Abstract
Persistence of Bacillus atrophaeus subsp. globigii spores on corroded iron coupons in drinking water was studied using a biofilm annular reactor. Spores were inoculated at 106 CFU/ml in the dechlorinated reactor bulk water. The dechlorination allowed for observation of the effects of hydraulic shear and biofilm sloughing on persistence. Approximately 50% of the spores initially adhered to the corroded iron surface were not detected after 1 month. Addition of a stable 10 mg/liter free chlorine residual after 1 month led to a 2-log10 reduction of adhered B. atrophaeus subsp. globigii, but levels on the coupons quickly stabilized thereafter. Increasing the free chlorine concentration to 25 or 70 mg/liter had no additional effect on inactivation. B. atrophaeus subsp. globigii spores injected in the presence of a typical distribution system chlorine residual (∼0.75 mg/liter) resulted in a steady reduction of adhered B. atrophaeus subsp. globigii over 1 month, but levels on the coupons eventually stabilized. Adding elevated chlorine levels (10, 25, and 70 mg/liter) after 1 month had no effect on the rate of inactivation. Decontamination with elevated free chlorine levels immediately after spore injection resulted in a 3-log10 reduction within 2 weeks, but the rate of inactivation leveled off afterward. This indicates that free chlorine did not reach portions of the corroded iron surface where B. atrophaeus subsp. globigii spores had adhered. B. atrophaeus subsp. globigii spores are capable of persisting for an extended time in the presence of high levels of free chlorine.
Contamination of the drinking water distribution system infrastructure with microbiological agents is a current homeland security concern. Should pathogens adhere, persist, detach, and survive in the water column, drinking water safety would be compromised. Vegetative bacterial bioterror agents have been shown to be more susceptible to disinfectants than spores in the planktonic phase (30, 32). However, adhered pathogens detaching from biofilm or corrosion material could be associated with particles, thereby increasing disinfection resistance and the likelihood that they could reach a consumer's tap (4, 16). Spore-forming bacteria could even survive boiling if the procedure were not performed properly (31). Even if pathogen density in the water column were low enough that infection of consumers did not occur, uncertainty surrounding the fate of injected pathogens present in the pipe material and biofilm would assuredly reduce the number of people utilizing municipal potable water. Therefore, understanding how long allochthonous pathogens survive in biofilm and/or pipe material under oligotrophic drinking water conditions, and decontamination of that material, is an important research topic.
It is well known that biofilm-associated microorganisms survive longer in the presence of disinfectants than planktonic organisms (8, 20-22, 34). However, information on persistence of microbiological agents in water distribution system pipe material is limited. Research available in the open literature mostly focuses on bacteria, including coliforms (7, 12, 25, 37), Helicobacter pylori (23, 28), Legionella pneumophila (3, 11, 19, 26, 36), Aeromonas hydrophila (5), and Salmonella enterica serovar Typhimurium (3). Other researchers have investigated the persistence of injected viruses (19, 29, 35) as well as wastewater cross-connections with drinking water pipes (13, 24). These studies focused on vegetative cells and viruses but did not address bacterial spores adhered to biofilm.
The literature cited above focuses mostly on drinking water biofilms grown on smooth substratum materials, such as glass, polyvinyl chloride (PVC), or stainless steel. Although these materials are easily obtainable and conducive to sampling, the nation's drinking water infrastructure is predominantly composed of different substratum materials. An American Water Works Association survey of drinking water utilities quantified the composition of pipe materials in the United States and Canada and found that 18% of the total pipe miles are unlined cast and ductile iron (2). The remainder of the pipe miles are 50% cement lined iron, 19% cement and concrete pipes, 11% PVC or polyethylene, and 2% steel (2).
These literature sources indicate that persistence of spore-forming bacteria adhered to corroded iron pipe material following contamination of a drinking water system is unclear. This study addresses the topic by establishing a “corroding biofilm,” or a mixture of cells, extracellular polymeric substances, and corrosion products, on iron coupons in biofilm annular reactors (BARs) with finished tap water flowing through them. Bacillus atrophaeus subsp. globigii, a surrogate for Bacillus anthracis, was pulse-injected into the reactors in spore form, and the B. atrophaeus subsp. globigii density on the coupons was monitored over time under dechlorinated and chlorinated bulk conditions. Decontamination of the coupons with high levels of free chlorine was also investigated.
MATERIALS AND METHODS
Experimental drinking water system.
Typical conditions of a drinking water distribution system pipe, including temperature, hydraulic shear, and surface composition, were simulated using BARs (BioSurface Technologies Corporation, Bozeman, MT). The experimental setup is identical to that described by Szabo et al. (37), which was based on previous studies (7, 25). The biofilm annular reactors contain 20 flush-mounted rectangular polycarbonate slides attached to a rotating polycarbonate cylinder, which is inside a stationary glass outer cylinder. The rotational speed of the inner drum was set to 100 rpm for all experiments. This condition generates shear on the inner drum similar to 30.5 cm/s (1 ft/s) flow in a 10.2-cm (4-in.) pipe. This calculation is only valid for a smooth inner drum. The biofilm and corrosion layer protruded from the polycarbonate slide surface as it formed. Therefore, it was not possible to determine the exact shear at the rough biofilm/corrosion surface; thus, these values are estimates of the flow conditions.
Iron coupons (Goodfellow Cambridge Ltd., Huntingdon, United Kingdom) were attached to the polycarbonate slides, as described below, and a “corroding biofilm” was formed. The mixture of corrosion products and microorganisms from Cincinnati tap water that colonized the iron surface formed the corroding biofilm. Iron sheets (99.5% pure Fe) were cut into 1-cm2 by 0.5-mm coupons, and the surface in contact with tap water was roughed with medium grit emery paper. Abrading the surface allowed for uniform corrosion and biofilm development over the entire coupon surface. The coupons were attached to the polycarbonate slides using acrylic cement (TAP Plastics, Oakland, CA). A steady-state biofilm was established before experiments began, and the entire coupon surface was covered with a rough layer of corrosion. The corroding biofilm has been described in detail by Szabo et al. (37).
Chlorinated and dechlorinated Cincinnati tap water was fed to the 1-liter BARs from a sterile polypropylene carboy at 0.5 liter/h, resulting in a mean retention time of 2 h. Sterile 10% (wt/vol) sodium thiosulfate was added to the carboy for dechlorinated experiments. Extra free chlorine was added to the reactors during chlorinated experiments as needed to keep the residual stable in the bulk phase. Syringe pumps added a concentrated sodium hypochlorite solution (approximately 10,000 to 50,000 mg/liter) to the reactors at 0.5 ml/h, which maintained the reactor bulk at a typical distribution system chlorine residual (∼0.75 mg/liter) or at decontamination concentrations of approximately 10, 25, or 70 mg/liter. Bulk-phase water in the reactor, which was similar in quality to tap water, was sampled daily for pH, temperature, and free chlorine, which ranged from 8.5 to 8.7, 21 to 24°C, and 0.6 to 1.0 mg/liter, respectively. Chlorinated and dechlorinated experiments were carried out in two independent but identical BARs. The standard N,N-diethyl-p-phenylenediamine colorimetric method was used for free chlorine analysis (1).
Reactors were disassembled and scrubbed with nonphosphate soap and water before experimentation. They were then reassembled, plumbed, and sterilized (121°C, 19 lb/in2 gauge, 25 min) with the polycarbonate slides inserted. Iron coupons were not autoclaved, since this weakened the adhesive holding them to the polycarbonate and resulted in excessive corrosion (7, 37). Instead, the slides with attached coupons were suspended for 30 seconds in 100 mg/liter sodium hypochlorite, rinsed with sterile water, and aseptically inserted into the autoclaved reactor. Since the coupons were only disinfected, sterility was checked by scraping the surfaces into 10 ml of sterile 0.05 M KH2PO4 buffer (pH 7.2), which was plated on R2A (20°C, 7 days) according to standard methods (1). No colonies (<1 CFU/0.1 ml) were detected on the plates after incubation, indicating that disinfection had removed attached cells from the coupons before experimentation.
Biofilm and corrosion sampling.
Polycarbonate slides with coupons attached were removed using a flame-sterilized slide removal tool (BioSurface Technologies Corporation, Bozeman, MT). Corroded coupons were removed from the polycarbonate with a flame-sterilized scalpel and tweezers. Biofilm and corrosion particles were removed from the coupon using a flame-sterilized scalpel and suspended in 10 ml of 0.05 M sterile KH2PO4 buffer (pH 7.2). The buffer was supplemented with 0.1 ml of sterile 10% (wt/vol) sodium thiosulfate when chlorine residual was present in the reactor bulk. Suspended biofilm and corrosion particles were homogenized for 30 seconds using a tissue homogenizer (Tekmar, Cincinnati, OH). The homogenized suspension was serially diluted and enumerated for B. atrophaeus subsp. globigii (described below).
Cultivation, preparation, and enumeration of B. atrophaeus subsp. globigii spores.
B. atrophaeus subsp. globigii spores were obtained from Dugway Proving Grounds (Dugway, Utah). Generic spore medium was inoculated with vegetative B. atrophaeus subsp. globigii cells and incubated for 5 days at 35°C with gentle shaking in a rotary shaker (9). Purified B. atrophaeus subsp. globigii endospores were produced using gradient separation as previously described by Nicholson and Setlow (27). The presence of spores was confirmed using phase-contrast microscopy (<0.1% vegetative cells). Spores were stored in 40% ethanol at 4°C until use. Biofilm annular reactors were seeded with these spore suspensions to achieve 106 CFU/ml inside the reactors.
B. atrophaeus subsp. globigii was enumerated in triplicate by spread plate technique (1) using trypticase soy agar plates (Becton Dickinson, Sparks, MD) incubated for 24 ± 2 h at 35°C. Homogenized corrosion material suspended in 10 ml of buffer (0.05 M KH2PO4, pH 7.2) and 10 ml of bulk water from the biofilm annular reactor were analyzed. Water and biofilm suspensions were heat shocked at 80°C for 10 min. B. atrophaeus subsp. globigii was easily detectable from the few other biofilm organisms which survived the heat shock, since it forms orange colonies.
RESULTS
Contamination experiments examined several important parameters that affect persistence of B. atrophaeus subsp. globigii on corroded iron with an established biofilm. In all experiments, spores were pulse-injected into the reactor in less than 1 minute, and the initial density in the bulk fluid was 106 CFU/ml. The experiments simulated the injection of B. atrophaeus subsp. globigii spores into a municipal drinking water system with (i) the tap water intentionally dechlorinated, (ii) a typical chlorine residual present, and (iii) decontamination as an attempt to remove the adhered spores.
Initially dechlorinated reactors.
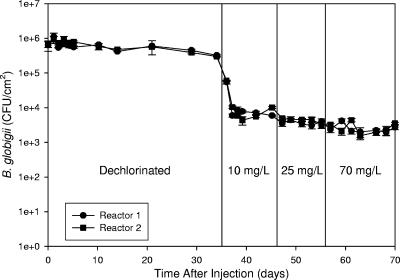
Data in the initial portion of Fig. 1 show spore densities on the coupons for approximately 1 month in dechlorinated water. This plot shows cell disappearance from the coupon through shear forces or sloughing of corrosion or biofilm material without the influence of a disinfectant. After 30 days, the spore concentrations on the coupon surfaces had dropped to 29 and 33% of the initial concentration (71 and 67% reduction). The spores remaining at day 34 were either strongly adhered to the surface or attached to an area on the coupon surface where they were not affected by shear forces or biofilm sloughing. Spores continued to be detected in the reactor bulk water (102 to 103 CFU/ml) during the dechlorinated portion of the study. As the drinking water environment is not favorable for germination (15), attachment and reattachment were the most likely cause for the presence of B. atrophaeus subsp. globigii in the bulk water over time.
FIG. 1.
Comparison of B. atrophaeus subsp. globigii spore persistence on corroded iron coupons in initially dechlorinated water. Decontamination started at day 35, with target decontamination chlorine levels of 10, 25, and 70 mg/liter. Error bars represent standard deviations from triplicate measurements carried out on samples from each reactor, and the data points represent the means.
Spore concentrations on the coupons were higher 1 day after injection than at 30 min after injection. The concentrations 30 min after injection were 6.4 × 105 and 6.7 × 105 CFU/cm2 in reactors 1 and 2, respectively, and 1.1 × 106 and 9.2 × 105 CFU/cm2 in the same reactors 1 day after inoculation. This represents an increase of 72 and 37% on the coupons in reactors 1 and 2, respectively. This trend indicates that adhesion was still taking place after the 30-min sample was taken. This trend was seen for all experiments, regardless of the chlorine concentration. Therefore, when log inactivation or Ct values were calculated, the spore concentration on the coupons determined at day 1 was considered the initial concentration, since it was the highest observed after inoculation and spore adhesion was still taking place through the first day.
Decontamination of initially dechlorinated reactors.
After 35 days in contact with dechlorinated water, the chlorine concentration was increased in the reactor bulk liquid with the intention of achieving 10 mg/liter. The actual average free chlorine concentrations achieved in the reactors over the 10-day decontamination period were 11.3 ± 1.5 and 12.5 ± 1.6 mg/liter (± standard deviation over the decontamination period). The results are shown in Fig. 1. An immediate sharp drop in B. atrophaeus subsp. globigii density on the coupons occurred after chlorination. B. atrophaeus subsp. globigii density on the coupons in the two reactors was approximately 3 × 105 CFU/cm2 before chlorination, and 2 days after introduction of 10 mg/liter free chlorine the spore concentration was on the order of 7.0 × 103 CFU/cm2. Spore density stabilized at this level for the remainder of the time in contact with the 10 mg/liter free chlorine in both reactors. The decrease after decontamination represents 99.2% B. atrophaeus subsp. globigii inactivation (2.1 logs) from the initial concentration that adhered to the coupons at day 1 and 97.7% inactivation (1.7 logs) from the concentration on the coupons immediately before chlorine was added to the reactor. However, 103 to 104 CFU/cm2 spores still remained on the coupons, and 10 mg/liter free chlorine did not effectively inactivate B. atrophaeus subsp. globigii after the initial decrease. B. atrophaeus subsp. globigii was not detected in the bulk phase after introduction of free chlorine.
After 10 days in the presence of approximately 10 mg/liter free chlorine, the bulk concentration was increased to approximately 25 mg/liter for an additional 10 days. The actual free chlorine concentrations in the reactors were 25.4 ± 2.7 and 26.6 ± 2.8 mg/liter. Figure 1 shows that little change in coupon spore density was observed. Free chlorine concentration was increased again to approximately 70 mg/liter and kept at this level for 14 more days. The same response was seen as with the previous disinfectant increase. Actual free chlorine concentrations in the reactors were 70.1 ± 5.2 and 73.5 ± 3.0 mg/liter.
Data in Fig. 2 show the log10 survivors of the B. atrophaeus subsp. globigii density on the coupons normalized by the day 1 density on the coupons. Log inactivation of adhered B. atrophaeus subsp. globigii ranged from 2.1 immediately after free chlorine addition to 2.6 at the end of the decontamination period. Trend lines generated by linear regression are plotted over the dechlorinated and decontamination portions of the plots. The gap between the lines represents the sharp decrease in spore concentration on the coupons that occurred when chlorine was added. The fit of the linear trend lines is acceptable (R2 of 0.77 and 0.82 for the dechlorinated and chlorinated portions, respectively), but the lines do show the decreasing trend in both sections. The average slopes of the trend lines between the two reactors were −0.0155 ± 0.0022 and −0.0172 ± 0.0009 day−1 for the dechlorinated and chlorinated portions, respectively (± standard deviation). This indicates that the rate of decrease in spore density was not dramatically different in the dechlorinated and chlorinated period after the initial steep decrease when chlorine was introduced.
FIG. 2.
B. atrophaeus subsp. globigii spore persistence on corroded iron coupons in initially dechlorinated water presented as the base log10 of the surviving fraction. Decontamination started at day 35. Data points represent the averages between two duplicate experiments, and bars represent the ranges between experiments.
Initially chlorinated reactors.
B. atrophaeus subsp. globigii spores were inoculated into biofilm annular reactors with a stable free chlorine residual in the bulk water. Free chlorine levels in the two biofilm annular reactors were 0.73 ± 0.16 and 0.77 ± 0.21 mg/liter during the course of the 1-month exposure. B. atrophaeus subsp. globigii in the bulk phase decreased to 5 CFU/ml 5 days after injection and was not detected in the bulk liquid thereafter.
Data in Fig. 3 show that spores persisted on the coupons soon after injection even though B. atrophaeus subsp. globigii spores were not present in the bulk water. In dechlorinated reactors the spores persisted on the coupons, but approximately 103 CFU/ml were present in the bulk. The initial portion of the curves in Fig. 3 shows the levels of B. atrophaeus subsp. globigii spores over the course of 33 days. The slope of the curve is initially sharp (days 1 to 5) but eventually levels off (days 5 to 33). In disinfection kinetics, this is known as “tailing off” (14, 17).
FIG. 3.
B. atrophaeus subsp. globigii spore persistence on corroded iron coupons in initially chlorinated water. Decontamination started at day 34. Error bars represent standard deviations from triplicate measurements on samples from each reactor, and the data points represent the means.
When the spore levels were plotted as log survivors (Fig. 4), two different trends appeared. From days 1 to 5, the trend was linear (R2 = 0.99) with a slope of −0.2221 ± 0.0342 day−1. For samples taken at day 10 and onward, the slope of the curve was noticeably flatter. The trend was also linear (R2 = 0.98), with a slope of −0.0389 ± 0.0091 day−1, which is six times lower than from the first 5 days. The trend lines represent the best fit after using linear regression for each curve. At day 5, roughly 1 log inactivation was achieved in the presence of 0.73 and 0.77 mg/liter free chlorine, but an additional 28 days were required before 2 logs inactivation was observed on the coupons. These curves indicate that the rate of inactivation/removal from the coupons changes with time.
FIG. 4.
B. atrophaeus subsp. globigii spore persistence on corroded iron coupons in initially dechlorinated water presented as the log10 of the surviving fraction. Decontamination started at day 34. Data points represent the averages between two duplicate experiments, and bars represent the ranges between experiments.
Decontamination of initially chlorinated reactors.
After 33 days in the chlorinated reactors, a 2-log inactivation was achieved, and the rate of spore decrease was constant. Chlorine levels were increased to a target concentration of 10 mg/liter, as in the dechlorinated reactors. Actual free chlorine concentrations in the two reactors were 9.3 ± 0.6 and 9.9 ± 1.1 mg/liter. Figures 3 and 4 show the level of B. atrophaeus subsp. globigii spores on the coupons as free chlorine concentration was increased. Immediately after introduction of 9.3 and 9.9 mg/liter free chlorine, the B. atrophaeus subsp. globigii levels on the coupons experienced no significant change in concentration. After 6 days at the elevated chlorine level, the mean spore density between the two reactors decreased approximately 5% from 3.9 × 103 to 3.7 × 103 CFU/cm2.
The bulk free chlorine concentration was further increased to target levels of 25 and 70 mg/liter, but actual chlorine concentrations were 30.2 ± 10.3 and 29.7 ± 3.6 mg/liter and 80.3 ± 19.7 and 80.1 ± 30.0 mg/liter in the two reactors, respectively. The higher standard deviation associated with the mean chlorine values during the 70-mg/liter period occurred because the actual chlorine concentrations in the reactors were initially higher than expected. The two reactors had chlorine levels of 124 and 148 mg/liter for 1 day after the levels were increased, but this did not affect the rate of decrease from the coupons.
Data in Fig. 4 show the rate of B. atrophaeus subsp. globigii decrease from the coupons from the time that the low chlorine residual (0.75 mg/liter) was present to the time when high levels of free chlorine (10, 25, and 70 mg/liter) were present. The fit of the curves (R2 = 0.93 for both reactors) shows that the rate of spore decrease did not change, even when elevated levels of free chlorine were added during the decontamination period. The slope of the regression line from days 10 to 58 in Fig. 4 is −0.0328 ± 0.0021 day−1. This is similar to the slope from days 10 to 33 (−0.0389 ± 0.0091 day−1), which only takes into account the period when the chlorine residual was 0.73 to 0.77 mg/liter. This provides strong evidence that increasing the chlorine concentration does not necessarily increase the rate of B. atrophaeus subsp. globigii inactivation on the coupons and that bacterial spores can reside on a corroded iron surface for an extended time.
Immediate decontamination.
Decontamination in the previous experiments took place after B. atrophaeus subsp. globigii had adhered to the coupons for approximately 1 month. Two additional experiments were conducted to determine the effect of increasing chlorine concentration soon after spore inoculation resulted in an increased log inactivation compared to the decontamination strategy performed previously. Data in Fig. 5 show the decrease in B. atrophaeus subsp. globigii spore levels on the coupons in the presence of various levels of free chlorine in the bulk water. The target chlorine concentrations were 0.0, 0.75, 10, and 25 mg/liter, but the actual levels were closer to those reported earlier. As expected, B. atrophaeus subsp. globigii initially decreased faster from the coupons at the 10- and 25-mg/liter levels. Still, the spore decrease eventually leveled off at 3-log inactivation at 24 and 15 days for the 10- and 25-mg/liter free chlorine levels, respectively (although the log inactivation for the 10-mg/liter level was 2.9 at day 15). This shows that a faster decrease can be initially achieved with elevated chlorine levels, but spore concentrations still plateau, even when the elevated chlorine levels remain constant.
FIG. 5.
B. atrophaeus subsp. globigii spore persistence on corroded iron coupons at different chlorine concentrations, presented as the log10 of the surviving fraction. Data points represent the averages between two duplicate experiments, and bars represent the ranges between experiments.
A 2-log inactivation was achieved in 30 days at 0.75 mg/liter free chlorine but approached 3-log inactivation at 60 days during the decontamination period (Fig. 3 and 4). Increasing the chlorine concentration in the decontamination phase did not increase the rate of spore inactivation. Figures 5 illustrates that B. atrophaeus subsp. globigii spore populations decrease on the coupon surface and eventually reach a plateau that is independent of chlorine concentration. High initial chlorine concentrations decreased the time necessary to reach this 3-log inactivation plateau, but it was reached at each chlorine concentration used in this study.
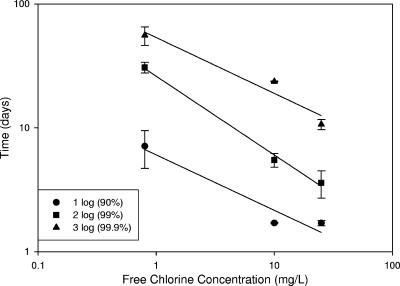
The two curves in Fig. 5 for 10 and 25 mg/liter free chlorine have very similar profiles. The time necessary for 1-log inactivation is 1.7 days at 10 and 25 mg/liter and 7.1 days at 0.75 mg/liter. The same trend holds for 2-log inactivation, but there is some separation for 3-log inactivation, with 25 mg/liter taking 11 days compared to 24 days at 10 mg/liter. These data are presented in Fig. 6, which is organized in a manner similar to a Watson's Law plot (14). Watson plots are common ways of summarizing disinfection data for planktonic organisms. In typical disinfection studies, the free chlorine concentration to which organisms are exposed is known, since they are freely suspended in the bulk water. The free chlorine concentration in Fig. 6 is the bulk concentration, but this is not necessarily what is reaching the cells on the coupon surface, as previous studies using microelectrode measurements have clearly shown (10, 18, 37).
FIG. 6.
Relationship between time and bulk free chlorine concentrations for inactivation of attached B. atrophaeus subsp. globigii spores. Actual bulk free chlorine concentrations were 0.73 and 0.77, 9.5 and 12.1, and 22.5 and 27.9 mg/liter in two independent experiments. The chlorine concentrations are the averages of two experiments (0.75, 10.8, and 25.2 mg/liter). Bars represent the ranges of duplicate experiments.
Data points in Fig. 6 actually fit a two-parameter power form (y = a·x−b) but are plotted on a log-log scale, and so they appear linear. Linearizing the plot yields slopes of 0.45, 0.63, and 0.45 with R2 values (sample coefficient of determination) of 0.93, 0.99, and 0.94 for 1, 2, and 3 log inactivation, respectively. Data points represent the mean of two independent experiments, with error bars representing the range. The data are also represented as the product of bulk disinfectant concentration and time necessary for a particular level of log inactivation, or the Ct value. Table 1 shows Ct values necessary for 1-, 2-, and 3-log inactivation. Ct values were determined by linear regression of appropriate segments of the decay curves (32).
TABLE 1.
Calculated Ct values for 1-, 2-, and 3-log inactivation of attached B. atrophaeus subsp. globigii spores
| Bulk free chlorine (mg/liter) | Avg Ct (mg·day/liter)a (SD)
|
||
|---|---|---|---|
| Ct90 | Ct99 | Ct99.9 | |
| 0.75 | 5.8 (1.7) | 22.4 (2.2) | 42.1 (7.7) |
| 10 | 5.9 (0.6) | 44.4 (7.5) | 178.3 (15) |
| 25 | 7.0 (0.1) | 40.4 (22) | 234.3 (25) |
Ct values represent chlorine concentrations multiplied by the time necessary for 90, 99, and 99.9% reduction in adhered spores. Data are averages from two independent experiments.
DISCUSSION
B. atrophaeus subsp. globigii that adhered to simulated distribution pipe material and biofilm persisted in the presence of a free chlorine residual. Decontamination with high levels of free chlorine proved ineffective at quickly removing/inactivating adhered B. atrophaeus subsp. globigii spores. The results provide interesting insights into how free chlorine affects the adhered bacteria. A sharp drop in B. atrophaeus subsp. globigii density on the coupons was observed when the chlorine was introduced into the initially dechlorinated reactor (Fig. 1 and 2). However, the B. atrophaeus subsp. globigii density quickly stabilized, and the rate of decrease was close to that observed in dechlorinated water. The B. atrophaeus subsp. globigii spores on the coupons were quickly inactivated if free chlorine could reach them, unlike spores associated with biofilm and rough corrosion surfaces. The initially chlorinated reactor (Fig. 3 and 4) did not show the same dramatic drop, but the disinfectant residual had removed/inactivated exposed spores before the high levels of chlorine were introduced.
Microelectrode measurements have shown that disinfectants like free chlorine, monochloramine, and chlorine dioxide are dramatically reduced at a biofilm surface (10, 18, 34, 37). The rough surface of corroded iron water pipe also provides areas that free chlorine cannot reach. It appears that this type of surface provides safe areas where spores, and possibly other hardy cells like oocysts, can reside for long periods of time unless removed by phenomena such as sloughing or hydraulic shear or physical removal, like pigging.
Past studies differ on whether allochthonous cells can survive when attached to biofilms in a drinking water environment. Variability between studies comes from differences in experimental systems, substratum material on which the biofilms were formed, specific microorganisms used, and the presence of a disinfectant residual. Experiments conducted under conditions identical to this study with Klebsiella pneumoniae showed dramatically different results. K. pneumoniae did not persist under dechlorinated or chlorinated conditions on corroded iron, indicating that it could not compete with the established biofilm organisms. These results are supported by Fass et al. (12), who used Escherichia coli, but differ from those of Camper et al. (7) and Morin et al. (25). However, in the latter two studies K. pneumoniae was grown as part of the biofilm (not attached to an established biofilm) and aided by attachment to carbon fines, respectively.
Multiple studies have shown that Legionella pneumophila can persist in or on simulated drinking water biofilm (3, 11, 19, 26, 36), as can Helicobacter pylori, but the results are limited (23, 28). Virus persistence has also been demonstrated, but published results have been variable (19, 29, 35). It should be noted that past studies have focused on substratum material, such as inert plastic (PVC or polycarbonate), stainless steel, or glass, which do not have the rough surface of corroded iron. These studies also used dechlorinated conditions or concentrations of free chlorine and monochloramine of less than 1 mg/liter.
Inactivation of adhered B. atrophaeus subsp. globigii was limited to 2.5 to 3.0 log10, even at elevated chlorine levels. It was initially thought that the change in slope seen in Fig. 4 could be due to increased corrosion products building up on the surface of the coupon after contamination. Corrosion products accumulating after contamination could consume chlorine and protect the adhered cells from inactivation. This led to the immediate decontamination experiments (10- and 25-mg/liter levels) shown in Fig. 5. These experiments showed that adding chlorine at approximately 10 or 25 mg/liter 1 day after inoculation of the spores still resulted in limited inactivation on coupon-associated B. atrophaeus subsp. globigii.
Data in Fig. 6 are a useful tool for pipe decontamination, since they show the relationship between bulk disinfectant concentration and time necessary for multiple levels of log inactivation for adhered B. atrophaeus subsp. globigii spores. Watson's Law plots have primarily been developed for planktonic organisms, and the relationship between time and disinfectant concentration, as characterized by the Watson's Law coefficient of dilution, varies between microorganisms and disinfectants (14, 17). This variation may hold for attached organisms as well, but generating this type of data for biofilm-associated microorganisms, especially spore formers, is needed for decontamination.
Ct data displayed in Table 1 are presented in units of mg·day/liter. Ct values for planktonic bioterror agents, and most microorganisms, are usually plotted in mg·min/liter, since a 3- to 4-log reduction generally happens on the order of minutes for vegetative cells and hours for spores (6, 30, 32, 33). Ct values reported by Rice et al. (30) for planktonic B. anthracis Sterne, Bacillus cereus, and Bacillus thuringiensis ranged from 130 to 250 mg·min/liter for 2-log inactivation and 190 to 370 mg·min/liter for 3-log inactivation at pH 8, 23°C, and 2 mg/liter free chlorine. These parameters are similar to the conditions in this study, but 2- and 3-log inactivation took approximately 22 and 42 mg·day/liter (Table 1), which translates to 31,680 and 60,480 mg·min/liter in the presence of 0.75 mg/liter free chlorine. Ct values for attached spores found in this study are 2 to 3 orders of magnitude higher than those for planktonic Bacillus spores found in the literature. More importantly, under the conditions in this study, planktonic cells were completely inactivated if the disinfectant residual was maintained, but this was not true for the attached spores.
Results presented in this study indicate that spores persist on corroded iron with an established biofilm in a drinking water environment. Three-log inactivation of attached spores was the maximum inactivation observed, indicating that some spores were situated on the coupons in places which could not be reached by free chlorine. Although 2- and 3-log inactivation can be achieved faster if 10 or 25 mg/liter free chlorine is introduced soon after contamination, decontamination at these chlorine residuals may not be feasible in a drinking water distribution system. The same log inactivation was achieved with typical distribution system chlorine residuals if given sufficient exposure. More importantly, the same 3-log plateau was reached regardless of the chlorine residual present, and so decontaminating spores that can be removed with the existing residual may be the most feasible option. As no chlorine concentration resulted in complete inactivation of spores from the coupons, decontamination with alternative disinfectants and physical removal of corrosion are good avenues for future research.
Acknowledgments
We thank Noreen Adcock of the U.S. Environmental Protection Agency's National Risk Management Research Laboratory, Water Supply and Water Resources Division, for preparing the B. atrophaeus subsp. globigii spores and William Shane of the University of Cincinnati's Department of Civil and Environmental Engineering for assistance in the lab. We also thank John Wright, U.S. Army, Dugway Proving Grounds, for providing the B. atrophaeus subsp. globigii spores.
This research was funded by U.S. Environmental Protection Agency contract 68-C-00-159.
Footnotes
Published ahead of print on 16 February 2007.
REFERENCES
- 1.American Public Health Association. 1998. Standard methods for the examination of water and wastewater, 20th ed. American Public Health Association, Washington, DC.
- 2.American Water Works Association. 2002. WATER:\STATS 2002 distribution survey. American Water Works Association, Denver, CO.
- 3.Armon, R., J. Starosvetsky, T. Arbel, and M. Green. 1997. Survival of Legionella pneumophila and Salmonella typhimurium in biofilm systems. Water Sci. Technol. 35:293-300. [Google Scholar]
- 4.Berman, D., E. W. Rice, and J. C. Hoff. 1988. Inactivation of particle-associated coliforms by chlorine and monochloramine. Appl. Environ. Microbiol. 54:507-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bomo, A. M., M. V. Storey, and N. J. Ashbolt. 2004. Detection, integration and persistence of aeromonads in water distribution system biofilms. J. Water Health 2:83-96. [PubMed] [Google Scholar]
- 6.Brazis, A. R., J. E. Leslie, P. W. Kabler, and R. L. Woodward. 1958. The inactivation of spores of Bacillus globigii and Bacillus anthracis by free available chlorine. Appl. Environ. Microbiol. 6:338-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camper, A. K., W. L. Jones, and J. T. Hayes. 1996. Effect of growth conditions and substratum composition on the persistence of coliforms in mixed-population biofilms. Appl. Environ. Microbiol. 62:4014-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu, C., C. Lu, C. M. Lee, and C. Tasi. 2003. Effects of chlorine level on growth of biofilm in drinking water pipes. Water Sci. Technol. Water Supply 3:171-177. [DOI] [PubMed] [Google Scholar]
- 9.Coroller, L., I. Leguerinel, and P. Mafart. 2001. Effect of water activities of heating and recovery media on apparent heat resistance of Bacillus cereus spores. Appl. Environ. Microbiol. 67:317-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Beer, D., R. Srinivasan, and P. S. Stewart. 1994. Direct measurement of chlorine penetration into biofilms during disinfection. Appl. Environ. Microbiol. 60:4339-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donlan, R., R. Murga, J. Carpenter, E. Brown, R. Besser, and B. Fields. 2002. Monochloramine disinfection of biofilm-associated Legionella pneumophila in a potable water model system, p. 406-410. In R. Marre, Y. Abu Kwaik, C. Bartlett, N. P. Cianciotto, B. S. Fields, M. Frosch, J. Hacker, and P. C. Luck (ed.), Legionella. ASM Press, Washington, DC.
- 12.Fass, S., M. L. Dincher, D. J. Reasoner, D. Gatel, and J. C. Block. 1996. Fate of Escherichia coli experimentally injected into a drinking water distribution pilot system. Water Res. 30:2215-2221. [Google Scholar]
- 13.Gibbs, S. G., M. C. Meckes, and P. V. Scarpino. 2003. The effect of long-term wastewater cross-connection on the biofilm of a simulated water distribution system. J. Environ. Eng. Sci. 2:85-98. [Google Scholar]
- 14.Haas, C. N., and G. R. Finch. 2001. Methodologies for the determination of disinfection effectiveness. Project 439. American Water Work Research Foundation, Denver, CO.
- 15.Hamouda, T., A. Y. Shih, and J. R. Baker. 2002. A rapid staining technique for the detection of the initiation of germination of bacterial spores. Lett. Appl. Microbiol. 34:86-90. [DOI] [PubMed] [Google Scholar]
- 16.Herson, D. S., B. McGonigle, M. A. Payer, and K. H. Baker. 1987. Attachment as a factor in the protection of Enterobacter cloacae from chlorination. Appl. Environ. Microbiol. 53:1178-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoff, J. C. 1986. Inactivation of microbial agents by chemical disinfectants. EPA/600/2-86/067. U.S. Environmental Protection Agency, Cincinnati, OH.
- 18.Jang, A., J. Szabo, A. A. Hosni, M. Coughlin, and P. L. Bishop. 2006. Measurement of chlorine dioxide penetration in dairy process pipe biofilms during disinfection. Appl. Microbiol. Biotechnol. 72:368-376. [DOI] [PubMed] [Google Scholar]
- 19.Långmark, J., M. V. Storey, N. J. Ashbolt, and T. A. Stenström. 2005. Accumulation and fate of microorganisms and microspheres in biofilms formed in a pilot-scale water distribution system. Appl. Environ. Microbiol. 71:706-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LeChevallier, M. W., C. D. Cawthon, and R. G. Lee. 1988. Factors promoting the survival of bacteria in chlorinated water supplies. Appl. Environ. Microbiol. 54:649-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeChevallier, M. W., C. D. Cawthon, and R. G. Lee. 1988. Inactivation of biofilm bacteria. Appl. Environ. Microbiol. 54:2492-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LeChevallier, M. W., C. D. Lowry, R. G. Lee, and D. L. Gibbon. 1993. Examining the relationship between iron corrosion and the disinfection of biofilm bacteria. J. Am. Water Works Assoc. 85:111-123. [Google Scholar]
- 23.Mackay, W. G., L. T. Gribbon, M. R. Barer, and D. C. Reid. 1998. Biofilms in drinking water systems—a possible reservoir for Helicobacter pylori. Water Sci. Technol. 38:181-185. [DOI] [PubMed] [Google Scholar]
- 24.McMath, S. M., C. Sumpter, D. M. Holt, A. Delanoue, and A. H. L. Chamberlain. 1999. The fate of environmental coliforms in a model water distribution system. Lett. Appl. Microbiol. 28:93-97. [DOI] [PubMed] [Google Scholar]
- 25.Morin, P., A. Camper, W. Jones, D. Gatel, and J. C. Goldman. 1996. Colonization and disinfection of biofilms hosting coliform-colonized carbon fines. Appl. Environ. Microbiol. 62:4428-4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murga, R., T. S. Forster, E. Brown, J. M. Pruckler, B. S. Fields, and R. M. Donlan. 2001. Role of biofilms in the survival of Legionella pneumophila in a model potable-water system. Microbiology 147:3121-3126. [DOI] [PubMed] [Google Scholar]
- 27.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination and outgrowth, p. 391-429. In C. R. Harwood and S. M. Cutting (ed.), Molecular biology methods for Bacillus. John Wiley and Sons, New York, NY.
- 28.Park, S. R., W. G. Mackay, and D. C. Reid. 2001. Helicobacter spp. recovered from drinking water biofilm sampled from a water distribution system. Water Res. 35:1624-1626. [DOI] [PubMed] [Google Scholar]
- 29.Quignon, F., M. Sardin, L. Kiene, and L. Schwartzbrod. 1997. Poliovirus-1 inactivation and interaction with biofilm: a pilot-scale study. Appl. Environ. Microbiol. 63:978-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rice, E. W., N. J. Adcock, M. Sivaganesan, and L. J. Rose. 2005. Inactivation of spores of Bacillus anthracis Sterne, Bacillus cereus, and Bacillus thuringiensis subsp. israelensis by chlorination. Appl. Environ. Microbiol. 71:5587-5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rice, E. W., L. J. Rose, C. H. Johnson, L. A. Boczek, M. J. Arduino, and D. J. Reasoner. 2004. Boiling and Bacillus spores. Emerg. Infect. Dis. 10:1887-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rose, L. J., E. W. Rice, B. Jensen, R. Murga, A. Peterson, R. M. Donlan, and M. J. Arduino. 2005. Chlorine inactivation of bacterial bioterrorism agents. Appl. Environ. Microbiol. 71:566-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sivaganesan, M., N. J. Adcock, and E. W. Rice. 2006. Inactivation of Bacillus globigii by chlorination: a hierarchical Bayesian model. J. Water Supply Res. 55:33-43. [Google Scholar]
- 34.Stewart, P. S., J. Rayner, F. Roe, and W. M. Rees. 2001. Biofilm penetration and disinfection efficacy of alkaline hypochlorite and chlorosulfamates. J. Appl. Microbiol. 91:525-532. [DOI] [PubMed] [Google Scholar]
- 35.Storey, M. V., and N. J. Ashbolt. 2001. Persistence of two model enteric viruses (B40-8 and MS-2 bacteriophages) in water distribution pipe biofilms. Water Sci. Technol. 43:133-138. [PubMed] [Google Scholar]
- 36.Storey, M. V., J. Långmark, N. J. Ashbolt, and T. A. Stenström. 2004. The fate of legionellae within distribution pipe biofilms: measurement of their persistence, inactivation and detachment. Water Sci. Technol. 49:269-275. [PubMed] [Google Scholar]
- 37.Szabo, J. G., E. W. Rice, and P. L. Bishop. 2006. Persistence of Klebsiella pneumoniae on simulated biofilm in a model drinking water system. Environ. Sci. Technol. 40:4996-5002. [DOI] [PubMed] [Google Scholar]