Abstract
Fructose utilization by wine yeasts is critically important for the maintenance of a high fermentation rate at the end of alcoholic fermentation. A Saccharomyces cerevisiae wine yeast able to ferment grape must sugars to dryness was found to have a high fructose utilization capacity. We investigated the molecular basis of this enhanced fructose utilization capacity by studying the properties of several hexose transporter (HXT) genes. We found that this wine yeast harbored a mutated HXT3 allele. A functional analysis of this mutated allele was performed by examining expression in an hxt1-7Δ strain. Expression of the mutated allele alone was found to be sufficient for producing an increase in fructose utilization during fermentation similar to that observed in the commercial wine yeast. This work provides the first demonstration that the pattern of fructose utilization during wine fermentation can be altered by expression of a mutated hexose transporter in a wine yeast. We also found that the glycolytic flux could be increased by overexpression of the mutant transporter gene, with no effect on fructose utilization. Our data demonstrate that the Hxt3 hexose transporter plays a key role in determining the glucose/fructose utilization ratio during fermentation.
During wine alcoholic fermentation, yeasts convert most of the glucose and fructose present into alcohol and CO2. Grape musts contain equal amounts of glucose and fructose, and the total hexose concentrations typically range from 160 to 300 g/liter. Saccharomyces cerevisiae is the preferred species of yeast for winemaking, and selected strains of S. cerevisiae are used as starters for inoculation of grape musts and for alcoholic fermentation. S. cerevisiae is a glucophilic yeast, preferring glucose to fructose. During fermentation, glucose at a higher rate than fructose, and the proportion of fructose therefore increases as fermentation progresses. Consequently, fructose becomes the main sugar present during the late stages of alcoholic fermentation, and wine yeasts have to ferment this nonpreferred sugar after long periods of starvation and in the presence of large amounts of ethanol. The stress associated with these conditions may be amplified by nutritional imbalances which may alter yeast activity, resulting in sluggish or stuck fermentations (1, 4, 5). In such situations, the low fructose utilization capacity of S. cerevisiae is thought to contribute to the low fermentation rate (9, 26, 27). The ability of wine yeasts to ferment fructose is therefore critically important for the maintenance of a high rate of fermentation at the end of the process and for fermentation of the must to dryness.
The reasons for the difference between the glucose fermentation rate and the fructose fermentation rate are unclear, but one of the first steps in hexose metabolism is generally thought to be involved. Sugar transport across the plasma membrane is the primary step in hexose metabolism. Another potential source of the difference is hexose phosphorylation, as glucose and fructose are both phosphorylated by the hexokinases Hxk1 and Hxk2 but with different efficiencies and the glucokinase Glk1 phosphorylates glucose but not fructose (8). The potential contributions of hexose transport and phosphorylation to the rates of glucose and fructose utilization are not known. Furthermore, other mechanisms may also be involved in limiting fructose utilization.
Hexose uptake in Saccharomyces is mediated by specific transporters that belong to a superfamily of monosaccharide facilitators (23). To date, 20 HXT genes encoding these transporters have been identified (29). Analyses of the effect of HXT gene inactivation have shown that the hexose carriers Hxt1 to Hxt7 are the main transporters (24). The various hexose transporters differ considerably in substrate specificity and affinity. Hxt1 and Hxt3 are low-affinity transporters (Km for glucose, ∼50 to 100 mM), Hxt4 is a moderately low-affinity transporter, and Hxt2, Hxt6, and Hxt7 are high-affinity transporters (Km for glucose, ∼1 to 4 mM) (18, 24). Hxt5 has been shown to be a transporter with intermediate to high affinity (6). Both high- and low-affinity carriers have been shown to have a higher affinity for glucose than for fructose (e.g., 2.1 mM versus 4.6 mM for Hxt7 and 65 mM versus 125 mM for Hxt3) (24). Such differences in affinity may affect the rates of utilization of the two sugars.
The expression of individual HXT genes depends on environmental factors, such as the hexose concentration sensed by the yeast cell. High-affinity carriers are induced by small amounts of glucose and are repressed by large amounts of glucose, whereas low-affinity transporters either are induced by high glucose concentrations (HXT1) or are only weakly regulated by the glucose concentration (HXT3) (16, 19, 20). We have previously shown that the hexose transporter genes HXT1, HXT2, HXT3, HXT6, and HXT7 are expressed during wine fermentation and that Hxt3 has the highest capacity to support fermentation (17, 21).
Although some variation in the ability of Saccharomyces strains to ferment fructose has been reported, the reasons for the potential differences and the underlying molecular mechanisms remain unknown (3). We characterized the fructose fermentation properties of a commercial wine yeast, Fermichamp, and found that it had a higher fructose fermentation capacity than other wine yeasts. We therefore investigated this strain further to determine the molecular basis of this enhanced fructose utilization capacity. We found that the enhanced fructose fermentation capacity of Fermichamp depended on expression of a mutated HXT3 allele. Here we provide the first evidence that the nature of the hexose transporter expressed by a wine yeast can influence the pattern of fructose utilization.
MATERIALS AND METHODS
Strains and culture conditions.
Fermichamp is an industrial S. cerevisiae wine strain. V5 (MATa ura3 gal) was derived from the Champagne wine strain 8130. The V5 hxt1-7Δ strain (MATa ura3 gal hxt1-7Δ) cannot grow on glucose or fructose, as it lacks HXT1 to HXT7 (18). Strains V5hxt1-7ΔHXT3V5 (MATa ura3 gal hxt1-7ΔHXT3V5) and V5hxt1-7ΔHXT3Fmp (MATa ura3 gal hxt1-7ΔHXT3Fmp) were obtained by integration of HXT3 from either V5 (HXT3-V5) or Fermichamp (HXT3-Fmp) into V5 hxt1-7Δ (this study).
Yeast strains were grown at 28°C on YP medium containing either 2% glucose or 2% maltose (V5 hxt1-7Δ). The growth phenotypes of the various integration mutant strains were assessed on synthetic medium (0.67% yeast nitrogen base without amino acids, 25 mg/liter uracil, 5% glucose). Yeast strains transformed with the p4H7 plasmid containing the HXT3 gene were grown on synthetic medium.
Batch fermentation experiments in enological conditions were carried out with a synthetic must (MS300) containing 100 g/liter glucose, 100 g/liter fructose, and an extra 115 mg/liter methionine and 25 mg/liter uracil (not used for transformed yeast strains) (2). In some cases the medium contained only glucose (200 g/liter) or only fructose (200 g/liters). Fermentors with a working volume of 1.1 liters, equipped with fermentation locks, were inoculated with cell cultures at a density of 106 cells/ml. Fermentations were carried out at 28°C with continuous stirring (500 rpm). These conditions resulted in fermentation kinetics similar to those found in industrial-scale winemaking.
Integration of HXT3 into the V5 hxt1-7Δ strain.
The HXT3 genes of V5 and Fermichamp were reintroduced into the V5 hxt1-7Δ strain by genomic integration at the site corresponding to the initial location of the corresponding gene cluster before its deletion. The HXT3 gene was amplified by PCR using primers HXT3P1 and I2HXT3. The PCR amplification products were used for genomic integration. Transformants were selected on the basis of their capacity to grow on glucose and were selected on synthetic medium containing 20 g/liter glucose. The HXT3 gene was integrated as a single copy downstream from its own promoter, including 1,128 bp upstream from ATG. Correct integration was checked by PCR with primers C1HXT3ORF and C2HXT3p. All the primers used are listed in Table 1.
TABLE 1.
Primers used for HXT3 cloning and integration in V5 hxt1-7Δ
| Primer | Sequence (5′ to 3′)a | Localization |
|---|---|---|
| HXT3P1 | GTGCGGGATccGAAGGCAATATC | −1128 |
| HXT3P2 | gatcggATCCATCATCACGTTCCTAGC | 2096 |
| I2HXT3 | aagtgacgggcgatgagtaagaaagaaataactgactcattagaCCATCATCACGTTCCTAGC | 2095 |
| C1HXT3ORF | GACACAGTGACATATGCACC | 168 |
| C2HXT3p | TTAAGCATGATCGTCTAGGC | −1689 |
| HXT3p426 | AacacaaaaacaaaaagtttttttaattttaatcaaaaaCTGAGTTAAACAATCATGAATTCAACTCC | −15 |
| HXT3t426 | GaatgtaagcgtgacataactaattacatgactcgagACGGTTTAGCGTGAAATTATTTCTTGCC | 1694 |
| C1HXT3ORF | GACACAGTGACATATGCACC | 168 |
| C′2HXT7p426 | gccaatacttcacaatgttcg | −125 |
Underlining indicates homology with the HXT7 terminator, uppercase letters indicate homology with HXT3, double underlining indicates homology with the p4H7 promoter, italics indicate homology with the p4H7 terminator, and boldface type indicates homology with the HXT7 promoter.
Cloning of HXT3 genes in multicopy plasmid p4H7.
HXT3 genes were amplified by PCR from V5 or Fermichamp genomic DNA using primers HXT3p426 and HXT3t426. These genes were inserted into p4H7 by in vivo recombination in S. cerevisiae, as described by Hamacher et al. (13). p4H7 is a multicopy plasmid that drives expression from the very strong, constitutive HXT71-392 promoter fragment. It was first linearized by digestion with BamHI and EcoRI. The 5′ end of HXT3p426 is homologous to the BamHI end (HXT7 promoter) of p4H7 linearized with BamHI and EcoRI. The 5′ end of HXT3t426 is homologous to the EcoRI end (terminator side) of the linearized plasmid. Yeast cells were transformed with the PCR amplification products of HXT3 and with p4H7 linearized with EcoRI and BamHI. Transformants were selected on the basis of their ability to grow on a minimal medium containing glucose as the sole carbon source. The recombined plasmids contained the HXT3 open reading frame behind the truncated and unregulated HXT7 promoter, resulting in HXT3 overexpression. The primers used are listed in Table 1. All constructs were checked by sequencing.
Glucose uptake assays.
Glucose transport was measured using a previously described procedure essentially as described by Walsh et al. (28). Glucose uptake experiments were performed with yeast cells collected during growth on synthetic must (MS300). Briefly, cells were harvested, washed, and incubated in phosphate buffer (0.1 M potassium phosphate, pH 6.5) for 3 min at 28°C. The uptake of d-[U-14C]glucose and d-[U-14C]fructose was measured by incubating an aliquot of cells with a radioactive solution for 5 s and then diluting it into a large volume of ice-cold quenching buffer. The cells were rapidly harvested by vacuum filtration, washed, and subjected to scintillation counting. Uptake measurements were obtained in triplicate. Kinetic parameters were determined using Eady-Hofstee plots, and data were analyzed with the Sigmaplot software.
Other methods. (i) Monitoring fermentation.
CO2 release was determined by automatic measurement of fermentor weight loss over successive 20-min periods. The rate of CO2 production was calculated automatically by polynomial smoothing of CO2 evolution. This method of monitoring fermentation gives highly reproducible results. The total amount of CO2 released was used to assess the completion of sugar fermentation. Experiments were carried out at least in duplicate, and representative results are shown below.
(ii) Monitoring glucose and fructose consumption.
The medium was sampled at least twice per day during fermentation. It was centrifuged to remove cells, and the supernatant was stored at −20°C for later determination of glucose and fructose contents by high-performance liquid chromatography.
(iii) Molecular techniques.
Standard molecular methods were used for DNA analysis and for transformation of Escherichia coli (25).
RESULTS
Capacity of Fermichamp to utilize fructose in wine fermentation conditions.
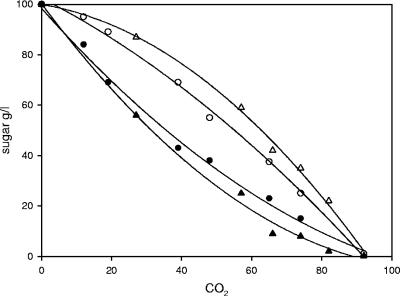
The wine yeast Fermichamp is known to ferment grape must sugars to dryness. We investigated the possible relationship between this capacity and a specific ability to utilize fructose by comparing the glucose and fructose consumption of Fermichamp with that of a standard strain, Fermivin, under enological alcoholic fermentation conditions in a synthetic must containing 100 g/liter of each hexose. The standard strain, Fermivin, displayed the marked difference between glucose utilization and fructose utilization classically reported for S. cerevisiae wine yeast strains (Fig. 1) and utilized glucose more rapidly than fructose. The difference between the amount of glucose consumed and the amount of glucose fructose consumed increased rapidly at the beginning of the fermentation and declined only in the last quarter of the process. Fermichamp had a very different sugar utilization profile. It consumed glucose more rapidly than it consumed fructose, but the difference in the rates of consumption of these two sugars was much smaller than the difference observed with Fermivin. The overall pattern, which showed differential utilization of the two sugars, was very similar, but Fermichamp had a much higher capacity for fructose fermentation than Fermivin had. Analysis of the sugar utilization profiles of other S. cerevisiae wine strains revealed patterns of hexose utilization very similar to that of Fermivin, confirming the exceptional nature of the fructose utilization capacity of Fermichamp (data not shown). Furthermore, our model strain, V5, a haploid strain derived from a wine yeast, also displayed the standard pattern of fructose utilization (data not shown).
FIG. 1.
Sugar utilization patterns of the Fermivin and Fermichamp strains during alcoholic fermentation. Consumption of sugars during fermentation was determined as a function of fermentation progress (CO2 released) with Fermivin (triangles) and Fermichamp (circles), using glucose (solid symbols) and fructose (open symbols).
Analysis of the sequences of the HXT genes of Fermichamp.
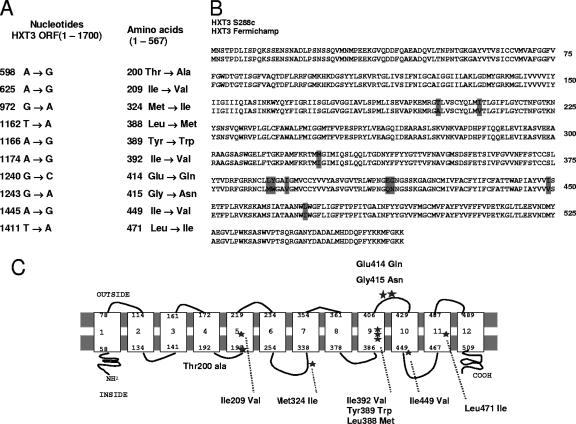
As hexose transport is known to be a critical step in hexose utilization, we hypothesized that differences in fructose transport properties might account for the higher fructose fermentation capacity of Fermichamp. The low-affinity carriers Hxt1 and Hxt3 were considered the most likely targets, given their known importance in alcoholic fermentation (17, 21). We investigated whether expression of mutated transporters was responsible for the greater fructose utilization capacity of Fermichamp. We amplified HXT1 and HXT3 by PCR and sequenced the PCR products. Analysis of the sequence of HXT1 revealed 24 mutations in the coding sequence, resulting in five amino acid substitutions compared with the type strain S288C sequence and no mutation in the promoter region (position −550 to position −1) (data not shown). However, these five amino acid substitutions were identical to those described previously for the sequence encoded by the HXT1 gene of V5 (17). As V5 exhibits no enhancement of fructose utilization capacity, we considered it unlikely that Hxt1 of Fermichamp was involved in the fructose utilization phenotype of this strain. Analysis of the HXT3 DNA sequence from Fermichamp revealed 38 mutations in the coding region, 10 of which resulted in amino acid substitutions compared with the type strain (S288C) or V5 sequences (14) (Fig. 2A) (the HXT3 sequence from V5 was previously shown to be 100% identical to the HXT3 sequence from S288C [17]). All but two of the changes in HXT3-Fmp (Thr-Ala at position 200 and Gly-Asn at position 415) were conservative substitutions. The mutations were not evenly distributed throughout the protein. Instead, the changes were found to be clustered in a region that included transmembrane domain 9 (TM9) and an external loop between TM9 and TM10 (Fig. 2B and C). The mutations in the Hxt3 carrier of Fermichamp may therefore result in a protein with modified transport properties potentially involved in determining the wine yeast phenotype. The HXT3 promoter sequence of Fermichamp (position −900 to position −1) had six changes: C to T at position −859, A to T at position −602, deletion of T at position −439, A to T at position −282, T to C at position −278, and C to T at position −88.
FIG. 2.
Distribution of mutations in the HXT3 transporter gene of Fermichamp. (A) Nucleotide and amino acid substitution positions. ORF, open reading frame. (B) Alignment of the Fermichamp HXT3 sequence and the standard HXT3 sequence. (C) Predicted topology of the Hxt3 transporter and positions of mutated residues (11). *, mutated amino acid.
Functional characterization of HXT3 from Fermichamp.
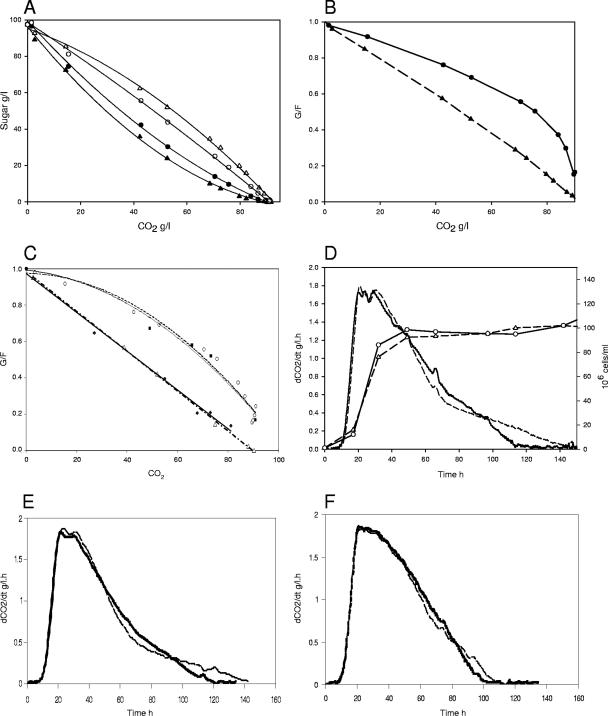
The mutated HXT3 allele was expressed in a strain lacking HXT1 to HXT7, V5hxt1-7Δ (17), for functional characterization. V5hxt1-7Δ cannot grow on or ferment glucose or fructose. This makes it possible to analyze the impact of an isolated HXT3 gene on sugar utilization. Two types of HXT3 genes were introduced by transformation of the tester strain: a standard gene (HXT3-V5) originating from V5 and the mutated allele from Fermichamp (HXT3-Fmp). Each of these genes was first integrated as a single copy at the original HXT3 locus under control of its own promoter (see Materials and Methods). The resulting recombinant yeasts, V5hxt1-7ΔHXT3V5 and V5hxt1-7ΔHXT3Fmp, each expressed only one type of transporter, making it possible to analyze the effects of that transporter on fructose utilization. We compared glucose and fructose utilization in the two strains during alcoholic fermentation. The sugar utilization profiles of the two strains were found to be very different (Fig. 3A). The strain expressing the HXT3-V5 gene had a much stronger preference for glucose than the strain expressing the Fermichamp allele had. The two behaviors could be clearly discriminated by the changes in the glucose/fructose ratio. The glucose/fructose ratio was consistently higher in the strain expressing the HXT3-Fmp allele (Fig. 3B). A comparison of the changes in the glucose/fructose ratios of the engineered strains with the changes in the glucose/fructose ratios of wine strains showed that the strain expressing the HXT3-Fmp allele had a profile similar to that of the Fermichamp strain, whereas the strain expressing the HXT3-V5 gene had a “standard” glucose/fructose profile similar to that of Fermivin or V5 (Fig. 3C). The fructose utilization phenotype depended directly on the nature of the HXT3 gene expressed. Expression of the HXT3 gene from Fermichamp in the V5 hxt1-7Δ strain was sufficient to reproduce the enhanced fructose utilization phenotype of Fermichamp. Remarkably, strains expressing only a single HXT3 gene displayed patterns of hexose utilization similar to those of the wild strains Fermivin, V5 (standard type), and Fermichamp (altered type), which have full sets of HXT genes.
FIG. 3.
Sugar utilization profiles of engineered and natural strains. (A) Glucose (solid symbols) and fructose (open symbols) consumption during alcoholic fermentation in strain V5 hxt1-7Δ expressing HXT3 from V5 (triangles) or Fermichamp (circles). (B) Changes in the glucose/fructose ratio (G/F) during fermentation with V5 hxt1-7Δ expressing HXT3 from V5 (triangles) or Fermichamp (circles). (C) Changes in the glucose/fructose ratio for strains Fermichamp (squares), Fermivin (diamonds), V5 (triangles), and V5 hxt1-7Δ expressing HXT3 from Fermichamp (circles). (D) Fermentation kinetic profiles of engineered strains with a single integrated HXT3 copy: fermentation rates of strain V5 hxt1-7Δ expressing HXT3 from V5 (dashed line) or Fermichamp (solid line) and cell number of strain with HXT3 from Fermichamp (circles) or from V5 (triangles). (E) Fermentation kinetic profiles of strains V5hxt1-7ΔHXT3V5 and V5hxt1-7ΔHXT3Fmp on medium containing only fructose (200 g/liter): fermentation rates of V5hxt1-7ΔHXT3V5 (dashed line) and V5hxt1-7ΔHXT3Fmp (solid line). (F) Fermentation kinetic profiles of strains V5hxt1-7ΔHXT3V5 and V5hxt1-7ΔHXT3Fmp on medium containing only glucose (200 g/liter): fermentation rates of V5hxt1-7ΔHXT3V5 (dashed line) and V5hxt1-7ΔHXT3Fmp (solid line).
Fermentation rate profiles were also significantly influenced by the HXT3 carrier gene expressed (Fig. 3D). No differences were observed in the first part of the fermentation (the maximum fermentation rates were the same), but the strain expressing HXT3-Fmp maintained a higher rate of fermentation toward the end of the process. Consistent with this finding, the fermentation time was shorter for the recombinant strain expressing this gene. V5hxt1-7ΔHXT3Fmp maintained a higher rate of fermentation only in the presence of small amounts of sugar and of a glucose/fructose ratio highly unfavorable for the strain expressing the standard allele. The higher fructose uptake capacity associated with HXT3-Fmp appeared to be advantageous at the end of fermentation.
To obtain additional information on the fructose and glucose utilization capacity of a strain with HXT3-Fmp, we examined the effect of expression of this gene in media containing only fructose or only glucose (Fig. 3E and F). In the media containing only fructose a higher fermentation rate was maintained in the last part of the fermentation and the fermentation time was reduced. This was consistent with an improved fructose transport capacity. In the medium containing only glucose expression of HXT3-Fmp had only a minor effect. However, a small increase in the fermentation rate was detected at the end of the process. This suggests that the Fermichamp Hxt3 carrier has some additional specific properties independent of the sugar transported that enable it to sustain a higher fermentation rate. Alternatively, one or more mutations in the promoter region might increase the expression of HXT3 in the stationary phase. Actually, no mutation is predicted to have such an effect. The only mutation located in a putative DNA binding domain of a known HXT3 regulator (T-439 deletion) eliminates 1 of 10 potential Rgt1 sites. Since Rgt1 is an activator in the presence of glucose (20), this mutation might lead to a reduction in HXT3 expression.
Effect of HXT3-Fmp overexpression.
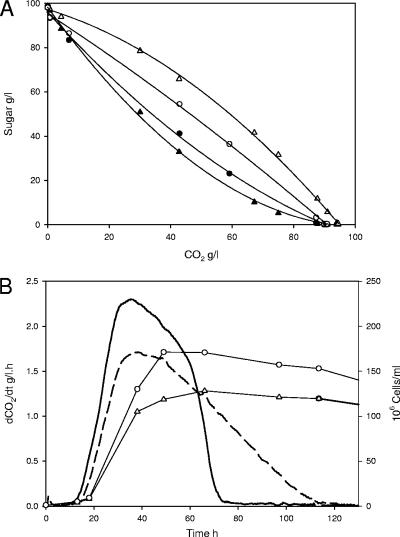
We investigated whether the level of expression of HXT3 genes affected hexose utilization profiles by overexpressing the standard and mutated alleles in the V5 hxt1-7Δ strain. This overexpression was achieved by inserting the genes into a multicopy plasmid under the control of a strong, unregulated promoter (13). HXT3 overexpression did not modify the glucose/fructose utilization profile of the yeast, which remained identical to that of the integrated, single-copy strains (Fig. 4A). The same difference in glucose and fructose utilization capacities was observed in strains overexpressing the HXT3-Fmp and HXT3-V5 genes. Indeed, overexpression of the HXT3-Fmp allele restored the fructose utilization phenotype of the Fermichamp strain. The enhanced fructose utilization associated with the Fermichamp allele appeared to be independent of the level of expression of HXT3-Fmp.
FIG. 4.
Sugar concentration profiles of engineered V5 hxt1-7Δ strains overexpressing the HXT3 gene from multicopy plasmid p4H7. (A) Changes in glucose (solid symbols) and fructose (open symbols) concentrations during fermentation of strain V5 hxt1-7Δ overexpressing HXT3 from V5 (triangles) or from Fermichamp (circles). (B) Fermentation kinetic profiles: fermentation rates of strain V5 hxt1-7Δ overexpressing HXT3 from V5 (dashed line) or Fermichamp (solid line) and cell number of strain with HXT3 from Fermichamp (circles) or from V5 (triangles).
The two alleles had very different effects on the fermentation rate when they were overexpressed (Fig. 4B). Overexpression of the HXT3-V5 gene increased the fermentation rate modestly compared to the rate with a single integrated copy and slightly reduced the fermentation time. Overexpression of the HXT3-Fmp allele greatly increased the fermentation rate and substantially reduced the fermentation time. The rate of fermentation remained high throughout most of the fermentation, decreasing only at the very end of the process, just before the sugar was exhausted. The overexpression of HXT3-Fmp led to a decrease in cell size (from 32 to 24 μm3), which was compensated for by a slight increase in the cell population. Indirect effects may therefore have contributed to the increase in sugar uptake in the strain overexpressing HXT3-Fmp. Additional experiments with HXT3-overexpressing strains and media containing either only glucose or only fructose showed that the increase in the fermentation rate was independent of the sugar fermented. A large increase in the fermentation rate was observed with both sugars (data not shown). Thus, the enhanced fermentation of the synthetic grape must did not result primarily from the specific fructose transport capacity of Hxt3-Fmp but resulted from other properties of the overexpressed transporter.
DISCUSSION
We show here that a commercial wine yeast, Fermichamp, has a higher fructose utilization capacity than standard S. cerevisiae wine strains have. This finding is consistent with the known capacity of this strain to restart stuck fermentations, as such fermentations occur in conditions in which the yeast is forced to ferment mainly fructose. Fermichamp harbors a mutated HXT3 allele with specific properties. Although the HXT1 gene from Fermichamp differs from the standard S288C allele, it is not involved in specific sugar utilization properties. It is identical to the HXT1 gene of another wine strain, suggesting that this allele is common in wine yeasts. Additional experiments confirmed that the HXT1 gene of Fermichamp was unable to improve fructose utilization (data not shown). The HXT3 gene from Fermichamp contains several previously unknown mutations.
We performed a functional analysis of the Fermichamp HXT3 allele by expressing it in an hxt1-7Δ deletion strain unable to ferment hexoses. Expression of the Fermichamp allele in this strain resulted in a higher fructose utilization capacity than expression of the standard HXT3 allele resulted in. Thus, the pattern of fructose utilization during alcoholic fermentation is directly influenced by the hexose transporter expressed by the yeast. This demonstrates the key role of hexose transport in determining the rates of fructose and glucose utilization during alcoholic fermentation. We also showed that expression of the HXT3 allele from Fermichamp was associated with a fructose consumption phenotype similar to that of the wine strain Fermichamp. We therefore reasonably inferred that the enhanced fructose utilization phenotype of this wine strain is dependent on the expression of a mutated HXT3 allele.
We also showed that expression of a single copy of the Fermichamp HXT3 allele improved fermentation. The fermentation rate at the end of the process, when fructose was the main sugar, was higher in the presence of this allele. This result is consistent with the hypothesis that fructose utilization is rate limiting at the end of fermentation. Whether additional properties of the Fermichamp carrier (e.g., greater stability of the protein under starvation conditions) or increased expression contributes to the improved fermentation deserves additional investigation.
Strains expressing a single HXT3 gene had hexose utilization patterns similar to those of strains with full sets of HXT genes (either the Fermichamp or standard type). Recovery of the fructose utilization pattern as a result of expression of a single Hxt3 transporter is consistent with the hypothesis that this transporter plays a key role in hexose transport and determination of the glucose/fructose utilization ratio (17, 21). The other transporters expressed during wine fermentation (Hxt1, Hxt2, Hxt6, and Hxt7) seem to contribute little to determining the glucose/fructose utilization ratio. However, the yeast genome harbors many HXT homologs, and we cannot rule out the possibility that in a given wine strain, increases in the expression of other transporters affect the rates of glucose and fructose utilization.
HXT3-Fmp overexpression led to a large increase in the fermentation rate not observed with the standard HXT3 allele. This improvement in fermentation was not due to specific fructose uptake capacity but was due to other, unidentified properties. The carrier encoded by this gene may have a higher capacity for folding or insertion into membranes when it is overexpressed than the protein encoded by the standard allele has. The increase in fermentation rate associated with HXT3-Fmp overexpression is consistent with the hypothesis that hexose transport is the main factor controlling the glycolytic flux (7, 10). Under enological conditions hexose transport is probably the rate-limiting step during much of the stationary phase (25).
We showed that the enhanced fructose utilization phenotype was preserved at the higher glycolytic fluxes associated with transporter overexpression. This indicates that other potentially critical steps that might affect the balance of glucose and fructose metabolism downstream from hexose transport were not limiting. One of these steps, sugar kinase activity, has been identified as a potentially critical process (8, 22). Our data suggest that the sugar phosphorylation capacity exceeds the amount of sugar transported and therefore does not make a significant contribution to determination of the glucose and fructose utilization rates.
Differences in the rates of glucose and fructose utilization by S. cerevisiae strains during wine fermentation can therefore be attributed to differences in transport efficiency between the two sugars. Given the essential role played by Hxt3, the transport kinetics of this transporter probably account for most of the differences in sugar utilization rates.
The Fermichamp strain expresses an Hxt3 carrier with mutations that may modify its fructose transport kinetics (Km and Vmax). We assessed the transport properties of Hxt3-Fmp by performing zero-trans uptake experiments. As shown in Table 2, Hxt3-Fmp had transport kinetic parameters similar to those of Hxt3-V5. The affinities for glucose (Km, 29 mM) were identical for the two carriers and consistent with previous findings (18, 24). Unexpectedly, the Km values for fructose were also found to be similar for the two carriers and considerably higher than the Km values for glucose (around 120 mM), in agreement with previously published data (24). We were not able to identify a difference in the transport kinetics of the two carriers. One likely explanation is that the differences in the fructose transport parameters of the two carriers are smaller than the errors in the determination analyses. Since the changes in hexose utilization rates during fermentation are small, small differences in sugar uptake are expected. Due to the very low affinity of the carrier for fructose and the low specific activity with labeled fructose at high concentrations, the transport parameters are not determined with great accuracy. Therefore, the transport properties of Hxt3-Fmp have to be characterized more thoroughly to draw firm conclusions about this point.
TABLE 2.
Transport kinetic data
| Protein | Glucose
|
Fructose
|
||
|---|---|---|---|---|
| Km (mM) | Vmax (nmol min−1 mg [dry wt]−1) | Km (mM) | Vmax (nmol min−1 mg [dry wt]−1) | |
| Hxt3-V5 | 28 ± 6 | 210 ± 30 | 116 ± 21 | 273 ±40 |
| Hxt3-Fmp | 29 ± 7 | 184 ±30 | 123 ± 22 | 242 ± 50 |
The 10 mutations in Hxt3-Fmp are clustered in TM9 and an external loop between TM9 and TM10. However, neither TM9 nor the external loop has been identified as a critical region for sugar transport in Hxt carriers (15). Three transmembrane domains have been shown to be critical for sugar recognition or translocation: TM5, TM7, and TM10. Only TM5 contains mutated residues in Hxt3-Fmp. In addition, comparison with the fructose/H+ symporter from Saccharomyces carlsbergensis FSY1 did not reveal conservation of the mutated residues (12). Further characterization of this transporter should make it possible to identify the residues essential for the fructose utilization phenotype.
In conclusion, this study provided insight into the molecular basis of a key property of wine yeasts: their capacity to utilize fructose. The higher fructose utilization capacity of the Fermichamp wine yeast strain results from the expression of an allelic variant of HXT3. This allele is clearly advantageous to the yeast under wine fermentation conditions. The stringent selection procedures used in the selection of wine yeasts therefore clearly lead to retention of highly relevant alleles. The identification of such alleles and the linking of genetic variation to specific wine yeast properties remain major challenges and should improve our exploitation of yeast biodiversity and improve fermentation processes. Our findings also show that fructose transport capacity is a relevant target for improving the fermentation performance of wine yeasts. Our results open up new possibilities for selection or engineering of wine yeasts with higher fructose utilization capacities.
Acknowledgments
We acknowledge DSM Food Ingredient Division for supporting this work.
Footnotes
Published ahead of print on 16 February 2007.
REFERENCES
- 1.Bauer, F. F., and I. S. Pretorius. 2000. Yeast stress response and fermentation efficiency: how to survive the making of wine—a review. S. Afr. J. Enol. 21:27-51. [Google Scholar]
- 2.Bely, L., J. Sablayrolles, and P. Barre. 1990. Description of alcoholic fermentation kinetics: its variability and significance. Am. J. Enol. Vitic. 40:319-324. [Google Scholar]
- 3.Berthels, N. J., R. R. Cordero Otero, F. F. Bauer, J. M. Thevelein, and I. S. Pretorius. 2004. Discrepancy in glucose and fructose utilisation during fermentation by Saccharomyces cerevisiae wine yeast strains. FEMS Yeast Res. 4:683-689. [DOI] [PubMed] [Google Scholar]
- 4.Bisson, L. F. 1999. Stuck and sluggish fermentations. Am. J. Enol. Vitic. 50:107-119. [Google Scholar]
- 5.Blatayron, L., and J. M. Sablayrolles. 2001. Stuck and slow fermentations in enology: statistical study of causes and effectiveness of combined additions of oxygen and diammonium phosphate. J. Biosci. Bioeng. 91:184-189. [DOI] [PubMed] [Google Scholar]
- 6.Buziol, S., J. Becker, A. Baumeister, S. Jung, K. Mauch, M. Reuss, and E. Boles. 2002. Determination of in vivo kinetics of the starvation-induced Hxt5 glucose transporter of Saccharomyces cerevisiae. FEMS Yeast Res. 2:283-291. [DOI] [PubMed] [Google Scholar]
- 7.Diderich, J. A., B. Teusink, J. Valkier, J. Anjos, Spencer-Martins, I. K. van Dam, and M. C. Walsh. 1999. Strategies to determine the extent of control exerted by glucose transport on glycolytic flux in the yeast Saccharomyces bayanus. Microbiology 145:3447-3454. [DOI] [PubMed] [Google Scholar]
- 8.Entian, K.-D. 1997. Sugar phosphorylation in yeast, p. 67-79. In F. K. Zimmerman and K.-D. Entian (ed.), Yeast sugar metabolism. Technomic Publishing Company, Lancaster, PA.
- 9.Gafner, J., and M. Schütz. 1996. Impact of glucose-fructose-ratio on stuck fermentations: practical experiences to restart stuck fermentations. Vitic. Enol. Sci. 51:214-218. [Google Scholar]
- 10.Gancedo, C., and R. Serrano. 1989. Energy-yielding metabolism, 2nd ed., vol. 3. Academic Press, London, England.
- 11.Gasteiger, E., A. Gattiker, C. Hoogland, I. Ivanyi, R. D. Appel, and A. Bairoch. 2003. ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 31:3784-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goncalves, P., H. Rodrigues de Sousa, and I. Spencer-Martins. 2000. FSY1, a novel gene encoding a specific fructose/H+ symporter in the type strain of Saccharomyces carlsbergensis. J. Bacteriol. 182:5628-5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamacher, T., J. Becker, M. Gárdonyi, B. Hahn-Hägerdal, and E. Boles. 2002. Characterization of the xylose-transporting properties of yeast hexose transporters and their influence on xylose utilization. Microbiology 148:2783-2788. [DOI] [PubMed] [Google Scholar]
- 14.Jacq, C., J. Alt-Morbe, B. Andre, W. Arnold, A. Bahr, J. P. Ballesta, M. Bargues, L. Baron, A. Becker, N. Biteau, H. Blocker, C. Blugeon, J. Boskovic, P. Brandt, M. Bruckner, M. J. Buitrago, F. Coster, T. Delaveau, F. del Rey, B. Dujon, L. G. Eide, J. M. Garcia-Cantalejo, A. Goffeau, A. Gomez-Peris, P. Zaccaria, et al. 1997. The nucleotide sequence of Saccharomyces cerevisiae chromosome IV. Nature 387(Suppl.):75-78. [PubMed] [Google Scholar]
- 15.Kasahara, T., M. Ishiguro, and M. Kasahara. 2004. Comprehensive chimeric analysis of amino acid residues critical for high affinity glucose transport by Hxt2 of Saccharomyces cerevisiae. J. Biol. Chem. 279:30274-30278. [DOI] [PubMed] [Google Scholar]
- 16.Liang, H., and R. F. Gaber. 1996. A novel signal transduction pathway in Saccharomyces cerevisiae defined by Snf3-regulated expression of HXT6. Mol. Biol. Cell 7:1953-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luyten, K., C. Riou, and B. Blondin. 2002. The hexose transporters of Saccharomyces cerevisiae play different roles during enological fermentation. Yeast 19:713-726. [DOI] [PubMed] [Google Scholar]
- 18.Maier, A., B. Völker, E. Boles, and G. F. Fuhrmann. 2002. Characterisation of glucose transport in Saccharomyces cerevisiae with plasma membrane vesicles (countertransport) and intact cells (initial uptake) with single Hxt1, Hxt2, Hxt3, Hxt4, Hxt6 Hxt7 or Gal2 transporters. FEMS Yeast Res. 2:539-550. [DOI] [PubMed] [Google Scholar]
- 19.Özcan, S., and M. Johnston. 1995. Three different regulatory mechanisms enable yeast hexose transporter (HXT) genes to be induced by different levels of glucose. Mol. Cell. Biol. 15:1564-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Özcan, S., and M. Johnston. 1999. Function and regulation of yeast hexose transporters. Microbiol. Mol. Biol. Rev. 63:554-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez, M., K. Luyten, R. Michel, C. Riou, and B. Blondin. 2004. Analysis of Saccharomyces cerevisiae hexose carrier expression during wine fermentation. Both low and high affinity Hxt transporters are expressed. FEMS Yeast Res. 5:351-361. [DOI] [PubMed] [Google Scholar]
- 22.Petit, T., J. A. Diderich, A. L. Kruckeberg, C. Gancedo, and K. Van Dam. 2000. Hexokinase regulates kinetics of glucose transport and expression of genes encoding hexose transporters in Saccharomyces cerevisiae. J. Bacteriol. 182:6815-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reifenberger, E., K. Freidel, and M. Ciriacy. 1995. Identification of novel HXT genes in Saccharomyces cerevisiae reveals the impact of individual hexose transporters on glycolytic flux. Mol. Microbiol. 16:157-167. [DOI] [PubMed] [Google Scholar]
- 24.Reifenberger, E., E. Boles, and M. Ciriacy. 1997. Kinetic characterization of individual hexose transporters of Saccharomyces cerevisiae and their relation to the triggering mechanisms of glucose repression. Eur. J. Biochem. 245:324-333. [DOI] [PubMed] [Google Scholar]
- 25.Salmon, J. M. 1989. Effect of sugar transportation inactivation in Saccharomyces cerevisiae on sluggish and stuck enological fermentations. Appl. Environ. Microbiol. 55:9535-9538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 27.Schutz, M., and J. Gafner. 1993. Sluggish alcoholic fermentation in relation to alteration of the glucose ratio. Chem. Mikrobiol. Technol. Lebensm. 15:73. [Google Scholar]
- 28.Walsh, M. C., H. P. Smits, M. Scholte, and K. van Dam. 1994. Affinity of glucose transport is Saccharomyces cerevisiae is modulated during growth on glucose. J. Bacteriol. 176:953-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wieczorke, R., S. Krampe, T. Weierstall, K. Freidel, C. P. Hollenberg, and E. Boles. 1999. Concurrent knock-out of at least 20 transporter genes is required to block uptake of hexoses in Saccharomyces cerevisiae. FEBS Lett. 464:123-128. [DOI] [PubMed] [Google Scholar]