackson ImmunoResearch Labs, Inc., West Grove, PA) by ELISA as described above.
Virus elution.
Virus was adsorbed to paraformaldehyde-fixed cells for 1 h at 37°C, free virus was rinsed off, and peptide or heparin was added for 1 h at 37°C. Virus remaining bound to cells was detected by ELISA as described above.
VP16 translocation.
Confluent Vero cell cultures on poly-l-lysine (Sigma; catalog no. 4747)-coated coverslips were exposed to virus (MOI, 10) for 80 min at 4°C. In two of the cultures, virus was not exposed to peptide and the cells were fixed either immediately following virus adsorption at 4°C (negative control) or following a 1-h entry period at 37°C (positive control). In a third culture, virus was exposed to 50 μM TAT-C− during the last 20 min of the adsorption period as well as during entry. A fourth culture was infected with virus that had been exposed to 300 μM TAT-C− for 1 h at 37°C. Dilution of the treated virus assured that the peptide was rendered ineffective during the adsorption and entry periods. After rinsing with PBS, all cells were fixed with 4% paraformaldehyde in serum-free DMEM (pH 7.4) overnight at 4°C and permeabilized by incubation with 0.2% Triton X-100 in PBS for 3 min at 4°C. Nonspecific binding was blocked with 10% FBS in PBS for 30 min before the cells were incubated with rabbit anti-VP16 IgG (Sigma; catalog no. V4388; 300-fold dilution) for 30 min at room temperature. The cells were rinsed three times with PBS containing 10% serum and then incubated with goat anti-rabbit IgG conjugated with Alexa Fluor 594 (Molecular Probes, Eugene, OR; catalog no. A-11037; 200-fold dilution) for 30 min at room temperature. After rinsing three times with PBS containing 10% FBS, the coverslips were mounted with M101 (Mount Biomedia, Foster City, CA) and viewed with a Zeiss Axioplan 2 microscope. Three representative fields of view were collected for each group, and 2-channel phase-contrast and fluorescent Z-stack images were collected, with an average of 16 sections per field of view. A total of 50 cells were analyzed for each group. For each cell, a series of Z-stack images passing through the center of the cell were selected, and the presence or absence of VP16 signal in the nucleus was recorded.
Virus-mediated cell-cell fusion.
Confluent Vero cell cultures in 96-well plates (2.0 × 105 cells/well) were infected with a syncytial variant of hrR3 (hrR3S; MOI, 0.02 to 0.05) for 1 to 2 h at 37°C. Free virus was rinsed off, and the cultures were exposed to neutralizing rabbit polyclonal anti-HSV-1 antibody (DAKO Cytomation; catalog no. B0114) diluted 50-fold into carbonate-buffered, serum-supplemented DMEM and incubated at 37°C for up to 24 h in the presence or absence of TAT peptides. Cell-cell fusion resulting in the formation of large syncytial complexes was monitored microscopically by staining cultures with X-Gal and quantified by measuring the initial rates of β-galactosidase activity in cell lysates as described above. The neutralization of extracellular virus by the antibody was measured in cultures exposed to hrR3S (MOI, 0.05) for 2 h at 4°C. After rinsing off free virus, these cultures were exposed for an additional 1 h at 4°C to various dilutions of antibody before entry was initiated at 37°C. Infection proceeded at 37°C in the presence of antibody for 6 h, at which time β-galactosidase activity was measured in cell lysates.
Cell-cell spread.
Vero cell cultures in 96-well plates (1 × 105 cells per well) were infected with HSV-1 KOS at a MOI of ≤0.01. The cultures were then exposed to inactivating anti-HSV antibody (Dako Cytomation; catalog no. B0114; 50-fold dilution) in the presence or absence of 100 μM TAT-Cd− for 24 h, and the cytopathic effect was scored microscopically.
HSV-2 plaque reduction assay.
Precooled confluent Vero cell cultures in 24-well plates were exposed to 150 PFU per well of HSV-2 strain 333 for 1 h at 4°C. Peptides were added at 4°C, and 10 min later, the cultures were shifted to 37°C. Alternatively, the cells were exposed to peptide for 1 h at 4°C and rinsed with cold peptide-free medium prior to the addition of virus. Following entry, extracellular virus was inactivated and the cells were cultured with an overlay of DMEM with 2% methylcellulose and 2.0% serum. After incubation for 2.5 days, the monolayers were fixed with 10% formaldehyde in PBS and stained with crystal violet, and the number of plaques was counted.
RESULTS
In studies aimed at improving the antiviral activity of the TAT PTD, we prepared a peptide, TAT-C, with a C-terminal cysteine as a potential coupling site for peptide additions that might enhance the activity. We tested the activity of TAT-C in three basic assays. The ability to inhibit entry was tested by adding peptide immediately after adsorption. The ability to inactivate virions in solution was tested by exposing virus to peptide followed by dilution or pelleting of virus to remove the peptide prior to infection. To determine if pretreatment of cells induced resistance to infection, we exposed cells to peptide followed by rinsing and infection. This combination of assays revealed that the addition of the C-terminal cysteine to the TAT PTD did little to enhance entry inhibition but enabled the peptides to inactivate virions in solution and induce cellular resistance to infection.
TAT-C peptides inhibit HSV-1 entry.
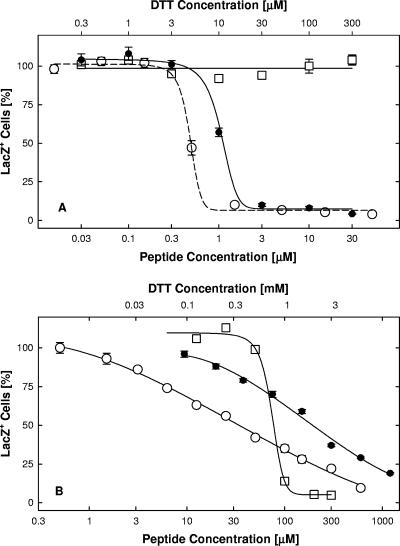
The results of the entry assay with TAT-Cd0 are shown in Fig. 1. Note that we previously showed that the TAT-C peptide was not toxic to several cell lines at concentrations up to 400 μM (1). In this case, the peptide was kept in the reduced (monomeric) form by the addition of a 10-fold molar excess of dithiothreitol (DTT). The addition of DTT alone at the concentrations used in these studies had no effect. As controls, we tested the unmodified TAT PTD (TAT0) and a peptide (TAT-Pd0), which was composed of d-enantiomers and also included an additional proline residue normally present in the TAT sequence. All three peptides displayed essentially identical activity, with EC50 values ranging from 0.3 to 1.0 μM. The results of these assays, and additional assays with variously modified TAT-C peptides, are summarized in Table 1 for comparison. For example, a comparison of TAT-Cd0 with TAT-C0 shows chirality is not critical for the activity of these peptides.
FIG. 1.
Entry inhibition. Virus (hrR3 at a MOI of 0.02) was adsorbed to confluent Vero cells in 96-well plates for 1 h at 4°C. Free virus was rinsed off, and entry was initiated by shifting the temperature to 37°C. One hour later, remaining extracellular virus was inactivated, and six hours later, cells were lysed and infectivity was estimated by measuring the initial rates of β-galactosidase activity relative to the rate in mock-treated controls. Inhibitors were added just before the temperature shift and remained present throughout the entry period: •, TAT0; ○, TAT-Pd0; ▵, TAT-Cd0 in the presence of a 10× molar excess of DTT; □, DTT alone. The data points represent the means of triplicate determinations with standard errors of the means.
To determine if net positive charge was important for the activity of TAT peptides (9), we tested the activities of peptides in which the terminal amide was replaced with a carboxyl group and/or pairs of lysines (n50,51TAT-C−) or arginines (n55,56TAT-C−) were substituted with norleucine (Table 1). A negative charge at the C terminus increased the EC50 value for entry inhibition 3- to 10-fold, whereas substitution of the paired lysines or arginines reduced entry-inhibiting activity 10-fold (Table 1). The substitution of positively charged amino acids and the addition of a negatively charged carboxyl group appeared to act synergistically in reducing the entry-blocking activity of TAT-C peptides (n50,51TAT-C− versus TAT-C0) and independently demonstrated that the net positive charge of these peptides is critical for antiviral activity.
Entry assays were unaffected by any postentry or cytotoxic effects of the peptides. Postentry effects were examined by attaching virus to cells for 1 h at 4°C and then shifting the cultures to 37°C for 1 h. Extracellular virus was then inactivated with pH 3 citrate buffer, peptides were added at various concentrations, and β-galactosidase activity was assayed 6 to 8 h later. Only one of the peptides, bTAT-C−, had any inhibitory effect and only at high concentrations (EC50, 175 μM). None of the peptides were found to be toxic, as determined by trypan blue exclusion (data not shown).
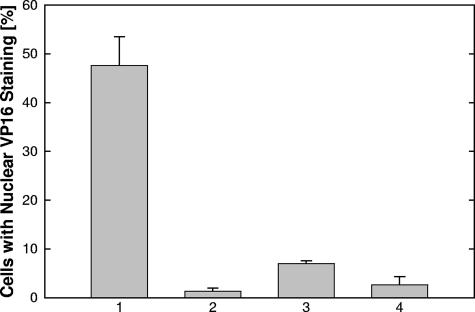
The fact that TAT peptides could block entry following virus attachment suggested that these peptides might interfere with entry itself. We therefore expected that an early event, VP-16 translocation to the nucleus, which had previously been used as a marker for viral entry, would be inhibited (10, 48). When virus was adsorbed at 4°C and allowed to enter cells at 37°C, we found 48% of the nuclei were positive for VP16 (Fig. 2, bar 1). When the cells were fixed at 4°C immediately following adsorption, only 1.3% of the nuclei were positive (Fig. 2, bar 2). If TAT-C− was added postadsorption, but prior to the temperature shift, only 6% of the nuclei were positive (Fig. 2, bar 3). Preincubation of virus with peptide prior to cell exposure resulted in only 2.5% of the cells staining positive (Fig. 2, bar 4). These results are consistent with TAT-C peptides inhibiting virus-cell fusion but did not exclude the possibility that these peptides might be internalized and block VP16 translocation directly.
FIG. 2.
TAT-C− blocks translocation of VP16 to the nucleus. Untreated virus (columns 1 to 3) and virus treated in solution with 300 μM TAT-C− for 1 h at 37°C (column 4) were adsorbed to precooled cells for 80 min at 4°C. Cells were either fixed immediately (column 2) or after a 1-h entry period at 37°C (columns 1, 3, and 4). In experiment 3, 50 μM TAT-C− was present from 20 min before shifting the cultures to 37°C through the end of the entry period. VP16 was detected by fluorescent microscopy following immunolabeling. As the result of peptide treatments (columns 3 and 4), nuclear VP16 labeling was significantly reduced compared to the positive control (column 1) and indistinguishable from background labeling (column 2) (two-tailed Student t test at 95% confidence level). The data points represent the means of triplicate determinations with standard errors of the means.
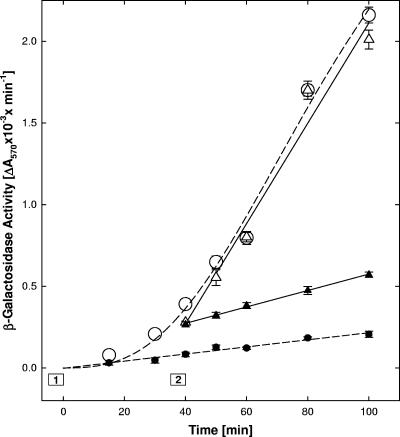
The TAT peptides are known to be internalized by endocytosis (44), which may involve the relatively slow caveolar pathway (17, 18). If peptide internalization was required for the antiviral activity, then entry inhibition would be expected to be delayed following the addition of peptide. We therefore measured the entry rates of adsorbed virus at 33°C in the absence or presence of n50,51TAT-C−. This peptide was chosen because it did not inactivate virions or induce cellular resistance to infection at the concentration used here (see below and Table 1). The n50,51TAT-C− peptide was also optimally active at low temperatures, which reduced entry rates by about 60% compared to the maximal rate measured at 37°C (8). Preliminary tests established that a temperature shift from 4 to 33°C initiated entry after a 20-min lag period (Fig. 3) and that addition of peptide just prior to the temperature shift (box 1) inhibited entry almost completely (Fig. 3). When n50,51TAT-C was added 35 min after the temperature shift (box 2), entry was inhibited immediately (Fig. 3). In cells that received mock peptide treatment 35 min after the temperature shift, entry proceeded at a rate identical to that in untreated control cells (Fig. 3). Thus, virus entry was arrested immediately. This, together with additional evidence (see Fig. 7D), indicated that peptide internalization was not required for entry inhibition.
FIG. 3.
Kinetics of entry inhibition. Virus was adsorbed for 1 h at 4°C to precooled cells plated in a strip-well plate, and entry was initiated by floating the plate in a 33°C water bath (0 time). Peptide (30 μM n50,51TAT-C−) was added either immediately before the temperature switch (box 1) or 35 min later (box 2; addition of peptide at ∼23°C, return to 33°C at 40 min). At the indicated times, any remaining external virus was inactivated with pH 3 citrate buffer, and 6 h later, cells were lysed and β-galactosidase activity was measured. Effects of peptide added early (•) or late (▴) were compared to mock-treated controls (○ versus ▵, respectively). The data points represent the means of triplicate determinations with standard errors of the means.
FIG. 7.
Rapid temperature-independent induction and persistence of cellular resistance to infection. Panel A: cell resistance is induced rapidly. Cultures in strip-well plates were exposed to 30 μM TAT-Cd− (•) or incubated with no peptide (○) at 37°C for the times indicated. Free peptide was removed by rinsing three times over a period of <5 min, and cells were infected with hrR3 (MOI, 0.04) in the absence of peptide, treated with pH 3 citrate buffer, and scored after X-Gal staining 8 h later. Cultures not incubated at 37°C (0 time points) were exposed to peptide at ∼23°C for <1 min before they were rinsed and infected. Panel B: induction of cellular resistance is not temperature dependent. Effects of cell pretreatments with TAT-Cd− for 1 h at 4 (○) or 37°C (•) were measured as described for panel A. Panel C: recovery of susceptibility to infection under normal culture conditions. Mock-treated cultures (○) and cultures exposed to 30 μM TAT-Cd− (•) for 1 h at 37°C were infected and scored for infectivity as described for panel A. Panel D: cell resistance is lost by rinsing with hypertonic medium. Cultures exposed to bTAT-Cd− for 1 h at 37°C were rinsed three times for 10 min at 4°C with peptide-free, serum-supplemented DMEM in the absence (○) or presence (•) of 0.5 M NaCl before they were infected as described for panel A. Infectivity was scored by measuring β-galactosidase activity in cell lysates 6 h postinfection. The data points represent the means of triplicate determinations with standard errors of the means.
Inactivation of virions in solution.
Although TAT-Cd0, TAT-Pd0, and TAT0 had the same ability to block entry, they differed significantly in the virus inactivation assay (Fig. 4A; Table 1). TAT-Cd0 inactivated virions with an EC50 of approximately 200 μM, whereas TAT-Pd0 required three to five times the amount of peptide and TAT0 was inactive. Peptide chirality was not important for virus inactivation, whereas charge was critical (Table 1). In contrast to their inhibitory effects on entry, lysine substitutions or the introduction of a carboxyl group to TAT-C0 enhanced virus inactivation three- to fourfold (Table 1; n50,51TAT-C0 versus TAT-C0 and TAT-C− versus TAT-C0, respectively). Only in combination did the substitution of pairs of positively charged amino acids and the addition of a negatively charged carboxyl group interfere with virus inactivation (Table 1; n50,51TAT-C− versus TAT-C0 and n55,56TAT-C− versus TAT-C0). Thus, both virus inactivation and entry inhibition were highly dependent on the charge of the TAT-C peptides but not necessarily in any coordinate manner.
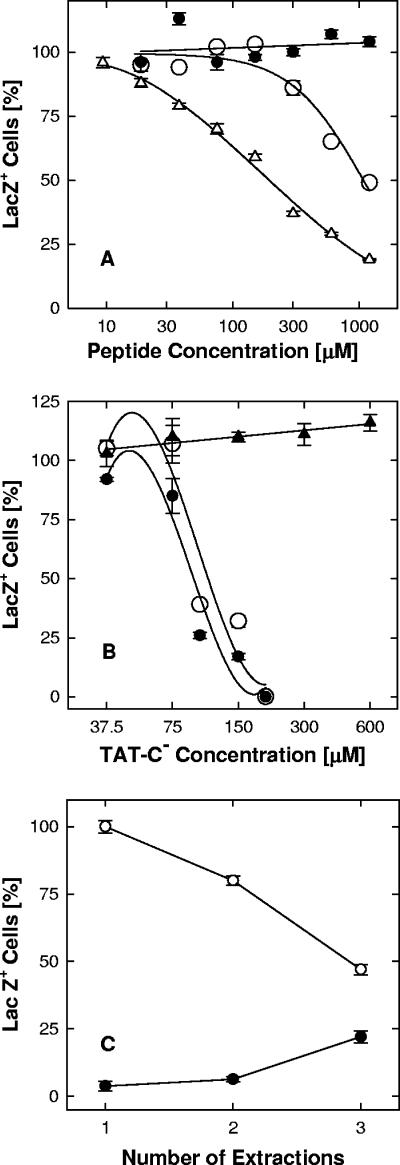
FIG. 4.
Virus inactivation in solution. Panel A: dose-dependent inactivation of virions. Virus in serum-free medium (1 × 106 PFU/ml) was exposed to TAT0 (•), TAT-Pd0 (○), or TAT-Cd0 (▵) for 1 h at 37°C. Incubation with monomeric TAT-Cd0 was done under strictly anaerobic conditions (argon atmosphere). Free peptide was removed by pelleting the virus in serum-supplemented medium, and the pelleted virus was resuspended by vortex treatment. Infectivity was scored in Vero cell cultures by measuring β-galactosidase activity in lysates. Panel B: temperature sensitivity of viral inactivation. Virus in serum-free medium (3 × 106 PFU/ml) was exposed to TAT-C− for 1 h at 4°C (▴) or 37°C (•, ○), and free peptide was either rendered ineffective by a 1,000-fold dilution of virus (▴, •) or removed by pelleting the virus as described for panel A (○). Infectivity was scored in X-Gal-stained cultures. Panel C: reversibility of viral inactivation. Virus in serum-free medium (6 × 106 PFU/ml) was mock treated (○) or exposed to 200 μM TAT-Cd− for 1 h at 37°C (•), pelleted in serum-supplemented medium, and resuspended by vortex treatment. Aliquots were assayed for infectivity by measuring β-galactosidase activity in lysates. As the result of the exposure to peptide, infectivity was reduced by 96% (extraction 1). The virus was subsequently extracted twice with 0.5 M NaCl in serum-supplemented medium for 30 min at 4°C, followed by sedimentation, resuspension by sonication, and measurements of infectivity in aliquots (extractions 2 and 3). As the result of the high-salt extractions, the peptide-treated virions regained some infectivity despite the loss of infectivity in mock-treated controls. The data points represent the means of triplicate determinations with standard errors of the means.
Viral inactivation by all of the TAT-C peptides was temperature dependent and did not occur below 10°C. For example, TAT-C− had no effect on infectivity at 4°C at concentrations up to 600 μM but completely inactivated virions at 37°C at a concentration of 200 μM regardless of whether the peptide was removed by dilution or by pelleting the virions (Fig. 4B).
Viral inactivation was difficult to reverse. Virions incubated with TAT-C− for 1 h at 37°C regained less than 1% of the original titer when incubated overnight in peptide-free medium at 4°C in the presence or absence of 0.5 M NaCl (data not shown). Mild trypsinization of treated virions under conditions that completely inactivated soluble TAT-C− also failed to restore infectivity. Only after repeated high-salt extractions did we see any recovery of infectivity, and this was only partial (Fig. 4C). Taking into account that the centrifugation and sonication steps reduced control titers by 54%, the infectivity of TAT-Cd−-inactivated virions was increased from 3.7 to 46% as the result of two salt extractions.
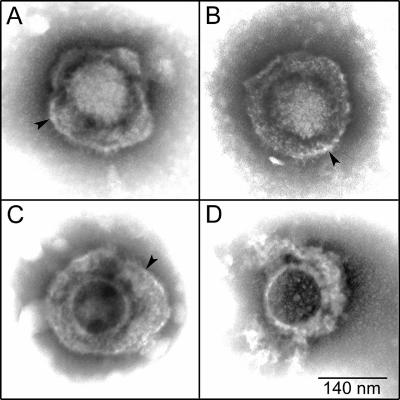
To test whether inactivation of virions in solution involved disruption of the structural integrity of virions, virus was adsorbed on grids, exposed to 11.4 mM TAT-C− or n50,51TAT-C−, negatively stained, and examined by electron microscopy. Virions treated with either peptide were indistinguishable from untreated controls (Fig. 5A to C). Most importantly, viral envelopes of treated virions were intact, as opposed to virions treated with 2% Triton X-100. In the detergent-treated virions, envelopes were disrupted and only the nucleocapsid with irregularly attached tegument material remained (Fig. 5D). Thus, even high concentrations of TAT-C peptides did not grossly alter the structure of virions.
FIG. 5.
Structure of inactivated virions. Virus (HSV-1 KOS) adsorbed to coated grids was treated with 11.4 mM TAT-C− (A) or n50,51TAT-C− (B) in serum-free DMEM (pH 7.4) for 30 min at 37°C and negatively stained. A mock-treated control (C) and adsorbed virus treated with 2% Triton X-100 (D) are included for comparison. Arrowheads point to intact viral envelopes.
Induction of cellular resistance to infection.
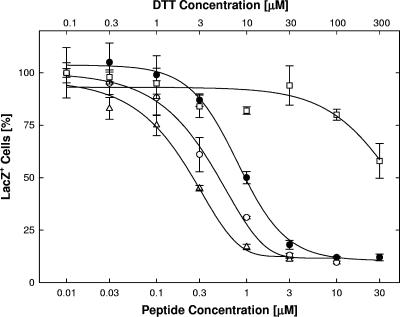
Peptides with similar entry-blocking activities differed substantially in their ability to induce cellular resistance to infection (Fig. 6; Table 1). The TAT-Cd0 peptide was an effective inducer of cellular resistance with an EC50 value of approximately 2 μM, whereas both TAT0 and TAT-Pd0 enhanced infection of the cells. The TAT-Cd0 peptide induced resistance to infection in HeLa cells and primary human foreskin fibroblasts as effectively as in Vero cells (data not shown).
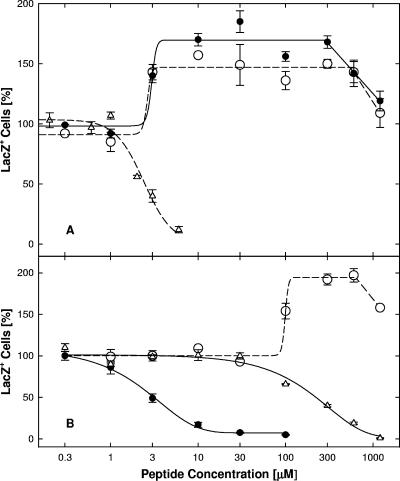
FIG. 6.
Modification of cellular susceptibility to infection. Panel A: pretreatments with TAT0 (•), TAT-Pd0 (○), or TAT-Cd0 (▵). TAT-Cd0 was administered in the presence of a 10-fold molar excess of DTT to keep the peptide in the reduced or monomeric form. Panel B: pretreatments with TAT-C− (•), n50,51TAT-C− (○), or n55,56TAT-C− (▵). Following pretreatments of Vero cells for 1 h at 37°C, free peptide was rinsed off and cultures were infected with hrR3 (MOI, 0.01) in the absence of peptide for 1 h at 37°C, treated with pH 3 citrate buffer, and scored for β-galactosidase activity 6 h later. The data points represent the means of triplicate determinations with standard errors of the means.
The presence of a carboxyl group at the C terminus of TAT-C peptides had little or no effect on the induction of cell resistance. In contrast, substituting the two lysines in TAT-C− not only eliminated the ability of the peptide to induce cell resistance but actually enhanced infectivity (n50,51TAT-C−; Fig. 6B). Changing a pair of arginines to norleucine (n55,56TAT-C−) also reduced the ability to induce resistance, increasing the EC50 over 50-fold, but did not enhance infection (Fig. 6B). These results indicate that induction of cellular resistance, like inhibition of entry or virus inactivation, strongly depended on the net positive charge of the peptides but not on chirality. In addition, these results suggest that the nature and/or position of charged residues may contribute to antiviral activity. The fact that TAT-Cd0 and TAT-Pd0 affected cellular susceptibility to infection in opposite directions confirms that sequence changes unrelated to charge or chirality are important.
The induction of cellular resistance occurred rapidly in a temperature-independent and reversible manner and could be reinduced once the cells had recovered susceptibility to infection. Following pretreatments of cultures with 30 μM TAT-Cd− at 37°C, infectivity was reduced by 70% within five minutes and maximum protection from infection was acquired by 20 min (Fig. 7A). In cultures exposed to peptide for less than 1 min at 23°C, the number of infected cells (Fig. 7A, 0 time point) was indistinguishable from the mock-treated controls (Fig. 7A), indicating that free peptide had been completely rinsed off. After removal of free peptide, the cells retained significant protection from infection for at least 8 h with a half-life of 5 to 6 h, but by 24 h, susceptibility had fully recovered (Fig. 7C). Following recovery of susceptibility to infection, a second exposure of the cells to the same peptide reinduced resistance without any loss of efficacy (data not shown).
In contrast to the relatively slow recovery of susceptibility under normal culture conditions, cellular resistance to infection was rapidly lost if peptide-treated cells were rinsed with hypertonic medium (Fig. 7D). Control cells exposed to bTAT-Cd− for 1 h at 37°C and rinsed with normal culture medium were rendered resistant to infection (EC50, 5.5 μM). Following rinses with hypertonic culture medium, resistance was lost except in cells treated with peptide concentrations exceeding 30 μM. Rinses as short as 5 min could restore nearly normal levels of susceptibility to infection, and rinses with media containing 50 μM heparin were as effective as hypertonic medium (data not shown). These results imply that even after exposure of the cells to peptide for 1 h at 37°C, resistance to infection remains dependent on extracellular peptide.
TAT-C peptides are inefficient adsorption inhibitors.
Both HSV-1 and the TAT PTD bind to cell surface HS (50, 51, 55, 64). It was therefore possible that the antiviral effects were mediated through the binding of TAT peptides to HS with subsequent inhibition of adsorption. We first examined the binding of biotinylated TAT peptides to intact and HS-deprived cells. It should be noted that the addition of a biotin group to the amino terminus of the peptides had little or no effect on entry inhibition (Table 1).
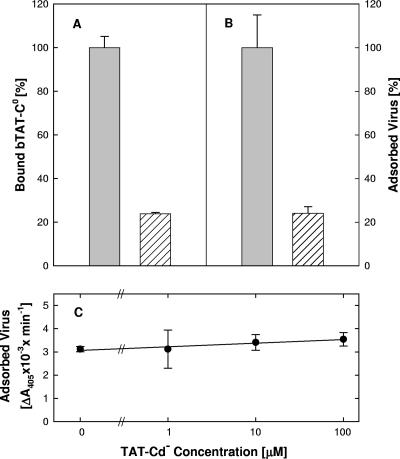
The bTAT-C0 peptide bound to paraformaldehyde-fixed cells at 37°C in a concentration-dependent manner approaching a maximum at 30 μM. Replacing a pair of arginines (bn55,56TAT-C−) reduced binding approximately fivefold, indicating that positive charge was important. Digestion of the fixed cells with heparinase III reduced bTAT-C0 binding (Fig. 8A) to the same extent that it blocked viral adsorption (Fig. 8B). Heparinase III had the same effect over a range of concentrations (0.002 to 0.01 units/ml), indicating that the maximum amount of HS had been removed (data not shown). Considering that about 10% of the total binding was to the sides of the culture wells, we estimated that over 80% of the peptide was bound to cell surface HS and expected bTAT-C0 to be an effective competitor of adsorption.
FIG. 8.
Peptide binding in competition with virus adsorption. Panels A, B: binding of 10 μM bTAT-C0 for 30 min at 37°C (A) and adsorption of hrR3 virus (MOI, ∼2) for 1 h at 37°C (B) to fixed cells, which had been mock treated (gray bars) or digested with 0.01 U/ml heparinase III for 2 h at 37°C (hatched bars) to remove HS. Panel C: cell pretreatment with peptide does not affect virus adsorption. Precooled live cells were exposed to TAT-Cd− for 1 h at 4°C. Free peptide was rinsed off and virus (MOI, ∼2) was added for 1 h at 4°C, when free virus was rinsed off and cells were fixed with 4% paraformaldehyde in PBS. The bound biotinylated peptide or adsorbed virus was detected in ELISAs. The data points represent the means of triplicate determinations with standard errors of the means.
When bTAT-C0 or bTAT-Cd− were present during adsorption, virus attachment to fixed cells at 37°C was inhibited by 50% at most and only at peptide concentrations exceeding 10 μM. With live cells, 100 μM bTAT-Cd− inhibited attachment of only 25% of the virus, whereas heparin, at the same concentration, blocked binding of more than 80% of the virus (data not shown). Competition for viral adsorption was also unrelated to the induction of cell resistance, as indicated by the finding that pretreatments of live cells with TAT-Cd− at 4°C had no effect on virus adsorption in the absence of peptide (Fig. 8C).
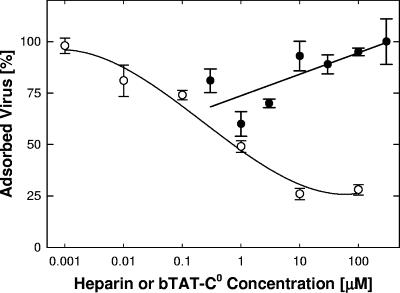
The ability of the peptides to elute virus was tested by adsorbing virions to paraformaldehyde-fixed cells, followed by addition of peptide, and by monitoring the amount of virus retained on cells. Heparin treatment released up to 75% of the virus (Fig. 9), whereas bTAT-C0 at concentrations up to 300 μM failed to elute any of the virus (Fig. 9) even though peptide was maximally bound to the cells (data not shown). We conclude from these results that TAT-C peptides are only weak competitors of adsorption and that inhibition of attachment is not a significant factor in the antiviral activity.
FIG. 9.
Virus elution by heparin and bTAT-C0. Virus was adsorbed to fixed cells for 1 h at 37°C, and wells were rinsed three times over a period of 30 min at 37°C. Heparin (○) or bTAT-C0 (•) was added, and cultures were incubated for 1 h at 37°C, rinsed, and assayed for retained virus by ELISA. Data points represent means and standard errors of the means of triplicate determinations.
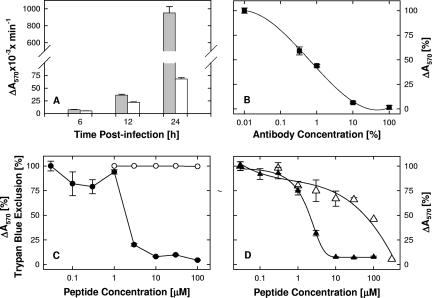
Inhibition of syncytium formation and cell-cell spread.
The finding that TAT-C peptides were inefficient attachment inhibitors and could block infection when added after attachment but before entry suggested that TAT-C peptides might inhibit the fusion step. This was further confirmed by determining if TAT-Cd− could block syncytium formation by hrR3S. Syncytium formation in the absence of peptide commenced between 10 and 12 h postinfection and by 24 h had increased 100-fold (Fig. 10A). In the presence of bTAT-C− (100 μM), β-galactosidase activity was reduced by 93% (Fig. 10A). Since the anti-HSV antibody present in the cultures prevented secondary infection (Fig. 10B), these results indicated that cell-cell fusion was inhibited. As shown in Fig. 10C, TAT-Cd− blocked fusion independent of cytotoxic effects (Fig. 10C) as efficiently as it blocked entry (Table 1). The TAT-C− peptide was as effective as TAT-Cd− (Fig. 10D), whereas n50,51TAT-C− was about 30-fold less effective (Fig. 10D), indicating that positive charge but not chirality was important for the ability of TAT-C peptides to block cell-cell fusion.
FIG. 10.
Fusion conditions and dose-dependent fusion inhibition. Panel A: time course of fusion. Cells were infected and then exposed to inactivating antibody in the absence (filled bars) or presence (open bars) of 100 μM TAT-Cd− as described in the legend to Fig. 10. β-Galactosidase activity was measured in lysates at the times indicated. Panel B: inactivating antibody concentrations. Antibody concentrations required for inactivation of extra cellular virus were determined by measuring inhibition of entry of hrR3 adsorbed to cells for 2 h at 4°C (MOI, 0.05). The infected cultures were exposed to various antibody dilutions for 1 h at 4°C and for 6 h at 37°C before β-galactosidase activity was measured in cell lysates (100% antibody concentration corresponds to a 10-fold antibody dilution). Panels C, D: dose-dependent inhibition of fusion. Cells were infected for 1 h at 37°C (MOI, 0.02) and then exposed to inactivating antibody and various concentrations of peptides for 24 h, at which time β-galactosidase activity was measured. (C) TAT-Cd− inhibited fusion (•; EC50, 1.9 μM) in the absence of cytotoxic effects (trypan blue exclusion; ○). (D) TAT-C− and n50,51TAT-C− inhibited fusion with EC50s of 2.1 (▴) and 60 μM (▵), respectively. The data points represent the means of triplicate determinations with standard errors of the means.
The TAT-C peptides also blocked cell-cell spread of wild-type virus. Vero cells were infected and cell-cell spread was monitored in the presence of anti-HSV antibody. At one day postinfection, small clusters of up to 100 cells showing cytopathic effect were present in cultures lacking peptide but no clusters were seen in the presence of TAT-Cd− (100 μM) (data not shown).
The effect of peptide dimerization.
To test whether the free sulfhydryl group in TAT-C was required for the antiviral activity, we oxidized the TAT-Cd0 peptide and examined the effect of the TAT-Cd0 dimer on cells and virions. Following pretreatment of both cells and virions, the TAT-Cd0 dimer proved to be at least as effective as the monomer (Fig. 11). To prevent the oxidation of monomers under aerobic conditions, cells were pretreated in freshly prepared peptide solutions in the presence of a 10-fold molar excess of DTT (Fig. 11A) (EC50, 1.1 μM). Exposure to DTT alone, at concentrations up to 300 μM, had no effect on cellular susceptibility to infection (Fig. 11A). Pretreatment with dimeric TAT-Cd0 in the absence of DTT blocked infection as effectively as, or more effectively than, the monomer (Fig. 11A) (EC50, 0.4 μM).
Virus inactivation by monomers or dimers was done under strictly anaerobic conditions in the absence of DTT, because pretreatment of virions with DTT alone blocked infection with an EC50 of 90 μM (Fig. 11B). Freshly prepared solutions of monomeric and dimeric peptide were flushed with argon and kept under argon throughout the pretreatment period. Under these conditions, dimers were approximately six times more effective than monomers (Fig. 11B) (EC50, 34 μM versus 200 μM, respectively). The availability of a free sulfhydryl group was therefore not essential for the induction of cellular resistance to infection nor for virus inactivation; however, the presence of the sulfhydryl group may enhance these antiviral activities through peptide dimerization.
Inhibition of HSV-2.
To determine if TAT peptides could block HSV-2 infection, we used a plaque reduction assay with HSV-2 strain 333. When TAT-Cd− was present during the entry period, infection was inhibited with an EC50 value of 1.3 μM. When cells were pretreated with TAT-Cd− for 1 h at 4°C, infection was also inhibited (EC50, 7.7 μM). An amidated form of this peptide, which also had the two lysines replaced with two norleucines (n50,51TAT-Cd0), was nearly as active as TAT-Cd− when present during entry (EC50, 2.5 μM) but was more than 10-fold less efficient in inducing cell resistance (EC50, 81 μM).
DISCUSSION
The most significant finding of the present study is that with the addition of a C-terminal cysteine residue, peptides derived from the TAT PTD gain the ability to inactivate virions in solution and to induce a state of cellular resistance to infection. The TAT-C peptides, to our knowledge, are the first cationic agents shown to render cells resistant to viral infection. The unmodified TAT PTD (TAT0) failed to induce resistance and also failed to inactivate virions even though it was an effective entry inhibitor.
The antiviral activities of TAT-C primarily depended on ionic rather than stereospecific or covalent interactions. When the net positive charge of the amidated TAT-C peptide (TAT-C0) was reduced from +9 to +6 by replacing pairs of lysine or arginine residues with norleucines and by replacing the amide with a carboxyl group, entry inhibition, virus inactivation, and induction of cellular resistance to infection were severely reduced or eliminated (Table 1; TAT-C0 versus n50,51TAT-C− or n55,56TAT-C−). There was, however, no simple correlation between net charge and antiviral activity, nor were the three antiviral activities always coordinately affected when the charge was changed. Thus, lysine substitutions without replacement of the amide in TAT-C0 reduced entry inhibition and the induction of cell resistance by ≥10-fold but enhanced virus inactivation three- to fourfold (Table 1; n50,51TAT-C0 versus TAT-C0). Some stereospecific interactions must also contribute to antiviral activities and account for the differential effects of lysine and arginine substitutions (Fig. 6B). Such stereospecific interactions, however, must play a limited role, since peptide chirality had little or no effect on any of the three antiviral activities of TAT-C. Covalent reactions of TAT-C are also not critical for activity. The free sulfhydryl group in TAT-C is not essential, since peptide dimers proved to be as active as, or even more active than, monomers. Peptide dimerization was not critical for the antiviral activity though, because TAT-C monomers could induce cellular resistance in the presence of excess DTT and inactivate virions under strictly anaerobic conditions (Fig. 11).
Several lines of evidence suggest that the TAT-C peptides are acting at the cell surface and inhibiting HSV entry at the fusion step. We found that TAT-C peptides were inefficient attachment competitors and could not elute bound virus from the cells. Peptides added after attachment of virus to cells at 4°C, but prior to the temperature shift to initiate entry, blocked infection. The TAT-C peptides blocked VP16 translocation to the nucleus without being required to enter cells. When we lowered the temperature to slow down the entry process, peptides added after entry initiation blocked infection immediately. The kinetics ruled out the possibility that the inhibitory effect was due to any slow endocytic process (17, 18). More-rapid peptide internalization (56) is also unlikely to contribute to the antiviral activity, because even after incubation of cells with peptide for an hour at 37°C, cellular resistance to infection remained dependent on extracellular peptide and resistance was lost following exposure to hypertonic medium or heparin. The most compelling data showing that TAT-C is a fusion inhibitor came from studies showing that TAT-C peptides block syncytium formation and cell-cell spread.
The primary receptor for TAT peptides on the cell surface is HS (55, 64), and consistent with this, we found that 80% of the bound peptide could be removed by treatments with heparinase III. HS may also determine the turnover rate of TAT-C at the cell surface, as suggested by the finding that susceptibility to infection recovered with a half-time of 5 to 6 h, which is comparable to the half-life of cell surface HS proteoglycans (62). The functional significance, if any, of the TAT-C binding to HS for the antiviral activity is not clear. Our observation that TAT-C is not effective in preventing attachment yet it binds to HS suggests that virus and TAT-C are recognizing different structural features. In addition, we have shown that TAT-C can induce cellular resistance to infection even after HS has been enzymatically removed from cells (data not shown). Thus, TAT-C binding to HS does not appear to be involved in the antiviral activity. Given the complexity of the cell surface, identifying the component or components involved will require additional studies.
Our observation that TAT-C inhibits infection in three ways raises the question of whether independent or related mechanisms are involved. Since the net positive charge of the peptide was critical for all activities, ionic interactions with negatively charged components of cells and virions are likely to be a common theme. However, several observations suggest that different targets are involved in the three antiviral activities. Changes in net charge did not always coordinately affect the three antiviral activities. Virus inactivation was essentially irreversible, whereas the induction of cellular resistance was readily reversed. Finally, virus inactivation typically required 30- to 150-fold higher TAT-C concentrations than entry inhibition or induction of cellular resistance (Table 1).
Preliminary studies suggest that TAT-C binds to sialic acid and that removal of sialic acid from virions reduces, or eliminates, TAT-C binding (unpublished data). Thus, it is possible that the inactivation of virions involves peptide binding to sialic acids on the viral glycoproteins. In this respect, TAT-C may be acting in a manner similar to positively charged defensins, some of which have been shown to bind to negatively charged carbohydrates (5, 15, 33, 49, 63). The binding of TAT-C to sialic acids on viral glycoproteins could inhibit infection in several ways. For example, TAT-C binding to envelope glycoproteins could prematurely trigger conformational changes thought to be required for assembly of the fusion complex or block protein-protein interactions critical for attainment of the fusogenic state. The fact that virus inactivation was temperature dependent and did not occur below 10°C (Fig. 4B) would be consistent with an effect on glycoprotein conformation or protein-protein interactions, which have previously been shown to be temperature dependent in influenza virions (32). Alternatively, TAT-C could be acting as a cross-linking agent that prevents conformational changes required for entry.
Previous studies with the modified θ-defensin RC2 showed that it acts by blocking attachment and some postadsorption step in HSV infection by binding to carbohydrates and functioning as a “minilectin” (57, 58, 63). Since TAT-C appears to bind to sialic acid (unpublished data), there may be similarities between TAT-C and RC2. However, the two peptides are not equivalent because RC2 does not irreversibly inactivate virions in solution whereas our data clearly show that virus inactivation by TAT-C is essentially irreversible. In addition, TAT-C primarily blocked entry at the fusion step rather than by inhibiting attachment. It is not known whether RC2 is capable of inducing cell resistance to infection. The TAT-C peptide also has the advantage of being easier to synthesize than RC2.
In summary, we have shown that the addition of a C-terminal cysteine residue to the TAT PTD peptide improves the antiviral activity against HSV by enabling the peptide to inactivate virions in solution and to induce cellular resistance to infection while retaining entry-blocking activity. The combination of these activities in the TAT-C peptide is unique among cationic antiviral peptides. In other work, we have shown that TAT-C shows little, if any, toxicity in cell culture or when applied topically to the mouse cornea (1). The TAT-C peptides may be useful as a topical agent to block sexual transmission of HSV, but this will require additional animal studies that are currently under way. The low toxicity, combined with the ability to inhibit HSV infection in multiple ways, makes TAT-C a strong candidate for further development as a drug to block HSV infection.
Acknowledgments
We thank Randall J. Massey and Ben August of the University of Wisconsin Medical School Electron Microscopy Facility for their help with the electron microscopy studies. We also thank Gary Case and Nina E. Porcaro from the University of Wisconsin Biotechnology Center for peptide synthesis, purification, and analysis.
These studies were supported by grants from the NIH (PO1-AI52049, RO1-EYO7336), a Core Grant for Vision Research, and DARPA (MDS 972097-1-0005); the University of Wisconsin University-Industry Relations Program, the University of Wisconsin—Madison Graduate School, the University of Wisconsin School of Medicine and Public Health, and by an unrestricted grant from Research to Prevent Blindness, Inc., to the Department of Ophthalmology and Visual Sciences.
Two patents relating to this work have been filed and the rights are assigned to the Wisconsin Alumni Research Foundation (WARF).
Footnotes
Published ahead of print on 29 January 2007.
REFERENCES
- 1.Akkarawongsa, R., A. E. Cullinan, A. Zinkel, J. Clarin, and C. R. Brandt. 2006. Corneal toxicity of cell-penetrating peptides that inhibit herpes simplex virus entry. J. Ocul. Pharmacol. Ther. 22279-289. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, J. H., H. Jenssen, K. Sandvik, and T. J. Guttenberg. 2004. Anti-HSV activity of lactoferrin and lactoferricin is dependent on the presence of heparin sulfphate at the cell surface. J. Med. Virol. 74262-271. [DOI] [PubMed] [Google Scholar]
- 3.Asbell, P. A., S. P. Epstein, J. A. Wallace, D. Epstein, C. C. Stewart, and R. M. Burger. 1998. Efficacy of cobalt chelates in the rabbit eye model for epithelial herpetic keratitis. Cornea 17550-557. [DOI] [PubMed] [Google Scholar]
- 4.Baghian, A., J. Jaynes, F. Enright, and K. G. Kousoulas. 1997. An amphipathic α-helical synthetic peptide analogue of melittin inhibits herpes simplex virus-1 (HSV-1)-induced cell fusion and virus spread. Peptides 18117-183. [DOI] [PubMed] [Google Scholar]
- 5.Belaid, A., M. Aouni, R. Khelifa, A. Trabelsi, M. Jemmali, and K. Hani. 2002. In vitro antiviral activity of dermaseptins against herpes simplex virus type 1. J. Med. Virol. 66229-234. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein, D. I., L. S. Loo, and S. Kohl. 1988. Antibody to cloned HSV glycoproteins B and D plus adult human leukocytes protect neonatal mice from lethal HSV infection. Antivir. Res. 10279-284. [DOI] [PubMed] [Google Scholar]
- 7.Brown, Z. A., S. Selke, J. Zeh, J. Kopelman, A. Maslow, R. L. Ashley, D. H. Watts, S. Berry, M. Herd, and L. Corey. 1997. The acquisition of herpes simplex virus during pregnancy. N. Engl. J. Med. 337509-515. [DOI] [PubMed] [Google Scholar]
- 8.Bultmann, H., and C. R. Brandt. 2002. Peptides containing membrane transiting motifs inhibit virus entry. J. Biol. Chem. 27736018-36023. [DOI] [PubMed] [Google Scholar]
- 9.Bultmann, H., J. S. Busse, and C. R. Brandt. 2001. Modified FGF4 signal peptide inhibits entry of herpes simplex virus type 1. J. Virol. 752634-2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheshenko, N., B. Del Rosario, C. Woda, D. Marcellino, L. M. Satlin, and B. C. Herold. 2003. Herpes simplex virus triggers activation of calcium-signalling pathways. J. Cell Biol. 163283-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corey, L., A. G. M. Langenberg, R. Ashley, R. E. Sekulovich, A. E. Izu, J. M. Douglas, Jr., H. H. Handsfield, T. Warren, L. Marr, S. Tyring, R. DiCarlo, A. A. Adimora, P. Leone, C. L. Dekker, R. L. Burke, W. P. Leong, and S. E. Straus. 1999. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. JAMA 282331-340. [DOI] [PubMed] [Google Scholar]
- 12.Corey, L., A. Wald, R. Patel, S. L. Sacks, S. K. Tyring, T. Warren, J. M. J. Douglas, J. Paavonen, R. A. Morrow, K. R. Beutner, L. S. Stratchounsky, G. Mertz, O. N. Keene, H. A. Watson, D. Tait, M. Vargas-Cortes, et al. 2004. Once daily valacyclovir to reduce the risk of transmission of genital herpes. N. Engl. J. Med. 35011-20. [DOI] [PubMed] [Google Scholar]
- 13.Dan, K., K. Miuashita, Y. Seto, S. Fujita, and T. Yamase. 2002. The memory effect of heteropolyoxotungstate (PM-19) pretreatment on infection by herpes simplex virus at the penetration state. Pharmacol. Res. 46357-362. [DOI] [PubMed] [Google Scholar]
- 14.Davis, W. B., J. A. Taylor, and J. E. Oakes. 1979. Ocular infection with herpes simplex virus type 1: prevention of acute herpetic encephalitis by systemic administration of virus-specific antibody. J. Infect. Dis. 140534-540. [DOI] [PubMed] [Google Scholar]
- 15.Egal, M., M. Conrad, D. L. Macdonald, W. L. Maloy, M. Motley, and C. A. Genco. 1999. Antiviral effects of synthetic membrane-active peptides on herpes simplex virus, type 1. Int. J. Antimicrob. Agents 1357-60. [DOI] [PubMed] [Google Scholar]
- 16.Eis-Hubinger, A. M., D. S. Schmidt, and K. E. Schneweis. 1993. Anti-glycoprotein B monoclonal antibody protects T cell-depleted mice against herpes simplex virus infection by inhibition of virus replication at the inoculated mucous membranes. J. Gen. Virol. 74379-385. [DOI] [PubMed] [Google Scholar]
- 17.Ferrari, M., V. Pellegrini, C. Arcangeli, A. Fittipaldi, M. Giacca, and F. Beltram. 2003. Caveolae-mediated internalization of extracellular HIV-1 fusion proteins visualized in real time. Mol. Ther. 8284-294. [DOI] [PubMed] [Google Scholar]
- 18.Fittipaldi, A., M. Ferrari, M. Zoppe, C. Arcanqeli, V. Pellegrini, F. Beltram, and M. Giacca. 2003. Cell membrane lipid rafts mediate caveolar endocytosis of HIV-1 Tat fusion proteins. J. Biol. Chem. 27834141-34149. [DOI] [PubMed] [Google Scholar]
- 19.Fukuma, M., Y. Seto, and T. Yamase. 1991. In vitro antiviral activity of polyoxotungstate (PM-19) and other polyoxometalates against herpes simplex virus. Antivir. Res. 16327-339. [DOI] [PubMed] [Google Scholar]
- 20.Geraghty, R. J., C. Krummenacher, G. Cohen, R. J. Eisenberg, and P. G. Spear. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor related protein 1 and poliovirus receptor. Science 2801618-1620. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein, D. J., and S. K. Weller. 1998. Herpes simplex virus type-1 ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. J. Virol. 62196-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gong, Y., B. Matthews, D. Cheung, T. Tam, I. Gadawski, D. Leung, G. Holan, J. Raff, and S. L. Sacks. 2002. Evidence of dual sites of action of dendrimers: SPL-2999 inhibits both virus entry and late stages of herpes simplex virus replication. Antivir. Res. 55319-329. [DOI] [PubMed] [Google Scholar]
- 23.Herold, B. C., S. I. Gerber, B. J. Belval, A. M. Siston, and N. Shulman. 1996. Differences in the susceptibility of herpes simplex virus types 1 and 2 to modified heparin compounds suggest serotype differences in viral entry. J. Virol. 703461-3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herold, B. C., S. I. Gerber, T. Polonsky, B. J. Belval, P. N. Shaklee, and K. Holme. 1995. Identification of structural features of heparin required for inhibition of herpes simplex virus type 1 binding. Virology 2061108-1116. [DOI] [PubMed] [Google Scholar]
- 25.Herold, B. C., I. Scordi-Bello, N. Cheshenko, D. Marcellino, M. Dzuzelewski, F. Francois, R. Morin, V. Mas Casullo, R. A. Anderson, C. Chany, D. P. Walker, L. J. D. Zaneveld, and M. E. Klotman. 2002. Mandelic acid condensation polymer: novel candidate microbicide for prevention of human immunodeficiency virus and herpes simplex virus entry. J. Virol. 7611236-11244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herold, B. C., A. Siston, J. Bremer, R. Kirkpatrick, G. Wilbanks, P. Fugedi, C. Peto, and M. Cooper. 1997. Sulfated carbohydrate compounds prevent microbial adherence by sexually transmitted disease pathogens. Antimicrob. Agents Chemother. 412776-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herold, B. C., R. J. Visalli, N. Susmarski, C. R. Brandt, and P. G. Spear. 1994. Glycoprotein-C independent binding of herpes simplex virus to cells requires cell surface heparan sulphate and glycoprotein B. J. Gen. Virol. 751211-1222. [DOI] [PubMed] [Google Scholar]
- 28.Herold, B. C., D. Wudunn, N. Soltus, and P. G. Spear. 1991. Glycoprotein C of herpes simplex virus type 1 plays a principle role in the adsorption of virus to cells and infectivity. J. Virol. 651090-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Highlander, S., S. L. Sutherland, P. J. Gage, D. C. Johnson, M. Levine, and J. C. Glorioso. 1987. Neutralizing monoclonal antibodies specific for herpes simplex virus glycoprotein D inhibit virus penetration. J. Virol. 613356-3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jenssen, H., J. Andersen, D. Mantzilas, and T. J. Gutteberg. 2004. A wide range of medium-sized, highly cationic, α-helical peptides show antiviral activity against herpes simplex virus. Antivir. Res. 64119-126. [DOI] [PubMed] [Google Scholar]
- 31.Jenssen, H., J. H. Andersen, L. Uhlin-Hansen, T. J. Gutteberg, and O. Rekdal. 2004. Anti-HSV activity of lactoferricin analogues is only partly related to their affinity for heparan sulfate. Antivir. Res. 61101-109. [DOI] [PubMed] [Google Scholar]
- 32.Junakar, P. R., and R. J. Cherry. 1986. Temperature and pH dependence of the haemolytic activity of influenza virus and of the rotational mobility of the spike glycoproteins. Biochim. Biophys. Acta 854198-206. [DOI] [PubMed] [Google Scholar]
- 33.Leikina, E., H. Delanoe-Ayri, K. Melikov, M.-S. Cho, A. Chen, A. J. Waring, W. Wang, Y. Xie, J. A. Loo, R. I. Lehrer, and L. V. Chernomordik. 2005. Carbohydrate-binding molecules inhibit viral fusion and entry by crosslinking membrane glycoproteins. Nat. Immunol. 6995-1001. [DOI] [PubMed] [Google Scholar]
- 34.Liesegang, T. J. 2001. Herpes simplex virus epidemiology and ocular importance. Cornea 201-13. [DOI] [PubMed] [Google Scholar]
- 35.Maguire, R. A., N. Bergman, and D. M. Phillips. 2001. Comparison of microbicides for efficacy in protecting mice against vaginal challenge with herpes simplex virus type 2, cytotoxicity, antibacterial properties, and sperm immobilization. Sex. Transm. Dis. 28259-265. [DOI] [PubMed] [Google Scholar]
- 36.Marchetti, M., E. Trybala, F. Superti, M. Johansson, and T. Bergstrom. 2004. Inhibition of herpes simplex virus infection by lactoferrin is dependent on interference with the virus binding to glycosaminoglycans. Virology 318405-413. [DOI] [PubMed] [Google Scholar]
- 37.Mertz, G. J., R. Ashley, R. L. Burke, J. Benedetti, C. Critchlow, C. C. Jones, and L. Corey. 1989. Double-blind, placebo-controlled trial of a herpes simplex virus type 2 glycoprotein vaccine in persons at high risk for genital herpes infection. J. Infect. Dis. 161653-660. [DOI] [PubMed] [Google Scholar]
- 38.Montgomery, R. I., M. S. Warner, B. J. Lum, and P. G. Spear. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87427-436. [DOI] [PubMed] [Google Scholar]
- 39.Nealon, K., W. W. Newcomb, T. R. Pray, C. S. Craik, J. C. Brown, and D. H. Kedes. 2001. Lytic replication of Kaposi's sarcoma-associated herpesvirus results in the formation of multiple capsids species: isolation and molecular characterization of A, B, and C capsids from a gammaherpesvirus. J. Virol. 752866-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nyberg, K., M. Eklblad, T. Bergstrom, C. Freeman, N. Bergman, C. R. Parish, V. Ferro, and E. Trybala. 2004. The low molecular weight heparan sulfate mimetic, PI-88, inhibits cell-to-cell spread of herpes simplex virus. Antivir. Res. 6315-24. [DOI] [PubMed] [Google Scholar]
- 41.Okazaki, K., and H. Kida. 2004. A synthetic peptide from a heptad repeat region of herpesvirus glycoprotein B inhibits virus replication. J. Gen. Virol. 852131-2137. [DOI] [PubMed] [Google Scholar]
- 42.Patel, R. 2004. Antiviral agents for the prevention of the sexual transmission of herpes simplex in discordant couples. Curr. Opin. Infect. Dis. 1745-48. [DOI] [PubMed] [Google Scholar]
- 43.Pope, L. E., J. F. Marcelletti, L. R. Katz, Y. Y. Lin, D. H. Katz, M. L. Parish, and P. G. Spear. 1998. The anti-herpes simplex virus activity of n-docosanol includes inhibition of the viral entry process. Antivir. Res. 4085-94. [DOI] [PubMed] [Google Scholar]
- 44.Richard, J. P., K. Melikov, E. Vives, C. Ramos, B. Verbeure, M. J. Gail, L. V. Chernomordik, and B. Lebleu. 2003. Cell penetrating peptides. J. Biol. Chem. 278585-590. [DOI] [PubMed] [Google Scholar]
- 45.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2460. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 46.Sacks, S. L., P. D. Griffiths, L. Corey, C. Cohen, A. Cunningham, G. M. Dusheiko, S. Self, S. Spruance, L. R. Stanberry, A. Wald, and R. J. Whitley. 2004. HSV-2 transmission. Antivir. Res. 63(Suppl. 1)S27-S35. [DOI] [PubMed] [Google Scholar]
- 47.Sanna, P. P., A. DeLogu, R. A. Williamson, Y.-L. Hom, S. E. Straus, F. E. Bloom, and D. R. Burton. 1996. Protection of nude mice by passive immunization with a type-common human recombinant monoclonal antibody against HSV. Virology 215101-106. [DOI] [PubMed] [Google Scholar]
- 48.Schwartz, J. A., E. K. Lium, and S. J. Silverstein. 2001. Herpes simplex virus type 1 entry is inhibited by the cobalt chelate complex CTC-96. J. Virol. 754117-4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sinha, S., N. Cheshenko, R. I. Lehrer, and B. C. Herold. 2003. NP-1, a rabbit α-defensin, prevents the entry and intercellular spread of herpes simplex virus type 2. J. Virol. 47494-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spear, P. G., R. J. Eisenberg, and G. H. Cohen. 2000. Three classes of cell surface receptors for alphaherpes virus entry. Virology 2751-8. [DOI] [PubMed] [Google Scholar]
- 51.Spear, P. G., and R. Longnecker. 2003. Herpesvirus entry: an update. J. Virol. 7710179-10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stanberry, L. R. 2004. Clinical trials of prophylactic and therapeutic herpes simplex virus vaccines. Herpes 11161A-169A. [PubMed] [Google Scholar]
- 53.Sumner-Smith, M., Y. Zheng, Y. P. Zhang, E. M. Twist, and S. C. Clime. 1995. Antiherpetic activities of N-α-acetyl-nona-d-arginine amide acetate. Drugs Exp. Clin. Res. XXI1-6. [PubMed] [Google Scholar]
- 54.Tam, J. P., C.-R. Wu, W. Liu, and J.-W. Zhang. 1991. Disulfide bond formation in peptides by dimethyl sulfoxide. Scope and applications. J. Am. Chem. Soc. 1136657-6662. [Google Scholar]
- 55.Tyagi, M., M. Rusnati, M. Presta, and M. Giacca. 2001. Internalization of HIV-1 tat requires cell surface heparan sulfate proteoglycans. J. Biol. Chem. 2763254-3261. [DOI] [PubMed] [Google Scholar]
- 56.Wadia, J. S., R. V. Stan, and S. F. Dowdy. 2004. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat. Med. 10310-315. [DOI] [PubMed] [Google Scholar]
- 57.Wang, W., A. M. Cole, T. Hong, A. J. Waring, and R. I. Lehrer. 2003. Retrocyclin, an antiretroviral θ-defensin, is a lectin. J. Immunol. 1704708-4716. [DOI] [PubMed] [Google Scholar]
- 58.Wang, W., S. M. Owen, D. L. Rudolph, A. M. Cole, T. Hong, A. J. Waring, R. B. Lal, and R. I. Lehrer. 2004. Activity of α- and θ-defensins against primary isolates of HIV-1. J. Immunol. 173515-520. [DOI] [PubMed] [Google Scholar]
- 59.Wender, P. A., D. J. Mitchell, K. Pattabiraman, E. T. Pelkey, L. Steinman, and J. B. Rothbard. 2000. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters. Proc. Natl. Acad. Sci. USA 9713003-13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Whaley, K. J., L. Zeitlin, R. A. Barratt, T. E. Hoen, and R. A. Cone. 1994. Passive immunization of the vagina protects mice against vaginal transmission of genital herpes infections. J. Infect. Dis. 169647-649. [DOI] [PubMed] [Google Scholar]
- 61.Whitley, R. J. 1996. Herpes simplex viruses, p. 2297-2342. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed., vol. 2. Lippincott-Raven, Philadelphia, PA. [Google Scholar]
- 62.Yanagishita, M., and V. C. Hascall. 1992. Cell surface heparin sulfate proteoglycans. J. Biol. Chem. 2679451-9454. [PubMed] [Google Scholar]
- 63.Yasin, B., W. Wang, M. Pang, N. Chesenko, T. Hong, A. J. Waring, B. C. Herold, E. A. Wagar, and R. I. Lehrer. 2004. θ-Defensins protect cells from infection by herpes simplex virus by inhibiting viral adhesion and entry. J. Virol. 785147-5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ziegler, A., and J. Seelig. 2004. Interaction of the protein transduction domain of HIV-1 TAT with heparan sulfate: binding mechanism and thermodynamic parameters. Biophys. J. 86252-263. [DOI] [PMC free article] [PubMed] [Google Scholar]