Abstract
Salmonella enterica serovar Typhimurium was isolated from the intestinal contents of Rattus rattus and Rattus norvegicus house rats captured at two buildings, designated buildings J and YS, in Yokohama City, Japan. From October 1997 to September 1998, 52 of 339 (15.3%) house rats were found to carry Salmonella serovar Typhimurium definitive phage type 104 (DT104). In building J, 26 of 161 (16.1%) house rats carried DT104 over the 1-year study period, compared to 26 of 178 (14.6%) rats in building YS. The isolation rates of DT104 from R. rattus and R. norvegicus were similar in the two buildings. Most DT104 strains from building J (24 of 26) showed resistance to ampicillin, chloramphenicol, streptomycin, sulfisoxazole, and tetracycline and contained both the 1.0- and 1.2-kbp integrons, carrying genes pse1, pasppflo-like, aadA2, sulI, and tet(G). All DT104 strains from building YS were resistant to ampicillin and sulfisoxazole, and had the 1.2-kbp integron carrying pse1 and sulI. Cluster analysis of pulsed-field gel electrophoresis patterns of BlnI-digested DT104 DNAs showed that 22 of 26 DT104 strains from building J and 24 of 26 strains from building YS could be grouped into separate clusters each specific for the building origin. These results indicated that DT104 strains were prevalent in house rat colonies in each building and suggest that house rats may play an important role in the epidemiology of DT104.
Human salmonellosis is a major public health problem. Salmonellosis caused by Salmonella enterica serovar Enteritidis is the most prevalent cause of bacterial food poisoning in Japan (27). In the 1990s, Salmonella enterica serovar Typhimurium definitive phage type 104 (DT104) emerged as a significant problem in both Europe and the United States because this strain has acquired multidrug resistance (10, 13). DT104 strains are found internationally in many animal species (1, 4, 8, 22, 24, 29, 37) that are considered to be reservoirs for human infection.
Rodents are public health hazards since they can be reservoirs for human salmonellosis (9, 12, 26, 30-32). We have previously reported the prevalence of Salmonella enterica in Rattus norvegicus and Rattus rattus rats captured at two buildings located in Yokohama City, Japan, during 1997 and 1998 (25). In that study, 60 of 339 (17.7%) house rats carried S. enterica, and of these 60 rats, 58 (96.7%) carried serovar Typhimurium and two carried serovar Enteritidis, pointing to the importance of rats as reservoirs of S. enterica serovar Typhimurium.
While there are some reports about salmonellae in rats (9, 12, 25, 26, 30-32), no information has been published regarding the incidence of DT104. The lack of information about DT104 is partially due to the difficulty of performing definitive phage typing. Although phage typing is the “gold standard” for DT104 identification, PCR has been used recently to identify DT104-specific DNA sequences (11, 28) and could facilitate studies of the DT104 prevalence in animal reservoirs. However, more comparative data on DT104 identification by PCR are needed.
A major problem for the investigation of DT104 prevalence in animal reservoirs is the experimental difficulty of determining genetic relationships among DT104 isolates. For example, molecular epidemiological analysis using pulsed-field gel electrophoresis (PFGE) analysis cannot readily determine genetic relationships (1, 4, 23). Recently, variable-number tandem repeat (VNTR) typing was reported to be a new molecular epidemiological method for differentiating pathogenic bacteria (21). Furthermore, VNTR typing has been used to differentiate DT104 strains having the same PFGE patterns (20).
In this study, we have investigated both the prevalence and the genetic properties of DT104 strains among serovar Typhimurium isolates from house rats by studies of antimicrobial resistance, detection of class I integrons, and antimicrobial resistance genes. Molecular epidemiological analysis was done using PFGE and VNTR typing.
MATERIALS AND METHODS
Bacterial strains and identification of DT104.
A total of 58 serovar Typhimurium strains were previously isolated from the intestinal contents of house rats. Trapping was carried out from October 1997 to September 1998 in two buildings, designated buildings J and YS, located in Yokohama City, Kanagawa, Japan (Tables 1 and 2). Buildings J and YS were multitenant buildings containing some restaurants, and they were 300 m apart, on opposite sides of the Yokohama main train station. Yokohama City is near metropolitan Tokyo, and its population in 1998 was over 3,300,000.
TABLE 1.
Antimicrobial resistance patterns of serovar Typhimurium DT104 isolates from house rats in building J
| Sampling date | Rat
|
No. of DT104 isolates
|
||||
|---|---|---|---|---|---|---|
| Species | No. captured | Total | With antimicrobial resistance pattern:
|
|||
| ACSSuT | SSu | ASu | ||||
| 17 October | R. rattus | 39 | 9 | 9 | 0 | 0 |
| 1997 | R. norvegicus | 11 | 0 | 0 | 0 | 0 |
| 20 November | R. rattus | 14 | 0 | 0 | 0 | 0 |
| 1997 | R. norvegicus | 7 | 2 | 2 | 0 | 0 |
| 18 December | R. rattus | 19 | 3 | 2 | 1 | 0 |
| 1997 | R. norvegicus | 13 | 2 | 1 | 1 | 0 |
| 26 March | R. rattus | 13 | 3 | 3 | 0 | 0 |
| 1997 | R. norvegicus | 9 | 2 | 2 | 0 | 0 |
| 23 April | R. rattus | 25 | 2 | 2 | 0 | 0 |
| 1998 | R. norvegicus | 11 | 3 | 3 | 0 | 0 |
| Total | R. rattus | 110 | 17 (15.5%a) | 16 | 1 | 0 |
| R. norvegicus | 51 | 9 (18.0%a) | 8 | 1 | 0 | |
DT104-carrying rats as a percentage of captured rats.
TABLE 2.
Antimicrobial resistance patterns of serovar Typhimurium DT104 isolates from house rats in building YS
| Sampling date | Rat
|
No. of DT104 isolates
|
||||
|---|---|---|---|---|---|---|
| Species | No. captured | Total | With antimicrobial resistance pattern:
|
|||
| ACSSuT | SSu | ASu | ||||
| 24 October | R. rattus | 16 | 1 | 0 | 0 | 1 |
| 1997 | R. norvegicus | 2 | 0 | 0 | 0 | 0 |
| 26 November | R. rattus | 14 | 0 | 0 | 0 | 0 |
| 1997 | R. norvegicus | 5 | 1 | 0 | 0 | 1 |
| 23 January | R. rattus | 10 | 2 | 0 | 0 | 2 |
| 1998 | R. norvegicus | 6 | 3 | 0 | 0 | 3 |
| 20 February | R. rattus | 18 | 1 | 0 | 0 | 1 |
| 1998 | R. norvegicus | 7 | 1 | 0 | 0 | 1 |
| 21 May | R. rattus | 6 | 1 | 0 | 0 | 1 |
| 1998 | R. norvegicus | 6 | 0 | 0 | 0 | 0 |
| 19 June | R. rattus | 20 | 1 | 0 | 0 | 1 |
| 1998 | R. norvegicus | 3 | 1 | 0 | 0 | 1 |
| 24 July | R. rattus | 17 | 3 | 0 | 0 | 3 |
| 1998 | R. norvegicus | 3 | 1 | 0 | 0 | 1 |
| 25 August | R. rattus | 16 | 7 | 0 | 0 | 7 |
| 1998 | R. norvegicus | 0 | 0 | 0 | 0 | 0 |
| 18 September | R. rattus | 29 | 3 | 0 | 0 | 3 |
| 1998 | R. norvegicus | 0 | 0 | 0 | 0 | 0 |
| Total | R. rattus | 146 | 19 (13.0%a) | 0 | 0 | 19 |
| R. norvegicus | 32 | 7 (21.9%a) | 0 | 0 | 7 | |
DT104-carrying rats as a percentage of captured rats.
Definitive phage typing of serovar Typhimurium strains was carried out as previously described (16). DT104 identification was also done using two PCR methods: (i) the PCR method described by Pritchett et al. (28) was used to detect DT104 and U302 rRNA sequences, and (ii) the PCR method described by Hermans et al. (11) was used to detect irsA, the HldD homologue, fragment 84, fragment 168, and fragment 180 in DT104. For these studies, 2-ng DNA samples were amplified using FastStart Taq DNA polymerase (Roche, Basel, Switzerland). The GeneAmp 9700 PCR system (Applied Biosystems, Foster City, CA) was used for PCRs. Each PCR involved one cycle at 94°C for 5 min; 30 cycles with the reaction conditions for each cycle being 94°C for 30 s, 60°C for 1 min, and 72°C for 45 s; and one cycle at 72°C for 7 min.
Characterization of DT104 strains.
Antimicrobial sensitivities of the DT104 strains were determined by the Kirby-Bauer single disk method (2). Thirteen different Sensi-Discs (Becton Dickinson, Franklin Lakes, NJ) were used, and the manufacturer's instructions were followed for testing. Each disk contained one of the following: 10 μg ampicillin, 30 μg cephalothin, 30 μg cefotaxime, 30 μg kanamycin, 10 μg gentamicin, 10 μg streptomycin, 30 μg tetracycline, 30 μg chloramphenicol, 5 μg ciprofloxacin, 250 μg sulfisoxazole, 30 μg nalidixic acid, 50 μg fosfomycin, and 5 μg trimethoprim.
The presence of class I integrons and antibiotic resistance genes was investigated by PCR as described by Lai-King et al. (18), using primers for the 5′ and 3′ conserved segments of class I integrons and pse1, aadA2, sulI, pasppflo-like, and tet(G) genes. For each PCR, 2 ng DNA was amplified using FastStart Taq DNA polymerase (Roche) with a GeneAmp 9700 PCR system (Applied Biosystems). Each PCR involved one cycle at 94°C for 5 min; 30 cycles with the reaction conditions for each cycle being 94°C for 30 s, 58°C for 30 s, and 72°C for 2 min; and one cycle at 72°C for 7 min. Class I integron amplicon images were saved as tagged image format files and analyzed using BioNumerics version 4 software (Applied Maths, Sint-Martens-Latem, Belgium) to calculate amplicon size.
PFGE analysis.
PFGE analysis was performed as previously described (40) with some modifications. Briefly, the plug containing bacteria from an overnight culture was made with Seakem gold agarose (Cambrex, Rockland, ME) using a sample plug caster (Bio-Rad, Hercules, CA). The plug was treated for 18 h at 50°C with a solution of 1 mg proteinase K (Roche) per ml. After incubation, the plug was treated twice for 20 min each with Tris-EDTA (TE) buffer containing 4 mM Pefabloc (Roche) at 50°C and then washed twice on ice for 20 min each with TE buffer. The plug was digested for 18 h at 37°C with either XbaI (Roche) or BlnI (Roche). Salmonella enterica serovar Braenderup H9812 was used as the molecular weight standard (14). The standard was set at every fourth lane for the normalization of PFGE patterns (40).
The cluster analysis method used in these studies has been previously described (40). Briefly, PFGE patterns were imported into BioNumerics software (Applied Maths). Similarities among the PFGE patterns were calculated using the Pearson product-moment correlation coefficient (Pearson correlation) with a 0.5% optimization parameter. Dendrograms were made using the unweighted pair group method using arithmetic averages (UPGMA). Variations in grouping DT104 strains were investigated by changing the cutoff value from 95% to values at which all strains were grouped. A cluster was defined as a group of strains with higher similarity values than the cutoff value for cluster analysis of XbaI-digested PFGE patterns and BlnI-digested PFGE patterns.
VNTR typing.
DNA was isolated from DT104 strains using InstaGene Matrix (Bio-Rad). Two-nanogram DNA samples were amplified using FastStart Taq DNA polymerase (Roche). Primers for five VNTR loci, described by Lindstedt et al. (20), were used. A GeneAmp 9700 PCR system (Applied Biosystems) was used for PCR. Each PCR involved one cycle at 94°C for 5 min; 30 cycles with the reaction conditions for each cycle being 94°C for 30 s, 58°C for 30 s, and 72°C for 1 min; and one cycle at 72°C for 7 min. PCR products were partially purified using EXOSAP-it (USB, Cleveland, OH), and 10-fold dilution samples were made using 0.1× TE buffer. The samples were analyzed by capillary electrophoresis using an ABI-310 Genetic Analyzer (Applied Biosystems) with POP4 polymer (Applied Biosystems) and GeneMark 1000 Fluorescent DNA Ladder Rox (Northernbiotech, Weston, WI) as the internal control. Electrophoresis was carried out at 60°C for 40 min. The sizes of the DNA fragments in the samples were measured using GeneScan version 3.12 (Applied Biosystems), and the number of repeats in each VNTR locus was calculated as described by Lindstedt et al. (20). The number of repeats was rounded to two decimal places (41).
The number of repeats in each sample was imported into BioNumerics software (Applied Maths), and similarities among strains were calculated using the Pearson correlation. A dendrogram was made using the UPGMA method with the “use square root” option (41). The percent similarities based on the “use square root” option were calculated as follows:
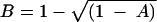 |
where A is the similarity calculated by the Pearson correlation and B is the similarity with the “use square root” option.
Variations in grouping DT104 strains were investigated following the same methods described above under “PFGE analysis.” A cluster was defined as a group of strains with higher similarity values than the cutoff value for cluster analysis of VNTR types.
Correlation between the three clustering methods.
The congruence among the results of three cluster analyses of XbaI- and BlnI-digested PFGE patterns and VNTR data was measured using BioNumerics software (Applied Maths). The pairwise comparison of DT104 strains using one cluster analysis was compared to pairwise comparison of the isolates using another cluster analysis. The values of the similarity matrices of the two typing methods were plotted on an xy graph. Each point in the graph was the corresponding similarity value for the two typing methods. Such a plot shows the degree of correlation between the two methods. The congruence values among the three cluster analyses were calculated using BioNumerics software (Applied Maths) with Kendall's tau correlation under the option of 0% of “minimum similarity” and 100% of “maximum similarity” and without the option of “included self matches.”
Of the three cluster analysis methods used in these studies (i.e., XbaI, BlnI, and VNTR cluster analysis), the method showing the most consistency with epidemiological information was used as the first step in a two-step cluster analysis. The clusters made by this first method were further analyzed using each of the two other cluster analysis methods to produce a two-step cluster analysis.
Statistical analysis.
Statistical analysis was performed using the Statcel2 (OMS Inc., Saitama, Japan) add-on package for Microsoft Excel. Chi-square analysis was used to compare numbers of rats carrying DT104 at each of the two study sites, as well as the numbers of R. rattus and R. norvegicus house rats carrying DT104 in each building. A P level of <0.05 was considered to be significant for all analyses.
RESULTS
Detection of DT104.
In this study, 52 of 58 (89.7%) serovar Typhimurium strains isolated from rats in the two buildings (26 from building J and 26 from building YS) were identified as DT104 strains by both phage typing and the two PCR methods used in these studies. One strain from building J was identified as DT104 by the two PCR methods although the strain was typed “not DT104” by phage typing (data not shown). In building J, 26 (16.1%) of 161 captured rats carried DT104, while 26 (14.6%) of 178 captured rats in building YS carried DT104. The period prevalence of DT104 was not significantly different between rats captured in building J and those captured in YS at different times during this study (chi-square test, P = 0.05). In building J, 17 of 110 (15.5%) R. rattus rats and 9 of 51 (18.0%) R. norvegicus rats carried DT104 (Table 1), while in building YS, 19 of 146 (13.0%) R. rattus rats and 7 of 32 (21.9%) R. norvegicus rats carried DT104 (Table 2). The isolation rates of DT104 from R. rattus and R. norvegicus were not significantly different between the two buildings (chi-square test, P = 0.05).
Characteristics of DT104.
The 52 DT104 strains showed antimicrobial resistance against at least two drugs. Three antibiotic resistance patterns were observed: resistance to ampicillin, chloramphenicol, streptomycin, sulfisoxazole, and tetracycline (ACSSuT); resistance to ampicillin and sulfisoxazole (ASu); and resistance to streptomycin and sulfisoxazole (SSu). The ACSSuT resistance pattern was found only among DT104 strains isolated from house rats in building J. Two DT104 strains isolated from the rats in building J showed the SSu resistance pattern (Table 1). All DT104 strains isolated from house rats in building YS had only the ASu resistance pattern (Table 2).
PCR analysis using primers to the 5′ and 3′ conserved segments of class I integrons showed that all DT104 strains with the ACSSuT resistance pattern produced both the 1.0- and 1.2-kbp amplicons, and by the use of primers for specific antibiotic resistance genes, pse1, pasppflo-like, aadA2, sulI, and tet(G) were identified. All the strains with the SSu resistance pattern produced the 1.0-kbp amplicon of the class I integron with aadA2 and sulI, and all the strains with the ASu resistance pattern produced the 1.2-kbp amplicon of the class I integron with pse1 and sulI.
Molecular epidemiological analysis of DT104 strains.
Cluster analysis of XbaI-digested PFGE patterns (XbaI cluster analysis) showed that most or all DT104 strains from the two buildings formed one large cluster at all cutoff values (Table 3; see the XbaI dendrogram in the supplemental material). At the 95% cutoff value, the large cluster (cluster 95-X1) was made up of 41 of the 52 DT104 strains: 21 of the 26 building J strains (80.8%) and 20 of the 26 building YS strains (76.9%). At the 90% cutoff value, a large cluster (90-X1) was formed by 22 building J strains and 22 building YS strains. At the 85%, 80%, and 75% cutoff values, only a large cluster was formed, containing 49, 51, and 52 strains, respectively.
TABLE 3.
Relationship between cutoff value and clustering of DT104 strains by use of three different cluster analysis methods
| Cluster analysis method and cutoff value (%) | No. of strains
|
No. of clusters | Cluster designation | No. of clustered strains isolated from building:
|
|||
|---|---|---|---|---|---|---|---|
| Unclustered | Clustered | J | YS | Total | |||
| XbaI | |||||||
| 95 | 9 | 43 | 2 | 95-X1 | 21 | 20 | 43 |
| 95-X2 | 2 | 0 | |||||
| 90 | 3 | 49 | 3 | 90-X1 | 22 | 22 | 49 |
| 90-X2 | 0 | 2 | |||||
| 90-X3 | 3 | 0 | |||||
| 85 | 3 | 49 | 1 | 85-X1 | 25 | 24 | 49 |
| 80 | 1 | 51 | 1 | 80-X1 | 26 | 25 | 51 |
| 75 | 0 | 52 | 1 | 75-X1 | 26 | 26 | 52 |
| BlnI | |||||||
| 95 | 4 | 48 | 3 | 95-B1 | 22 | 0 | 48 |
| 95-B2 | 0 | 24 | |||||
| 95-B3 | 2 | 0 | |||||
| 90 | 1 | 51 | 1 | 90-B1 | 26 | 25 | 51 |
| 85 | 0 | 52 | 1 | 85-B1 | 26 | 26 | 52 |
| VNTR | |||||||
| 95 | 3 | 49 | 3 | 95-V1 | 24 | 15 | 49 |
| 95-V2 | 0 | 8 | |||||
| 95-V3 | 2 | 0 | |||||
| 90 | 1 | 51 | 3 | 90-V1 | 26 | 15 | 51 |
| 90-V2 | 0 | 8 | |||||
| 90-V3 | 0 | 2 | |||||
| 85 | 1 | 51 | 2 | 85-V1 | 26 | 23 | 51 |
| 85-V2 | 0 | 2 | |||||
| 80 | 1 | 51 | 2 | 80-V1 | 26 | 23 | 51 |
| 80-V2 | 0 | 2 | |||||
| 75 | 0 | 52 | 2 | 75-V1 | 26 | 23 | 52 |
| 75-V2 | 0 | 3 | |||||
Cluster analysis of BlnI-digested PFGE patterns (BlnI cluster analysis) found that the strains from the two buildings formed separate large clusters at the 95% cutoff value (Table 3; see the BlnI dendrogram in the supplemental material). Of the building J strains, 22 formed a large cluster (95-B1), while 24 building YS strains formed another large cluster (95-B2). At the 90% and 85% cutoff values, only a large cluster was formed, containing 51 and 52 strains, respectively.
Cluster analysis of the VNTR data (VNTR cluster analysis) found one major and one minor cluster when the cutoff value was 95 or 90%. At the 95% cutoff value, one major cluster (95-V1) was formed by 24 building J strains and 15 building YS strains and one minor cluster (95-V2) was formed by eight building YS strains (Table 3; see the VNTR dendrogram in the supplemental material). At the 90% cutoff value, one major cluster (90-V1) was formed by all 26 building J strains and 15 building YS strains and one minor cluster (90-V2) was formed by eight building YS strains. At the 85%, 80%, and 75% cutoff values, 49 strains formed a large cluster at all values.
Correlation between the three clustering methods.
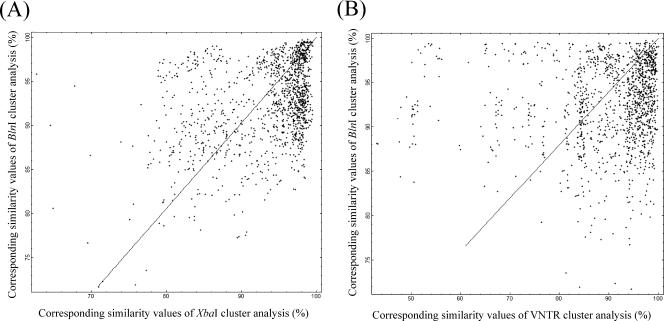
The congruence between the results of the XbaI and BlnI cluster analyses was low. The corresponding similarity values for these analyses are plotted in Fig. 1A, and the congruence value between the two analyses was 23.5 ± 1.83%. The congruence between the results of the BlnI and VNTR cluster analyses was also low. The corresponding similarity values for the two analyses are plotted in Fig. 1B, and the congruence value between the two analysis was 10.7 ± 1.83%.
FIG. 1.
Correlation of XbaI, BlnI, and VNTR cluster analyses. (A) Corresponding similarities of BlnI and XbaI cluster analysis. (B) Corresponding similarities of BlnI and VNTR cluster analysis.
For two-step cluster analysis, the clusters identified by BlnI cluster analysis were subjected to VNTR cluster analysis (Table 4). In the two-step analysis, the small cluster formed by two building J strains (cluster 95-B3) was not divided further at the 95% cutoff level. However, the two large clusters (cluster 95-B1 formed by 22 building J strains and cluster 95-B2 formed by 24 building YS strains) were each separated into two clusters at the 95% cutoff level. When two-step cluster analysis was done using XbaI cluster analysis, none of the clusters could be divided further at any cutoff value.
TABLE 4.
Relationship between cutoff value and clustering of DT104 strains in the clusters made by BlnI cluster analysis
| Secondary method of cluster analysis | Original cluster | Cutoff value (%) | No. of unclustered strains | No. of clusters | Cluster designation | No. of clustered strains |
|---|---|---|---|---|---|---|
| VNTR | 95-B1 | 95 | 0 | 2 | B1-95-V1 | 20 |
| B1-95-V2 | 2 | |||||
| 95-B2 | 95 | 3 | 2 | B2-95-V1 | 14 | |
| B2-95-V2 | 7 | |||||
| 90 | 1 | 2 | B2-90-V1 | 21 | ||
| B2-90-V2 | 2 | |||||
| 85 | 1 | 2 | B2-85-V1 | 21 | ||
| B2-85-V2 | 2 | |||||
| 80 | 1 | 2 | B2-80-V1 | 21 | ||
| B2-80-V2 | 2 | |||||
| 75 | 0 | 1 | B2-75-V1 | 24 | ||
| 95-B3 | 95 | 0 | 1 | B3-95-V1 | 2 | |
| XbaI | 95-B1 | 95 | 0 | 1 | B1-95-X1 | 2 |
| 95-B2 | 95 | 2 | 1 | B2-95-X1 | 20 | |
| 90 | 1 | 1 | B2-90-X1 | 21 | ||
| 85 | 1 | 1 | B2-85-X1 | 21 | ||
| 80 | 0 | 1 | B2-80-X1 | 22 | ||
| 95-B3 | 95 | 3 | 1 | B3-95-X1 | 21 | |
| 90 | 0 | 1 | B3-90-X1 | 24 |
DISCUSSION
Rodents are known to be carriers of zoonotic agents (26, 30). The study reported here shows that the prevalence of DT104 in house rats carrying serovar Typhimurium was high, suggesting an important role for rats in the transmission of this organism. Most rodents are thought to carry salmonellae in their spleen or liver, with less than 3% of the rats carrying the bacteria in the intestine, suggesting that rodents are not a major source of human salmonella infection (9). However, there were many reports indicating the detection of the organism in feces of rodents (12, 26, 30-32). In this study, DT104 strains were consistently isolated from the intestinal contents of house rats throughout the 1-year study period. It is well known that animals carrying salmonellae contaminate their environment, which can become the source of infection for both humans and animals (13). Therefore, it can be concluded that house rats carrying DT104 contaminate their environment and the contaminated environment can transmit the organism to other house rats.
The prevalence of DT104 strains in house rats, demonstrated in this study, supports the hypothesis of global expansion of DT104 by clonal dissemination (5, 29). Generally, the increase in antibiotic-resistant bacteria is due to selective pressure by antibiotics (7, 36). Since it is doubtful that such selective pressure could occur among house rats inhabiting buildings, dissemination by clonal expansion is a more likely conclusion. The hypothesis of clonal dissemination is supported by the molecular epidemiological results reported here. DT104 strains with similar PFGE patterns after XbaI digestion must have arisen from the same genetic clone (1, 4, 23). Most of the DT104 strains of this study had the same PFGE patterns following XbaI digestion (1, 16) and class I integrons with the same ACSSuT resistance pattern (18).
The global expansion of DT104 by clonal dissemination among house rats raises the difficult problem of tracing the local prevalence of the organism by comparison of PFGE patterns with XbaI digestion. Even though PFGE is the “gold standard” method for determining genetic relatedness of bacteria in molecular epidemiological studies (35), DT104 is highly clonal and XbaI digests of DT104 DNAs showed very similar PFGE patterns (1, 16). Therefore, other tools are necessary to investigate the molecular epidemiology of DT104 in house rats. Several molecular epidemiological methods have been investigated to differentiate DT104 strains (4, 19, 23). Plasmid profiling has been reported to be the most effective method for differentiating DT104 strains, compared to PFGE analysis, IS200 typing, and randomly amplified polymorphic DNA typing (1, 23). In contrast, plasmid profiling was not suitable for identifying DT104 clones in long-term studies because the plasmid studied was unstable (19). Since our study isolated DT104 strains over a 1-year period, we did not use plasmid profiling for molecular epidemiological analysis.
Another problem with the molecular epidemiological method is the question of the relationship between the similarity of PFGE patterns and the genetic relatedness of bacterial strains. Tenover et al. (35) recommended that the criteria for a genetic relationship could be based on differences in the numbers of restriction digest bands among PFGE patterns due to single-nucleotide polymorphisms at the restriction sites. However, the change of a PFGE pattern was due not primarily to the single-nucleotide polymorphism but to large-scale inversion (15, 17). Therefore, computer-based image analysis of PFGE patterns and cluster analysis of these data should be used to identify genetic relationships between bacterial strains. Cluster analysis can sort data into groups and reveal associations that are not otherwise evident. Although a number of studies have previously investigated cluster analysis of PFGE patterns, there were problems due to overlapping restriction bands (6) and the need to use a band matching coefficient, such as the Dice coefficient, for similarity calculations (40). In this study, the Pearson correlation, a densitometric curve-based coefficient, was used. Use of the Pearson correlation removes the problem of overlapping restriction bands, and the effectiveness of this approach has been confirmed by cluster analysis of PFGE patterns of other enteric bacteria (40).
In this study, XbaI cluster analysis could not differentiate DT104 strains. Most DT104 strains isolated from the two buildings studied were grouped in one large cluster. In contrast, analysis of the class I integron in these strains showed that the DT104 strains isolated from the two buildings contained different integrons, suggesting that different DT104 clones are prevalent in the house rats in the two buildings. The absence of DT104 strains with the ACSSuT or SSu resistance pattern in building YS suggests that DT104 strains with the ASu resistance pattern in building YS may have originated from ACSSuT strains by a single crossover at intI1 (3) before DT104 spread in this building. These results indicate that PFGE analyses with additional restriction enzymes will be required to trace the local prevalence of DT104 strains in house rats.
BlnI cluster analysis is more suitable than XbaI cluster analysis for tracing the local prevalence of DT104. PFGE analysis of BlnI digests has been reported to be more effective for differentiating DT104 strains than XbaI digestion (1, 23). In this study, BlnI cluster analysis showed that most DT104 strains from each building formed a single cluster per building at the 95% cutoff value. The BlnI cluster analysis results were in agreement with the integron analysis results for the DT104 strains in this study. All DT104 strains from building YS had the ASu resistance pattern, encoded by the same resistance genes in the same-size integron, while most strains from building J had the ACSSuT resistance pattern, encoded by the same resistance genes in the two same-size integrons. The BlnI cluster analysis and integron data and the circulation of salmonellae through habitats via carrier animals (13) suggest that different DT104 strains were prevalent in the house rat colonies of each building in this study. However, DT104 strains isolated from each of the two buildings showed similar BlnI-digested PFGE patterns throughout this 1-year study, indicating that it is impossible to follow the transmission of DT104 among house rats by this method.
Any single method of molecular analysis used in this study could not reveal the transmission of DT104 among house rats at a single location. DT104 strains derived from building YS were obviously divided into two distinct clusters using VNTR cluster analysis, but information about the appropriate dendrogram cutoff value is lacking. This result indicates that VNTR cluster analysis is useful for further differentiation of DT104 strains to follow the transmission of DT104 among house rats. However, strain differentiation among rats from the two buildings was not possible with only VNTR cluster analysis. Although the differentiating power of VNTR cluster analysis for DT104 strains will be improved by adding more VNTR loci (21), there is no information about the usefulness of VNTR loci other than those used in this study. One possible solution is to combine several analytical methods. The BioNumerics software can make composite data based on the results of several types of analytical methods. However, since similarities of PFGE patterns calculated by band matching coefficients are required to produce composite data (BioNumerics version 4 manual; Applied Maths), such a composite analysis could not be done in this study.
A two-step cluster analysis might partially trace the transmission of DT104 among house rats at a single location, with a VNTR cluster analysis of BlnI cluster analysis data. In this study, the congruence values of XbaI and VNTR cluster analysis results for BlnI cluster analysis results were low. These results indicated that clusters identified by BlnI cluster analysis could be further divided by XbaI or VNTR cluster analysis. Of the three clusters identified by BlnI cluster analysis, two were divided further into smaller clusters and unclustered strains by VNTR cluster analysis, while XbaI cluster analysis did not divide any of the BlnI clusters at any cutoff values. At the present time, it is unclear which cutoff values should be applied for VNTR cluster analysis. To determine the relatedness of bacterial strains, epidemiological information should be combined with genetic relatedness data (35). In this study, detailed epidemiological contact information among house rats was lacking. The efficiency of the two-step cluster analysis results for DT104 should be confirmed using epidemiological information.
It is unknown how many of the reported human cases of serovar Typhimurium in Japan are caused by DT104. Phage type is not always examined for human salmonellosis in Japan. However, DT104 has been expanding internationally (10). The DT104 strains isolated from human cases in Japan had the same genetic profile as the original DT104 clone (16), indicating that DT104 has already spread in Japan. Moreover, there have been reports that DT104 had also spread among livestock in Japan (8, 34), indicating a possible risk of carrier livestock contaminating food (13). Despite numerous reports of human DT104 infection in foreign countries (10), only one case was reported during a mass outbreak in Japan (33). During 1997-1998, one mass outbreak and three sporadic cases of serovar Typhimurium were reported in Yokohama City, but there were no phage type data (38, 39). Some cases of DT104 might be identified if serovar Typhimurium isolates from outbreaks were subjected to phage typing. PCR identification would be a useful tool to investigate whether serovar Typhimurium strains are DT104, although one strain showed contradictory results by PCR and phage typing. The positive reaction of the strain by Pritchett's methods suggested that the strain might be U302 (28). However, phage typing in this study was done to identify “DT104” or “not DT104,” rather than to identify U302. In the study by Hermans et al. (11), no U302 strains were investigated, so it is still unclear whether PCR identification can identify DT104 only or a DT104 complex including U302.
In conclusion, the house rat plays an important role in the epidemiology of DT104, since DT104 can be maintained in the house rat colonies for a long time. BlnI cluster analysis is useful for investigating the molecular epidemiology of DT104, and two-step cluster analysis using VNTR typing may reveal further molecular epidemiology of DT104.
Acknowledgments
We express our gratitude to H. Izumiya and H. Watanabe, National Institute of Infectious Disease, for the performance of definitive phage typing.
This work was supported by grants for Academic Frontier Project “Surveillance and Control for Zoonosis” from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Footnotes
Published ahead of print on 16 February 2007.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Baggesen, D. L., D. Sandvang, and F. M. Aarestrup. 2000. Characterization of Salmonella enterica serovar Typhimurium DT104 isolated from Denmark and comparison with isolates from Europe and the United States. J. Clin. Microbiol. 38:1581-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer, A. W., W. M. Kirby, J. C. Sherris, and M. Turck. 1966. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 36:493-496. [PubMed] [Google Scholar]
- 3.Boyd, D., A. Cloeckaert, E. Chaslus-Dancla, and M. R. Mulvey. 2002. Characterization of variant Salmonella genomic island 1 multidrug resistance regions from serovars Typhimurium DT104 and Agona. Antimicrob. Agents Chemother. 46:1714-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cloeckaert, A., and S. Schwarz. 2002. Molecular characterization, spread and evolution of multidrug resistance in Salmonella enterica Typhimurium DT104. Vet. Res. 32:301-310. [DOI] [PubMed] [Google Scholar]
- 5.Davis, M. A., D. D. Hancock, and T. E. Besser. 2002. Multiresistant clones of Salmonella enterica: the importance of dissemination. J. Lab. Clin. Med. 140:135-141. [DOI] [PubMed] [Google Scholar]
- 6.Davis, M. A., D. D. Hancock, T. E. Besser, and D. R. Call. 2003. Evaluation of pulsed-field gel electrophoresis as a tool for determining the degree of genetic relatedness between strains of Escherichia coli O157:H7. J. Clin. Microbiol. 41:1843-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeFrancesco, K. A., R. N. Cobbold, D. H. Rice, T. E. Besser, and D. D. Hancock. 2004. Antimicrobial resistance of commensal Escherichia coli from dairy cattle associated with recent multi-resistant salmonellosis outbreaks. Vet. Microbiol. 98:55-61. [DOI] [PubMed] [Google Scholar]
- 8.Esaki, H., A. Morioka, A. Kojima, K. Ishihara, T. Asai, Y. Tamura, H. Izumiya, J. Terajima, H. Watanabe, and T. Takahashi. 2004. Epidemiological characterization of Salmonella Typhimurium DT104 prevalent among food-producing animals in the Japanese Veterinary Antimicrobial Resistant monitoring Program (1999-2001). Microbiol. Immunol. 48:553-556. [DOI] [PubMed] [Google Scholar]
- 9.Healing, T. D. 1991. Salmonella in rodents: a risk to man. CDR 1(13):R114-R116. [PubMed] [Google Scholar]
- 10.Helms, M., S. Ethelberg, K. Mølbak, and the DT104 Study Group. 2005. International Salmonella Typhimurium DT104 infections, 1992-2001. Emerg. Infect. Dis. 11:859-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hermans, A. P., T. Abee, M. H. Zwietering, and H. J. M. Aarts. 2005. Identification of novel Salmonella enterica serovar Typhimurium DT104-specific prophage and nonprophage chromosomal sequences among serovar Typhimurium isolated by genomic subtraction hybridization. Appl. Environ. Microbiol. 71:4979-4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hilton, A. C., R. J. Willis, and J. S. Hickie. 2002. Isolation of Salmonella from urban wild brown rats (Rattus norvegicus) in the West Midlands, UK. Int. J. Environ. Health Res. 12:163-168. [DOI] [PubMed] [Google Scholar]
- 13.Humphery, T. 2001. Salmonella Typhimurium definitive type 104. A multi-resistant Salmonella. Int. J. Food Microbiol. 67:173-186. [DOI] [PubMed] [Google Scholar]
- 14.Hunter, S. B., P. Vauterin, M. A. Lambert-Fair, M. S. Van Duyne, K. Kubota, L. Graves, D. Wrigley, T. Barrett, and E. Ribot. 2005. Establishment of a universal size standard strain for use with the PulseNet standardized pulsed-field gel electrophoresis protocols: converting the national database to the new size standard. J. Clin. Microbiol. 43:1045-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iguchi, A., S. Iyoda, J. Terajima, H. Watanabe, and R. Osawa. 2006. Spontaneous recombination between homologous prophage regions causes large-scale inversions within the Escherichia coli O157:H7 chromosome. Gene 372:199-207. [DOI] [PubMed] [Google Scholar]
- 16.Izumiya, H., J. Terajima, S. Matsushita, K. Tamura, and H. Watanabe. 2001. Characterization of multidrug-resistant Salmonella enterica serovar Typhimurium isolated in Japan. J. Clin. Microbiol. 39:2700-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kudva, I. T., P. S. Evans, N. T. Perna, T. J. Barrett, F. M. Ausubel, F. R. Blattner, and S. B. Calderwood. 2002. Strains of Escherichia coli O157:H7 differ primarily by insertions or deletions, not single-nucleotide polymorphisms. J. Bacteriol. 184:1873-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai-King, N. G., M. R. Mulvey, I. Martin, G. A. Peters, and W. Johnson. 1999. Genetic characterization of antimicrobial resistance in Canadian isolates of Salmonella serovar Typhimurium DT104. Antimicrob. Agents Chemother. 43:3018-3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liebana, E., L. Garcia-Migura, C. Clouting, F. A. Clifton-Hadley, E. Lindsay, E. J. Threlfall, S. W. J. McDowell, and R. H. Davies. 2002. Multiple genetic typing of Salmonella enterica serotype Typhimurium isolates of different phage types (DT104, U302, DT204b, and DT49) from animals and humans in England, Wales, and Northern Ireland. J. Clin. Microbiol. 40:4450-4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindstedt, B.-A., T. Vardund, L. Aas, and G. Kapperud. 2004. Multiple-locus variable number tandem-repeats analysis of Salmonella enterica subsp. enterica serovar Typhimurium using PCR multiplexing and multicolor capillary electrophoresis. J. Microbiol. Methods 59:163-172. [DOI] [PubMed] [Google Scholar]
- 21.Lindstedt, B.-A. 2005. Multiple-locus variable number tandem repeats analysis for genetic fingerprinting of pathogenic bacteria. Electrophoresis 26:2567-2582. [DOI] [PubMed] [Google Scholar]
- 22.Low, J. C., M. Angus, G. Hopkins, D. Munro, and S. C. Rankin. 1997. Antimicrobial resistance of Salmonella enterica Typhimurium DT104 isolates and investigation of strains with transferable apramycin resistance. Epidemiol. Infect. 118:97-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malorny, B., A. Schroeter, C. Bunge, B. Hoog, A. Steinbeck, and R. Melmuth. 2001. Evaluation of molecular typing methods for Salmonella enterica serovar Typhimurium DT104 isolated in Germany from healthy pigs. Vet. Res. 32:119-129. [DOI] [PubMed] [Google Scholar]
- 24.Markogiannakis, A., P. T. Tassios, M. Lambiri, L. R. Ward, J. Kourea-Kremastinou, N. J. Legakis, The Greek Nontyphoidal Salmonella Study Group, and A. C. Vatopoulos. 2000. Multiple clones within multidrug-resistant Salmonella enterica serotype Typhimurium phage type DT104. J. Clin. Microbiol. 38:1269-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maruyama, S., A. Mitsuzane, K. Nakagawa, H. Kabeya, E. Yokoyama, and Y. Katsube. 2000. Prevalence of Salmonella species in house rats from the buildings in Yokohama city. J. Vet. Epidemiol. 4:27-32 (In Japanese.) [Google Scholar]
- 26.McKiel, J. A., D. E. Rappay, J. G. Cousineau, R. R. Hall, and H. E. McKenna. 1970. Domestic rats as carriers of leptospires and salmonellae in Eastern Canada. Can. J. Public Health 61:336-340. [PubMed] [Google Scholar]
- 27.National Institute of Infectious Disease. 2003. Recent trend of salmonellosis in Japan. Infect. Agent Serv. Rep. 24:179-180. [Google Scholar]
- 28.Pritchett, L. C., M. E. Konkel, J. M. Gay, and T. E. Besser. 2000. Identification of DT104 and U302 phage types among Salmonella enterica serotype Typhimurium isolates by PCR. J. Clin. Microbiol. 38:3484-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scalzo, S., J. E. Corkill, D. J. Shanks, T. G. Rowan, J. Delaval, A. Fleetwood, M. Murphy, and C. A. Hart. 2004. Phenotypic and genotypic changes in Salmonella enterica subsp. enterica serotype Typhimurium during passage in intestines of broiler chickens on diets that included ionophore anticoccidial supplement. J. Clin. Microbiol. 42:3399-3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seguin, B., Y. Boucaud-Maitre, P. Quenin, and G. Lorgue. 1986. Epidemiologic evaluation of a sample of 91 rats (Rattus norvegicus) captured in the sewers of Lyon. Zentbl. Bakteriol. Mikrobiol. Hyg. A 261:539-546. [PubMed] [Google Scholar]
- 31.Singh, S. P., M. S. Sethi, and V. D. Sharma. 1980. The occurrence of salmonellae in rodent, shrew, cockroach and ant. Int. J. Zoonoses 7:58-61. [PubMed] [Google Scholar]
- 32.Steffen, E. K., and J. E. Wagner. 1983. Salmonella enteritidis serotype Amsterdam in a commercial rat colony. Lab. Anim. Sci. 33:454-456. [PubMed] [Google Scholar]
- 33.Taguchi, M., K. Seto, M. Kanki, T. Tsukamoto, H. Izumiya, and H. Watanabe. 2005. Outbreak of food poisoning caused by lunch boxes prepared by a company contaminated with multidrug resistant Salmonella typhimurium DT104. Jpn. J. Infect. Dis. 58:55-56. [PubMed] [Google Scholar]
- 34.Takahashi, A., H. Kajita, T. Segawa, R. Shiraiwa, N. Hujita, M. Hiraga, and K. Oshima. 2001. Drug sensitivity of Salmonella Typhimurium isolated from broiler chickens at processing plants. J. Jpn. Vet. Med. Assoc. 54:797-800. (In Japanese with English summary.) [Google Scholar]
- 35.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van den Bogaard, A. E., and E. E. Stobberingh. 2000. Epidemiology of resistance to antibiotics. Links between animals and humans. Int. J. Antimicrob. Agents 14:327-335. [DOI] [PubMed] [Google Scholar]
- 37.Wall, P. G., E. J. Threllfall, L. R. Ward, and B. Rowe. 1996. Multiresistant Salmonella typhimurium DT104 in cats: a public health risk. Lancet 348:471. [DOI] [PubMed] [Google Scholar]
- 38.Yokohama City Institute of Health. 1998. Annual reports of bacteriological investigations in 1997. Ann. Rep. Yokohama Inst. Health 37:8. [Google Scholar]
- 39.Yokohama City Institute of Health. 1999. Annual reports of bacteriological investigations in 1998. Ann. Rep. Yokohama Inst. Health 38:14. [Google Scholar]
- 40.Yokoyama, E., and M. Uchimura. 2006. Optimal settings of fingerprint type analyzing software for the analysis of enterohemorrhagic Escherichia coli pulsed-field gel electrophoresis patterns. Epidemiol. Infect. 134:1004-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yokoyama, E., K. Kishida, M. Uchimura, and S. Ichinohe. 2006. Comparison between agarose gel electrophoresis and capillary electrophoresis for variable numbers of tandem repeat typing of Mycobacterium tuberculosis. J. Microbiol. Methods 65:425-431. [DOI] [PubMed] [Google Scholar]