Abstract
A human cervical explant culture was utilized for the preclinical assessment of anti-human immunodeficiency virus type 1 (HIV-1) activity and tissue toxicity of formulated, candidate topical microbicides. Products tested included cellulose acetate 1,2-benzene dicarboxylate (CAP), a carrageenan-based product (PC-515), a naphthalene sulfonate polymer (PRO 2000), a lysine dendrimer (SPL7013), a nonnucleoside reverse transcriptase inhibitor (UC781), and an antimicrobial peptide (D2A21), along with their placebos. Cervical explants were cultured overnight with HIV-1 with or without product, washed, and monitored for signs of HIV-1 infection. HIV-1 infection was determined by p24gag levels in the basolateral medium and by immunohistochemical analysis of the explant. Product toxicity was measured by the MTT [1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan] assay and histology. CAP, PRO 2000, SPL7013, and UC781 consistently prevented HIV-1 infection in all explants tested. PC-515 and D2A21 prevented HIV-1 infection in 50% or fewer of the explants tested. Placebos did not prevent infection in any of the explants tested. With the exception of PRO 2000 (4%), the MTT assay and histological analysis of the other products and placebos showed minimal toxicity to the epithelium and submucosa. Collectively, these data suggest that this culture system can be used for evaluating the safety and efficacy of topical microbicides designed for vaginal use.
Sexual transmission of human immunodeficiency virus type 1 (HIV-1) fuels the HIV/AIDS pandemic, with more than 90% of all adolescent and adult HIV-1 infections resulting from heterosexual intercourse (23). Worldwide, approximately half of the 42 million people living with HIV/AIDS are women, and that proportion is growing (23, 37). A variety of biological and socioeconomic factors contribute to women's vulnerability to infection. Studies in discordant couples indicate that the virus is more easily transmitted from male to female than from female to male (30, 33, 34). Anatomic and histological differences in the genital mucosa between men and women may account for the increased susceptibility of women to HIV-1 infection (14). The presence of sexually transmitted diseases, the exchange of sex for drugs or money, or limited control of their sexuality places many women at increased risk for acquiring HIV-1. Despite evidence suggesting that proper use of condoms is effective at decreasing sexual transmission of HIV-1 (11), they are not used consistently. As a result, women are at risk for acquiring HIV-1 due to exposure to infectious seminal fluid during intercourse with high-risk sex partners (12, 40).
Significant advances have been made in the development of antiretroviral drugs, resulting in enhanced quality of life and reduced morbidity and mortality. However, fueled by the lack of access to these drugs and the lack of a preventative vaccine, the spread of HIV-1 continues unabated. Over the past decade, an alternative strategy focused on the development of low-cost intravaginal formulations of anti-HIV-1 agents to curb mucosal and perinatal HIV-1 transmission, termed microbicides, has emerged (15, 21). While topical formulations of reverse transcriptase inhibitors (RTIs) such as N-[4-chloro-3-(3-methyl-2-butenyloxy)phenyl]-2-methyl-3-furancarbothioamide (UC781, a nonnucleoside RTI [NNRTI]) and 9-[2-(phosphonomethoxy)propyl]adenine (PMPA, a nucleoside RTI) are being considered, the majority of candidate topical microbicides are designed to disrupt the virus or block adherence of HIV-1 to target genital tissues (4). More novel approaches include plant-derived antibodies and lactobacilli engineered to secrete potential microbicides (4) to augment the vaginal environment.
The first product to be evaluated as a topical microbicide was one containing the surfactant nonoxynol-9 (N9), originally developed as a spermicide with known in vitro anti-HIV-1 activity. The results from the clinical trial (41) demonstrated the toxic effects of N9. Moreover, HIV-1 infection of the women using the N9 product was higher than that of the women receiving the placebo. This work has emphasized the need for improved preclinical testing of microbicides. Although several groups have used epithelial cell lines, peripheral blood mononuclear cells, and blood-derived macrophages to evaluate product toxicity and efficacy (5, 16), these cell types are not representative of the primary cells, both epithelial and immune target cells, present in the genital mucosa.
To better approximate the in vivo setting, we have developed a polarized cervical explant culture system to comparatively evaluate the toxicity and efficacy of topical microbicides that are formulated for use in humans. Six candidate topical microbicides and N9 were evaluated for their ability to prevent HIV-1 infection and for their possible toxicity on cervical tissue. The products include those that (i) maintain or enhance normal vaginal defense mechanisms (e.g., an antimicrobial peptide), (ii) disrupt or inactivate the pathogen (e.g., cellulose acetate 1,2-benzene dicarboxylate [CAP]), (iii) block binding and fusion of pathogens (e.g., a naphthalene sulfonate polymer, a lysine dendrimer, and CAP), and (iv) affect the pathogen life cycle (e.g., an NNRTI). The results presented here demonstrate that this cervical explant culture provides additional information for the preclinical assessment of topical microbicides to prevent HIV-1 transmission during vaginal intercourse.
(This work was presented in part at the following meetings: Microbicides 2002, May 2002, Antwerp, Belgium, and Microbicides 2004, March 2004, London, England.)
MATERIALS AND METHODS
Tissues.
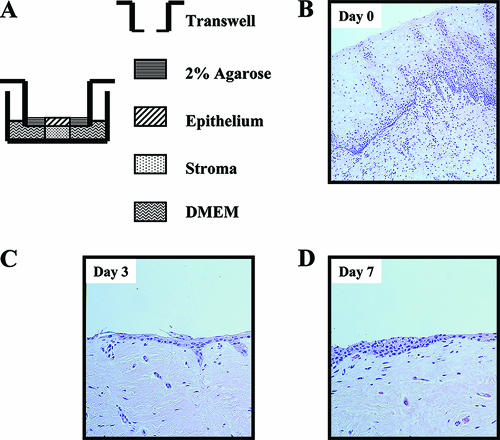
Unless otherwise stated, all tissue culture reagents were obtained from Invitrogen Corp. (Carlsbad, CA). After obtaining informed consent, normal ectocervical tissue (i.e., with no clinically observed disease) was acquired from premenopausal women undergoing routine hysterectomy. Tissues were transported on ice in L15 medium containing 10% fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2.5 μg/ml fungizone (amphotericin B). The cervix was washed twice with phosphate-buffered saline (PBS). Excess submucosal tissue was removed to produce a flat piece containing both epithelium and stromal tissue. Dermal biopsy punches (5-mm diameter) (Miltex Instrument Company, Inc., Bethpage, NY) were used to cut full-thickness tissue specimens. Each explant was inserted through a 4-mm-diameter hole in a transwell insert (12-mm diameter, polyester membrane; Corning, New York, NY) with the epithelium oriented upward in the apical chamber. The epithelial surface of the explant was surrounded with 2% agarose to maintain the tissue orientation. In the basolateral chamber, the stroma was cultured in 0.6 ml of Dulbecco's minimal essential medium containing 10% human AB serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and nonessential amino acids (cDMEM) (Fig. 1A). Tissues were cultured at 37°C under 7% CO2 in a humidified incubator.
FIG. 1.
Cervical explant culture. (A) Circular tissue explants were inserted through a hole in a transwell insert with the epithelium oriented upward in the apical chamber. The epithelial surface of the explant was surrounded with 2% agarose to maintain tissue orientation and minimize seepage of microbicides and/or virus around the tissue edges. The stroma was cultured in cDMEM in the basolateral chamber. HIV-1 alone in medium or mixed with microbicide was applied to the apical surface to simulate mucosal exposure in vivo. (B to D) Histology of a representative hematoxylin-and-eosin-stained normal human cervix at day 0 (day of surgery; B), day 3 (C), and day 7 (D) of culture. Original magnification, ×50.
Tissue permeability.
To evaluate the integrity of the explants, the transmission of fluorescent polymer microspheres (0.026-μm diameter; Duke Scientific Corp., Palo Alto, CA) was tested. After removal of the suspension solution, the microspheres were washed and suspended in PBS (1 × 1014 microspheres/ml). On the first day of culture, 0.5 ml of the microspheres was added to the epithelial surface. As controls, the same volume of microspheres was added to a blank well and a well containing 2% agarose. The basolateral medium was sampled at 1, 3, and 7 days. The fluorescence in the basolateral supernatant was measured using a Fluoroskan Ascent FL fluorometer (Thermo Electron Corp., Waltham, MA) using 485-nm- (excitation) and 538-nm- (emission) wavelength filters. The percent transmission was calculated by dividing the fluorescent signal in the basolateral supernatant from the explants and agarose control by that from the blank well.
Topical microbicides.
Six candidate microbicides were evaluated in formulations designed for use in humans (Table 1). Four percent N9 (Ortho-McNeil Pharmaceuticals, Inc., Raritan, NJ), an approved over-the-counter product, was used as a control for tissue toxicity. PC-515, a carrageenan-based product, was provided by the Population Council (New York, NY). PRO 2000, a naphthalene sulfonate polymer, was provided by Procept, Inc. (Cambridge, MA). CAP, a lysine dendrimer (SPL7013), an NNRTI (UC781), and an antimicrobial peptide (D2A21) were provided in response to a solicitation placed in the Federal Register by the Division of HIV/AIDS Prevention, CDC, which requested proposals for collaboration in the evaluation of potential microbicide agents (9, 10). CDC remains interested in testing new agents using these methods; potential agents should have demonstrated in vitro anti-HIV-1 activity and have been formulated for vaginal or rectal application (8).
TABLE 1.
Topical microbicide products and placebos tested
| Product (reference[s])a | Product description | Placebo | Placebo description | Product manufacturer |
|---|---|---|---|---|
| 3% carrageenan-based product (PC-515) (25) | Linear sulfated polysaccharide (lambda- and kappa-carrageenan) with antimicrobial activity | Methyl cellulose | 2.5% aqueous gel | Population Council (New York, NY) |
| 13% cellulose acetate CAP (29) | Pharmaceutical excipient; polycarboxylic polymer with antimicrobial activity | Methyl cellulose | 2.5% aqueous gel | Lindsley F. Kimball Research Institute (New York, NY) and Dow Pharmaceutical Sciences (Petaluma, CA) |
| 0.5% and 4% naphthalene sulfonate (PRO 2000) (28) | Sulfonated polymer with antimicrobial activity | Cross-linked polyacrylic acid polymer-based aqueous gel | 0.5% and 4% formulations contain 1.35% and 2% gelling agent, respectively, with pH buffering capacity | Indevus Pharmaceuticals, Inc. (Lexington, MA) |
| 5% lysine dendrimer (SPL7013) (7, 22) | Surface has been derivatized with sodium naphthalene disulfonate groups with antimicrobial activity | Cross-linked polyacrylic acid polymer-based aqueous gel | 5% gelling agent with pH buffering capacity | Starpharma Limited (Melbourne, Australia) |
| 0.1% and 1% NNRTI (UC781) (2) | Tightly binding NNRTI; anti-HIV-1 activity only | Cross-linked polyacrylic acid polymer-based aqueous gel | 1% gelling agent with pH buffering capacity | Biosyn, Inc. (Huntingdon Valley, PA) |
| 1% antimicrobial peptide (D2A21) (24) | Synthetic 23-amino-acid amphipathic peptide with antimicrobial activity | Hydroxyethyl cellulose | 3.25% aqueous gel | Demegen, Inc. (Pittsburgh, PA) |
The percent concentration of each formulated product is shown.
HIV-1 infection of cervical explants.
Explants were activated for 2 days in cDMEM containing 5 μg/ml phytohemagglutinin-P (PHA; Difco Laboratories, Detroit, MI) and 100 U/ml human interleukin-2 (Roche, Indianapolis, IN). HIV-1BaL was purchased from ABI (Columbia, MD) and supplied with a known endpoint titer calculated by the Karber method (17). On day 3, 0.5 ml of HIV-1BaL (5 × 104 50% tissue culture infective doses [TCID50]) in cDMEM was applied to the epithelium and incubated for 18 h. Afterwards, the epithelium was washed five times with PBS to remove residual virus, and the basolateral medium was replaced with cDMEM containing interleukin-2 without PHA. The culture medium was harvested twice weekly over a 14- to 17-day period and stored at −70°C. Viral replication was determined by using a p24gag enzyme-linked immunosorbent assay kit (Beckman Coulter, Miami, FL). To demonstrate growth of primary HIV-1 subtypes, 104 TCID50 of 96USSN20/A (CCR5/CXCR4 using), 97USSN54/A (CCR5 using), and 92US660/B (CCR5 using), obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH, were used to infect cervical explants from one tissue donor. Endpoint titers for the primary HIV-1 stocks were determined using the Reed and Muench method (38).
Efficacy of microbicides.
To facilitate the even spread of products over the tissues, microbicides or placebos were diluted 1:10 (vol/vol) in 0.5 ml cDMEM containing 5 × 104 TCID50 HIV-1BaL and mixed. After 18 h of exposure to the product and virus, the epithelium was washed five times with PBS to remove residual product and virus, and the tissues were cultured as described above. Virus replication was determined in harvested culture supernatants by Coulter p24gag enzyme-linked immunosorbent assay. HIV-1 p24 levels were determined from standard curves using a four-parameter logistic algorithm (DeltaSoft microplate analysis software; BioMetallics, Princeton, NJ). Samples with concentrations that were too high to measure accurately were diluted in cDMEM to produce values in the linear range of the assay. Each product was tested in tissues from at least two donors. Tissues from a total of 16 donors were used in this study. For each treatment condition (virus alone, microbicide plus virus, and placebo plus virus), one replicate per donor was tested in tissues from at least two donors. The following final concentrations were tested for efficacy and toxicity: (i) microbicides: 0.4% N9, 0.3% PC-515, 1.3% CAP, 0.05% and 0.4% PRO 2000, 0.5% SPL7013, 0.01% and 0.1% UC781, and 0.1% D2A21, and (ii) placebos: 0.25% methyl cellulose (for PC-515 and CAP), 0.2% cross-linked polyacrylic acid polymer (for PRO 2000), 0.5% cross-linked polyacrylic acid polymer (for SPL7013), 0.1% cross-linked polyacrylic acid polymer (for UC781), and 0.3% hydroxyethylcellulose (for D2A21).
Histology and IHC.
Tissues were fixed in formalin and processed to evaluate general tissue architecture and product toxicity. Following routine paraffin embedding, sections were cut and stained with hematoxylin and eosin. Normal ectocervical tissue contains ∼15 layers of epithelium, including the basal layer and stratified layers of epithelial cells that mature as they reach the surface. In tissue that is cultured ex vivo, there is a loss of the outer stratified layers and a retention of the basal layer. Toxicity was determined by examining the loss of this remaining basal layer of epithelium and necrosis in the submucosa. Epithelial necrosis, sloughing, and regeneration of the epithelium and necrosis of the submucosa were evaluated as indicators of toxicity. For evaluation of the tissues described above in the efficacy studies, at the study endpoint tissues were fixed and processed for histology to determine product toxicity and immunohistochemical (IHC) analysis for p24 antigen to determine product efficacy. For each tissue donor, one section from each treated explant was analyzed for p24 antigen by IHC.
Four-micron sections of paraffin-embedded explants were used for IHC assays. Sections were deparaffinized, rehydrated, digested in 0.1 mg/ml proteinase K (Roche Molecular Biochemicals, Indianapolis, IN) in 0.6 M Tris-HCl (pH 7.5)-0.1% CaCl2 for 15 min, washed, and incubated at room temperature for 1 h with anti-p24 (Dako Corp., Carpinteria, CA). Slides were then washed, and swine anti-mouse biotinylated link antibody, alkaline phosphatase-labeled streptavidin, and naphthol fast red chromogenic substrate (LSAB2 universal alkaline phosphatase system; Dako Corp.) were sequentially applied. Sections were then counterstained in Mayer's hematoxylin (Fisher Scientific, Pittsburgh, PA). Negative controls were tissue sections from each explant incubated with normal mouse ascitic fluid.
To determine which cells were HIV-1 infected, double-staining was performed by using the Envision double-stain system (Dako Corp.). Sections were pretreated as previously described and incubated for 1 h with anti-S-100, anti-CD3, or anti-CD68 (Dako Corp.). A horseradish peroxidase-labeled polymer was applied for 30 min, and detection was performed by incubating the specimens for 5 min with liquid diaminobenzidine plus substrate-chromogen. A double-stain block for endogenous alkaline phosphatase activity was applied for 3 min. Tissue sections were then incubated for 1 h with the antibody against p24 and followed by a 30-min application of an alkaline phosphatase-labeled polymer. Fast red substrate (15 min of incubation) was used to detect the second antigen. Sections were counterstained in Meyer's hematoxylin.
MTT assay.
Viability of the explants and assessment of microbicide toxicity was quantified by the reduction of the tetrazolium salt 1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan (MTT) by viable tissue into a methanol-soluble formazan product. Cervical explants (4-mm diameter) were incubated with or without product diluted 1:10 in cDMEM. For comparison, a tissue control that had been incubated in medium alone was used. After culturing for 18 h, explants were washed five times in PBS. For determination of toxicity, tissues were immediately cultured in cDMEM containing MTT (250 μg/ml) for an additional 3 h at 37°C. Tissue viability was determined by dividing the optical density of the formazan product (570 nm) by the dry weight of the explant. The effect of each microbicide on tissue viability was determined by comparing the viability of the treated explants to the untreated tissue control. Tissue from a minimum of two donors was tested for each product. A Tukey's multiple comparison test (GraphPad software, version 4) was used to determine significant differences in MTT levels.
RESULTS
Validation of the cervical explant culture system. (i) Tissue morphology, cellular distribution, and permeability.
The design of the culture is presented in Fig. 1A. Under these conditions, the outer stratified layers of epithelium had sloughed off by day 3 of culture (Fig. 1C; compare to day of surgery [Fig. 1B]). Although the basal epithelium showed signs of proliferation at day 7 (Fig. 1D), the normal thickness and differentiation of the stratified epithelium were not recuperated. The cells and architecture of the submucosa remained intact throughout the culture period, as shown by histology. Activation of the tissue with PHA did not adversely affect the tissue's architecture (data not shown).
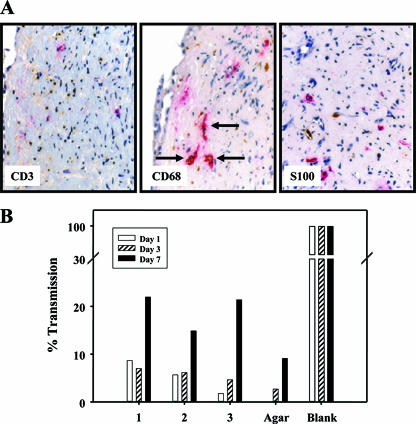
IHC was used to determine the presence of target cells for HIV-1 (CD3+ T cells, S100+ dendritic cells, and CD68+ macrophages) in the submucosa of the explants (Fig. 2A). CD3+ cells were the most abundant while S100+ cells were the least abundant. CD3+ cells showed small round nuclei and little cytoplasm, CD68+ cells had round nuclei with opened chromatin and abundant cytoplasm, and S100+ cells had round nuclei with opened chromatin and cytoplasm with fingerlike projections.
FIG. 2.
(A) Immunohistochemical analysis of cervical tissue infected with HIV-1BaL. Colocalization of HIV-1 p24 antigen (red) with cell markers (brown) for T cells (CD3, left), macrophages (CD68, middle), and dendritic cells (S100, right) in human cervical tissues. Arrows point to macrophages (CD68+ cells) with brown cytoplasmic staining that also stain red, denoting the presence of p24 antigen (original magnification, ×50). (B) The integrity of the cervical explant model system was evaluated by examining the transmission of fluorescent microspheres (0.026-μm diameter) across the tissues. On the first day of culture, 0.5 ml of the fluorescent microspheres (1 × 1014/ml) was added to the epithelial surface of three explants (1, 2, and 3; same tissue donor) and a well filled with 2% agarose. The basolateral medium was sampled at 1, 3, and 7 days, and the percent transmission was calculated by dividing the fluorescent signal in the basolateral supernatant from the explants and agarose control by that from the blank well.
To determine the integrity of the agar/explant interface, fluorescent microspheres one-fourth of the size of HIV-1 virions were applied to the epithelium on day 0 of culture. The basolateral supernatant was sampled at days 1, 3, and 7 of culture and tested for fluorescence (Fig. 2B). Although increased permeability was observed by day 7 (15 to 22% transmission), the amount of microspheres transmitted at day 3 was <10% of that in the blank well. The agar control well had ∼3% transmission by day 3 compared to the blank well.
(ii) HIV-1 infection.
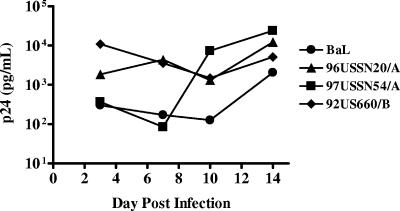
Initial experiments were performed to determine whether activation of the tissue was necessary for productive HIV-1 infection. Dilutions (103 to 106 TCID50) of HIV-1BaL were added to tissues with or without PHA activation. Reproducible virus replication was observed in all explants exposed to ≥5 × 104 TCID50 in PHA-activated tissues. Overnight infection with 5 × 104 TCID50 HIV-1BaL resulted in a steady rise of virus replication that typically peaked by day 12 to 15 of culture (Fig. 3 and 4A). Despite reproducible virus replication in PHA-activated tissues, inconsistent levels of virus replication (<500 pg/ml) were detected in resting tissues (data not shown). Therefore, to facilitate productive infection in subsequent experiments, all tissues were pretreated with PHA (48 to 72 h) and exposed to 5 × 104 TCID50 HIV-1 on day 3 of culture. To demonstrate the ability to infect cervical tissues with primary HIV-1 isolates, explants were successfully infected with two clade A isolates (96USSN20/A [CCR5/CXCR4] and 97USSN54/A [CCR5]) and one clade B isolate (92US660/B [CCR5]) (Fig. 3). These clades were chosen to show that primary HIV-1 isolates grow in a manner similar to that of laboratory-adapted HIV-1 and that there was no coreceptor preference in this explant culture system. Virus levels at day 14 postinfection were comparable to those observed after infection with HIV-1BaL. Further, HIV-1 replication occurred regardless of the coreceptor used (Fig. 3).
FIG. 3.
Growth kinetics of HIV-1 in cervical tissues. After an overnight incubation with 104 TCID50 HIV-1BaL or primary HIV-1 isolates (96USSN20/A CCR5/CXCR4-using, 97USSN54/A CCR5-using, and 92US660/B CCR5-using), the explants were washed and maintained in culture for 21 days. Basolateral supernatants were sampled every 3 to 4 days and assayed for p24gag protein.
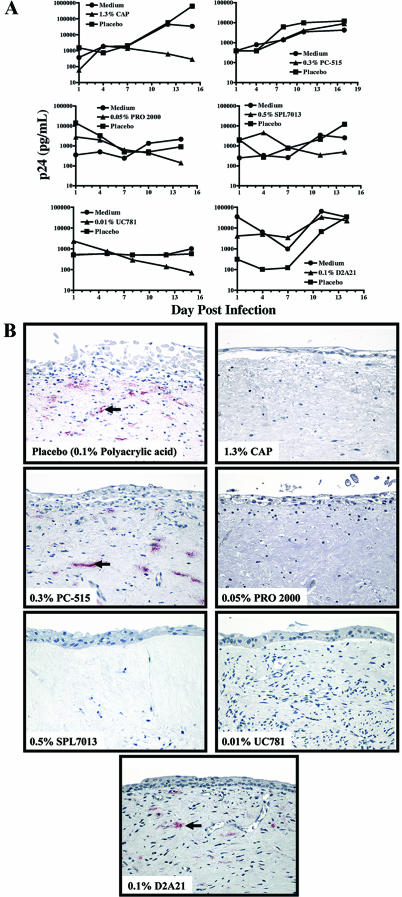
FIG. 4.
The effects of microbicides and placebos on HIV-1BaL infection in representative cervical explant cultures. (A) After overnight incubation with HIV-1BaL alone (5 × 104 TCID50) in medium or a 1:10 dilution of microbicide or placebo (final concentrations are indicated on the figure) with virus, the explants were washed and maintained in culture. Basolateral supernatants were sampled twice a week for up to 17 days and assayed for p24gag protein. (B) Comparison of HIV-1-infected cervical explant cultures treated with a representative placebo or each of the tested microbicides. Products were diluted 1:10 in culture medium (final concentrations are indicated on the figure) containing HIV-1 (5 × 104 TCID50) and added to the apical surface of the tissue. After overnight incubation, the tissues were washed and maintained in culture. At the study endpoint, tissues were fixed and examined by immunohistochemical analysis for the presence of p24 antigen (red). Representative photographs from an explant treated with a placebo (polyacrylic acid) (top left) or each of the microbicides are shown (original magnification, ×50).
To determine the phenotypes of infected cells, tissues infected with HIV-1 were analyzed by IHC for colocalization of p24 antigen with target cell markers (Fig. 2A). Although p24 antigen and T cells were observed in the same microscopic field, infected T cells were difficult to discern because of color deposition and their low cytoplasmic volume. p24 antigen did not colocalize with dendritic cells. Macrophages were the only infected cell type detected in these tissue sections (Fig. 2A).
Assessment of microbicide efficacy.
Microbicides and placebos were tested for their efficacy against HIV-1 by determining inhibition of p24 levels in explant supernatants and p24 antigen in endpoint tissues compared to infection controls. Microbicides that demonstrated efficacy in all tissue explants included CAP, SPL7013, PRO 2000, and UC781 (Table 2 and Fig. 4). Virus replication waned over time as demonstrated by a >80% inhibition of p24 levels (compared to the virus control in medium) by the end of culture. Moreover, these explants did not have any detectable HIV-1-infected cells at the study endpoint (Fig. 4B). Efficacy was only demonstrated by PC-515 in tissue from one of two donors and by D2A21 in tissue from one of three donors (Table 2 and Fig. 4). Although a reduction of p24 levels was demonstrated by the placebos (0.25% methyl cellulose, 42% inhibition; polyacrylic acid polymer [0.2%, 59% inhibition; 0.5%, 32% inhibition; 0.1%, 44% reduction]; 0.3% hydroxyethylcellulose, 63% inhibition), all placebos were considered nonefficacious since endpoint tissues tested positive for p24 antigen by IHC (Table 2 and Fig. 4B).
TABLE 2.
Summary of toxicities and efficacies of products and placebos in cervical explant cultures
| Producta | Concn (%) | Toxicityb | Presence of p24+ cells (%)c | Efficacy (%)d |
|---|---|---|---|---|
| CAP | 1.3 | No | 0/2 (0) | 2/2 (100) |
| PC-515 | 0.3 | No | 1/2 (50) | 1/2 (50) |
| Methyl cellulose (CAP and PC-515 placebo) | 0.25 | No | 4/4 (100) | 0/4 (0) |
| N9 | 0.4 | Yes | 0/2 (0) | 2/2 (100) |
| PRO 2000 | 0.4 | Yes | 0/2 (0) | 2/2 (100) |
| PRO 2000 | 0.05 | No | 0/2 (0) | 2/2 (100) |
| Polyacrylic acid (PRO 2000 placebo) | 0.2 | No | 3/3 (100) | 0/3 (0) |
| SPL7013 | 0.5 | No | 0/2 (0) | 2/2 (100) |
| Polyacrylic acid (SPL7013 placebo) | 0.5 | No | 2/2 (100) | 0/2 (0) |
| UC781 | 0.1 | No | 0/2 (0) | 2/2 (100) |
| UC781 | 0.01 | No | 0/2 (0) | 2/2 (100) |
| Polyacrylic acid (UC781 placebo) | 0.1 | No | 4/4 (100) | 0/4 (0) |
| D2A21 | 0.1 | No | 2/3 (66) | 1/3 (33) |
| Hydroxyethylcellulose (D2A21 placebo) | 0.3 | No | 3/3 (100) | 0/3 (0) |
Final concentration of each product and placebo.
Histology was used to evaluate tissue sections from treated explants. Products were considered toxic if damage (necrosis) to the epithelium or submucosa was observed.
IHC was used to detect p24 antigen in tissue sections from treated explants. Values represent numbers of tissue explants with detectable p24+ cells per number of explants tested.
Products were considered efficacious if tissues tested negative for p24 antigen by IHC. Values represent numbers of tissue explants with no detectable p24+ cells per number of explants tested.
Assessment of microbicide toxicity. (i) Acute toxicity (MTT assay).
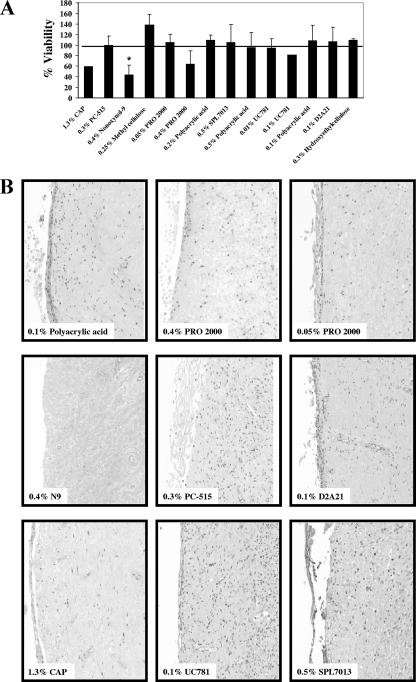
After overnight treatment with microbicide or placebo, the tissues were immediately assayed using the MTT assay. No toxicity was observed in tissues treated with the placebos (95 to 138%) (Fig. 5A). With the exception of CAP, 0.4% PRO 2000, and the N9 toxic control, treatment of tissues with PC-515, 0.05% PRO 2000, SPL7013, UC781, and D2A21 resulted in MTT levels similar to or above (82 to 105%) those of the untreated tissue controls after treatment (Fig. 5A). While reduced viability, as determined by reduced levels of MTT, was observed in tissues treated with CAP (59%), 0.4% PRO 2000 (64%), and N9 (44%), MTT levels were significantly lower only in the N9-treated tissues compared to the other microbicides and their placebos (P < 0.05).
FIG. 5.
(A) Effects of diluted microbicides and placebos on cervical explant viability as determined using the MTT assay. After an overnight (18-h) incubation, the effect of each product on tissue viability was determined by comparing the viability of the treated explants to that of the untreated tissue control (same donor). Data are expressed as the average percent viabilities (± standard deviations) for each treatment tested in two donors (*, P < 0.05). (B) Histopathologic comparison of the effect of the different microbicides in cervical tissues. Microbicides or their placebos were diluted 1:10 in culture medium and added to the apical surface of the tissue. After overnight incubation, the tissues were washed and maintained in culture for 21 days. At the study endpoint, tissues were fixed and examined histologically for morphological changes. Representative photographs for one placebo (polyacrylic acid) and each of the microbicides are shown (hematoxylin and eosin stain; original magnification, ×50). The final concentrations of each product and placebo are indicated.
(ii) Endpoint toxicity (histology).
Histology was used to evaluate product toxicity at the study endpoint (Fig. 5B). Tissues treated with CAP, PC-515, 0.05% PRO 2000, SPL7013, UC781, and D2A21 showed regenerated epithelium and an intact lamina propria. Denuded epithelium and focal necrosis of the lamina propria were observed in tissues treated with N9 and 0.4% PRO 2000. The tissue architecture appeared normal after treatment with the placebos. With the exception of the reduced MTT viability observed with CAP, the toxicity observed by histology confirmed the MTT results for N9 and 0.4% PRO 2000 (Fig. 5B and Table 2).
DISCUSSION
Ex vivo organotypic cultures offer a valuable link between in vitro culture systems and the clinic by providing a controlled format in which microbicides can be comparatively evaluated for (i) anti-HIV-1 activity in target cells present within the submucosa of human tissues and (ii) toxicity against the mucosal epithelium. Two other ex vivo ectocervical explant systems have been described (13, 19). In one method, tissues are completely embedded/submerged and HIV-1 with or without microbicide is applied apically (13). In the other method, the tissue is exposed to HIV-1 with or without microbicide in a nonpolarized manner, and the explants are cultured while submerged in medium (19). We describe a cervical explant culture for which tissue is maintained in a polarized state with the epithelial surface positioned at the air/tissue interface and the submucosa (stroma) submerged in medium. Unlike the other explant culture systems (13, 19), this positioning of the cervical tissue allows application of virus and candidate topical microbicides directly to the epithelium and allows access to the cells in the submucosa. As indicated in the present study and in descriptions of other published explant systems (13, 19), cervical explant cultures have several limitations. These include lack of hormone modulation, lack of recruitment of immune cells, loss of epithelium, and inability to regenerate/repair. However, the last two attributes make this system sensitive to any potential toxic effect by the topical microbicide. Further, this explant culture demonstrates the capacity to be infected with HIV-1 with the subsequent evaluation of efficacy of several topical microbicide compounds. It is important to note that there is currently no consensus among the laboratories using tissue explants regarding appropriate efficacy and toxicity endpoints. A multisite effort is currently under way to determine the optimum endpoints and to address tissue replicates (14a).
As reported elsewhere (19), there was a loss of the outer stratified layers of epithelium during the initial 24 to 72 h of culture of untreated tissues. Although the basal layer remained intact with gradual regeneration over the culture period, the epithelium of these explants did not recapitulate to that of the normal cervix. Since leakiness of the explant culture is a concern with embedded tissues, other investigators have analyzed the integrity of the system by measuring the transmission of blue dextran or fluorescent beads (7.2-μm diameter) across the mucosa (13, 20). Given the size of HIV-1 virions (∼100-nm diameter), smaller fluorescent beads (26-nm diameter) were used for the permeability studies and allowed to remain for 7 days. Because of the smaller bead size and the modifications to the embedded culture system, a higher rate of transmission of the fluorescent beads compared to the 7.2-μm-diameter beads was observed (20). Despite this, <10% transmission of the beads was detected by day 3 of culture. Although a true polarized tissue orientation may be difficult to achieve ex vivo, this does not negate use of this system. Tears and disruptions to the mucosal epithelium are likely to occur during sexual intercourse (39). Moreover, sexually transmitted pathogens can result in ulceration of the mucosa and increased acquisition of HIV-1 (24, 25). An ideal microbicide should have the capacity to prevent HIV-1 infection when the virus has its best opportunity to achieve infection: during the presence of a compromised epithelium and activated immune targets (i.e., inflammation). The cervical explant culture described here represents a unique system for the preclinical evaluation of candidate microbicides that takes into account the optimal environment for HIV-1 infection.
In this cervical explant culture, T cells, macrophages, and dendritic cells were detected by IHC within cervical tissues. However, HIV-1 p24 antigen only colocalized with macrophages. Macrophages were the primary target for infection in one culture system (19) while CD4+ T cells were the primary infected cell type in the other culture (20). Since the sensitivity of the IHC technique and the timing after infection can affect the ability to detect infected cells, the inability to detect T-cell infection based on the IHC data does not exclude infection of these or other nonmacrophage cell types in this cervical explant culture. Regardless of which cell type was infected, HIV-1 infection and replication were consistently observed after infection with HIV-1BaL, thus allowing for the comparative analysis of six topical microbicides for their anti-HIV-1 activity. Microbicides with significant anti-HIV-1 activity typically demonstrated >90% (1 to 2 log10) reduction in p24 levels at day 14. In instances where >80% reduction of p24 levels was observed, no p24 antigen could be detected in tissue sections by IHC. While the carrageenan (PC-515)- and peptide (D2A21)-based products showed some anti-HIV-1 activity, the CAP, naphthalene sulfonate (PRO 2000), lysine dendrimer (SPL7013), and NNRTI (UC781) products consistently blocked HIV-1 infection.
In previously described explant cultures, the toxicities of candidate topical microbicides were assessed using either biochemical (MTT) (19) or histopathological (43) methods. In the present study, both methods were employed. While MTT represents a convenient method for measuring tissue toxicity, it is a limited approach since it measures general toxicity. Conversely, histopathology allows determination of toxicity specific to the mucosal epithelium or the underlying submucosa. With the exception of CAP, both methods detected tissue toxicity after treatment with N9 and 4% PRO 2000. After treatment with CAP, reduced cell viability was observed by MTT assay, yet histology revealed no physical damage to the epithelium or submucosa. The reduced viability observed in the MTT assay may result from interference by CAP, suggesting a potential artifact of this biochemical assay. Alternatively, CAP could be affecting the mitochondrial metabolism without affecting the overall tissue structure/integrity. While the MTT assay may prove beneficial for initial toxicity testing in tissues, these data suggest that it should be used in conjunction with histology to confirm toxicity.
In the explant culture presented here, virus growth was evaluated by monitoring p24 levels over time (at multiple time points) and determining the presence of p24 antigen by IHC. It should be noted that efficacy data obtained for several other explant cultures are limited to one time point (19, 42), are sometimes presented as percentages of a virus control (thus not depicting actual p24 levels) (17-18), and/or rely on that one endpoint (without confirmation by a secondary method, such as IHC) (42). In the present study, compounds that were found to be active in both donors showed a >80% decrease in culture supernatant p24 levels at the study endpoint and no p24 staining by IHC.
Six topical microbicides were evaluated for toxicity and efficacy in a modified cervical explant culture system. With the exception of 4% PRO 2000, the remaining microbicides were relatively nontoxic, consistent with toxicity results from other explant cultures (19, 43), animal studies (5, 31), and safety and acceptability trials (3, 6, 27, 31, 35, 36, 42). With the exception of PC-515 and D2A21, the microbicides were efficacious against HIV-1 infection, consistent with efficacy data from other cervical explant cultures (1, 18, 19, 43) and preclinical and animal studies (16, 26, 32). The low toxicity and high anti-HIV-1 activity of CAP, 0.5% PRO 2000, SPL7013, and UC781 suggest the need for further testing of these products.
Despite the limitations described above, our study shows that the cervical explant culture is valuable for evaluating the efficacy and toxicity of potential microbicides. In addition, when used as a secondary confirmatory assay for compounds demonstrating activity in cell-based assays (14b, 15), this cervical explant culture can be a valuable tool for selecting priority candidates to advance to clinical trials.
Acknowledgments
We thank Ira Horowitz (Emory University) for his support as well as Rita Lloyd for coordinating collection of the cervical tissues at Grady Memorial Hospital (Atlanta, GA). We acknowledge Nicola Richardson-Harman at BioStat Solutions (Mt. Airy, MD) for performing the statistical analysis. We also thank Lisa Rohan and Robin Shattock for helpful discussions.
J.E.C. was supported by a National Research Service Award (5 F32 HD40727) from the National Institute of Child Health and Human Development, National Institutes of Health.
None of the authors has a significant financial interest in any of the commercial products used in this study. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the U.S. Department of Health and Human Services, the Public Health Service, or the Centers for Disease Control and Prevention. In addition, use of trade names is for identification only and does not imply endorsement by these government agencies.
Footnotes
Published ahead of print on 12 March 2007.
REFERENCES
- 1.Abner, S. R., P. C. Guenthner, J. Guarner, K. A. Hancock, J. E. Cummins, Jr., A. Fink, G. T. Gilmore, C. Staley, A. Ward, O. Ali, S. Binderow, S. Cohen, L. A. Grohskopf, L. Paxton, C. E. Hart, and C. S. Dezzutti. 2005. A human colorectal explant culture to evaluate topical microbicides for the prevention of HIV infection. J. Infect. Dis. 1921545-1556. [DOI] [PubMed] [Google Scholar]
- 2.Bader, J. P., J. B. McMahon, R. J. Schultz, V. L. Narayanan, J. B. Pierce, W. A. Harrison, O. S. Weislow, C. F. Midelfort, S. F. Stinson, and M. R. Boyd. 1991. Oxathiin carboxanilide, a potent inhibitor of human immunodeficiency virus reproduction. Proc. Natl. Acad. Sci. USA 886740-6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balzarini, J., L. Naesens, E. Verbeken, M. Laga, L. Van Damme, M. Parniak, L. Van Mellaert, J. Anne, and E. De Clercq. 1998. Preclinical studies on thiocarboxanilide UC-781 as a virucidal agent. AIDS 121129-1138. [DOI] [PubMed] [Google Scholar]
- 4.Beer, B. E., and J. E. Cummins, Jr. 2005. Novel strategies in HIV prevention—development of topical microbicides, p. 277-290. In A. M. Doherty (ed.), Annual reports in medicinal chemistry, vol. 40. Academic Press, San Diego, CA. [Google Scholar]
- 5.Beer, B. E., G. F. Doncel, F. C. Krebs, R. J. Shattock, P. S. Fletcher, R. W. Buckheit, Jr., K. Watson, C. S. Dezzutti, J. E. Cummins, E. Bromley, N. Richardson-Harman, L. A. Pallansch, C. Lackman-Smith, C. Osterling, M. Mankowski, S. R. Miller, B. J. Catalone, P. A. Welsh, M. K. Howett, B. Wigdahl, J. A. Turpin, and P. Reichelderfer. 2006. In vitro preclinical testing of nonoxynol-9 as potential anti-human immunodeficiency virus microbicide: a retrospective analysis of results from five laboratories. Antimicrob. Agents Chemother. 50713-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernstein, D. I., L. R. Stanberry, S. Sacks, N. K. Ayisi, Y. H. Gong, J. Ireland, R. J. Mumper, G. Holan, B. Matthews, T. McCarthy, and N. Bourne. 2003. Evaluations of unformulated and formulated dendrimer-based microbicide candidates in mouse and guinea pig models of genital herpes. Antimicrob. Agents Chemother. 473784-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourne, N., L. R. Stanberry, E. R. Kern, G. Holan, B. Matthews, and D. I. Bernstein. 2000. Dendrimers, a new class of candidate topical microbicides with activity against herpes simplex virus infection. Antimicrob. Agents Chemother. 442471-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 2004. Opportunity to collaborate in the evaluation of topical microbicides to reduce sexual transmission of human immunodeficiency virus. Fed. Regist. 6929138-29139. [Google Scholar]
- 9.Centers for Disease Control and Prevention. 2001. Opportunity to collaborate in the evaluation of topical microbicides to reduce heterosexual transmission of human immunodeficiency virus (HIV). Fed. Regist. 6634453. [Google Scholar]
- 10.Centers for Disease Control and Prevention. 2001. Opportunity to collaborate in the evaluation of topical microbicides to reduce transmission of human immunodeficiency virus (HIV) among men who have sex with men (MSM). Fed. Regist. 6634452. [Google Scholar]
- 11.Centers for Disease Control and Prevention. 1993. Update: barrier protection against HIV infection and other sexually transmitted diseases. Morb. Mortal. Wkly. Rep. 42589-597. [PubMed] [Google Scholar]
- 12.Coggins, C., F. Alvarez, V. Brache, I. S. Fraser, M. Lacarra, P. Lahteenmaki, R. Massai, D. R. Mishnell, D. M. Phillips, A. M. Salvatierra, and C. J. Elias. 1997. Female-controlled methods to prevent sexual transmission of HIV. Contraception 56387-389. [DOI] [PubMed] [Google Scholar]
- 13.Collins, K. B., B. K. Patterson, G. J. Naus, D. V. Landers, and P. Gupta. 2000. Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat. Med. 6475-479. [DOI] [PubMed] [Google Scholar]
- 14.Cummins, J. E., and C. S. Dezzutti. 2000. Sexual HIV-1 transmission and mucosal defense mechanisms. AIDS Rev. 2144-154. [Google Scholar]
- 14a.Cummins, J., N. Richardson-Harman, J. Bremer, P. Anton, C. Dezzutti, P. Gupta, N. Lurain, L. Margolis, R. Shattock, and P. Reichelderfer. 2006. Abstr. 13th Conf. Retrovir. Opportun. Infect., Denver, CO, p. 378.
- 14b.Cummins, J., M. Jones, C. Lackman-Smith, and B. Beer. 2006. Abstr. Microbicides 2006, Cape Town, South Africa, p. 212.
- 15.D'Cruz, O. J., and F. M. Uckun. 2004. Clinical development of microbicides for the prevention of HIV infection. Curr. Pharm. Des. 10315-336. [DOI] [PubMed] [Google Scholar]
- 16.Dezzutti, C. S., V. N. James, A. Ramos, S. T. Sullivan, A. Siddig, T. J. Bush, L. A. Grohskopf, L. Paxton, S. Subbarao, and C. E. Hart. 2004. In vitro comparison of topical microbicides for prevention of human immunodeficiency virus type 1 transmission. Antimicrob. Agents Chemother. 483834-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finney, D. J. 1952. Probit analysis: a statistical treatment of the sigmoid response curve. Cambridge University Press, Cambridge, United Kingdom.
- 18.Fletcher, P., Y. Kiselyeva, G. Wallace, J. Romano, G. Griffin, L. Margolis, and R. Shattock. 2005. The nonnucleoside reverse transcriptase inhibitor UC-781 inhibits human immunodeficiency virus type 1 infection of human cervical tissue and dissemination by migratory cells. J. Virol. 7911179-11186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenhead, P., P. Hayes, P. S. Watts, K. G. Laing, G. E. Griffin, and R. J. Shattock. 2000. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J. Virol. 745577-5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta, P., K. B. Collins, D. Ratner, S. Watkins, G. J. Naus, D. V. Landers, and B. K. Patterson. 2002. Memory CD4+ T cells are the earliest detectable human immunodeficiency virus type 1 (HIV-1)-infected cells in the female genital mucosal tissue during HIV-1 transmission in an organ culture system. J. Virol. 769868-9876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.International Working Group on Vaginal Microbicides. 1996. Recommendations for the development of vaginal microbicides. AIDS 101-6. [PubMed] [Google Scholar]
- 22.Jiang, Y. H., P. Emau, J. S. Cairns, L. Flanary, W. R. Morton, T. D. McCarthy, and C. C. Tsai. 2005. SPL7013 gel as a topical microbicide for prevention of vaginal transmission of SHIV89.6P in macaques. AIDS Res. Hum. Retrovir. 21207-213. [DOI] [PubMed] [Google Scholar]
- 23.Joint United Nations Program on HIV/AIDS (UNAIDS). 2005. AIDS epidemic update, December 2004. Joint United Nations Program on HIV/AIDS (UNAIDS), Geneva, Switzerland.
- 24.Lushbaugh, W. B., A. C. Blossom, P. H. Shah, A. K. Banga, J. M. Jaynes, J. D. Cleary, and R. W. Finley. 2000. Use of intravaginal microbicides to prevent acquisition of Trichomonas vaginalis infection in Lactobacillus-pretreated, estrogenized young mice. Am. J. Trop. Med. Hyg. 63284-289. [PubMed] [Google Scholar]
- 25.Maguire, R. A., N. Bergman, and D. M. Phillips. 2001. Comparison of microbicides for efficacy in protecting mice against vaginal challenge with herpes simplex virus type 2, cytotoxicity, antibacterial properties, and sperm immobilization. Sex. Transm. Dis. 28259-265. [DOI] [PubMed] [Google Scholar]
- 26.Manson, K. H., M. S. Wyand, C. Miller, and A. R. Neurath. 2000. Effect of a cellulose acetate phthalate topical cream on vaginal transmission of simian immunodeficiency virus in rhesus monkeys. Antimicrob. Agents Chemother. 443199-3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayer, K. H., S. A. Karim, C. Kelly, L. Maslankowski, H. Rees, A. T. Profy, J. Day, J. Welch, and Z. Rosenberg. 2003. Safety and tolerability of vaginal PRO 2000 gel in sexually active HIV-uninfected and abstinent HIV-infected women. AIDS 17321-329. [DOI] [PubMed] [Google Scholar]
- 28.Mohan, P., R. Singh, and M. Baba. 1991. Potential anti-AIDS agents. Synthesis and antiviral activity of naphthalenesulfonic acid derivatives against HIV-1 and HIV-2. J. Med. Chem. 34212-217. [DOI] [PubMed] [Google Scholar]
- 29.Neurath, A. R., N. Strick, Y. Y. Li, K. Lin, and S. Jiang. 1999. Design of a “microbicide” for prevention of sexually transmitted diseases using “inactive” pharmaceutical excipients. Biologicals 2711-21. [DOI] [PubMed] [Google Scholar]
- 30.Nicolosi, A., M. L. Correa Leite, M. Musicco, C. Arici, G. Gavazzeni, A. Lazzarin, et al. 1994. The efficiency of male-to-female and female-to-male sexual transmission of the human immunodeficiency virus: a study of 730 stable couples. Epidemiology 5570-575. [DOI] [PubMed] [Google Scholar]
- 31.Niruthisard, S., R. E. Roddy, and S. Chutivongse. 1991. The effects of frequent nonoxynol-9 use on the vaginal and cervical mucosa. Sex. Transm. Dis. 18176-179. [DOI] [PubMed] [Google Scholar]
- 32.Otten, R. A., D. R. Adams, C. N. Kim, E. Jackson, J. K. Pullium, K. Lee, L. A. Grohskopf, M. Monsour, S. Butera, and T. M. Folk. 2005. Multiple vaginal exposures to low doses of R5 simian-human immunodeficiency virus: strategy to study HIV preclinical interventions in nonhuman primates. J. Infect. Dis. 191164-173. [DOI] [PubMed] [Google Scholar]
- 33.Padian, N., L. Marquis, D. P. Francis, R. E. Anderson, G. W. Rutherford, P. M. O'Malley, and W. Winkelstein, Jr. 1987. Male-to-female transmission of human immunodeficiency virus. JAMA 258788-790. [PubMed] [Google Scholar]
- 34.Padian, N. S., S. C. Shiboski, S. O. Glass, and E. Vittinghoff. 1997. Heterosexual transmission of human immunodeficiency virus (HIV) in northern California: results from a ten-year study. Am. J. Epidemiol. 146350-357. [DOI] [PubMed] [Google Scholar]
- 35.Patton, D. L., Y. T. Cosgrove Sweeney, L. K. Rabe, and S. L. Hillier. 2002. Rectal applications of nonoxynol-9 cause tissue disruption in a monkey model. Sex. Transm. Dis. 29581-587. [DOI] [PubMed] [Google Scholar]
- 36.Phillips, D. M., C. L. Taylor, V. R. Zacharopoulos, and R. A. Maguire. 2000. Nonoxynol-9 causes rapid exfoliation of sheets of rectal epithelium. Contraception 62149-154. [DOI] [PubMed] [Google Scholar]
- 37.Quinn, T. C., and J. Overbaugh. 2005. HIV/AIDS in women: an expanding epidemic. Science 3081582-1583. [DOI] [PubMed] [Google Scholar]
- 38.Reed, L. J., and J. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27493-497. [Google Scholar]
- 39.Shattock, R. J., and J. P. Moore. 2003. Inhibiting sexual transmission of HIV-1 infection. Nat. Rev. Microbiol. 125-34. [DOI] [PubMed] [Google Scholar]
- 40.Stein, Z. A. 1990. HIV prevention: the need for methods women can use. Am. J. Public Health 80460-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Damme, L., G. Ramjee, M. Alary, B. Vuylsteke, V. Chandeying, H. Rees, P. Sirivongrangson, L. Mukenge-Tshibaka, V. Ettiegne-Traore, C. Uaheowitchai, S. S. Karim, B. Masse, J. Perriens, and M. Laga. 2002. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet 360971-977. [DOI] [PubMed] [Google Scholar]
- 42.Van Damme, L., A. Wright, K. Depraetere, I. Rosenstein, V. Vandersmissen, L. Poulter, M. McKinlay, E. Van Dyck, J. Weber, A. Profy, M. Laga, and V. Kitchen. 2000. A phase I study of a novel potential intravaginal microbicide, PRO 2000, in healthy sexually inactive women. Sex. Transm. Infect. 76126-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zussman, A., L. Lara, H. H. Lara, Z. Bentwich, and G. Borkow. 2003. Blocking of cell-free and cell-associated HIV-1 transmission through human cervix organ culture with UC781. AIDS 17653-661. [DOI] [PubMed] [Google Scholar]