Abstract
Influenza A virus particles (2 × 106) were inoculated onto copper or stainless steel and incubated at 22°C at 50 to 60% relative humidity. Infectivity of survivors was determined by utilizing a defined monolayer with fluorescent microscopy analysis. After incubation for 24 h on stainless steel, 500,000 virus particles were still infectious. After incubation for 6 h on copper, only 500 particles were active.
Influenza A is a viral pathogen that causes significant mortality and morbidity particularly in the elderly and in high-risk groups (14). During the 2005 and 2006 influenza seasons, the percentage of deaths attributed to pneumonia and influenza in the United States peaked twice at 7.8% (5). Information from the Centers for Disease Control and Prevention (CDC) suggests that influenza is transmitted from person to person primarily via large virus-laden droplets or through direct or indirect contact with respiratory secretions when touching surfaces contaminated with influenza virus (4). In support of this, one study used PCR to detect influenza A virus on over 50% of some 218 fomites tested in homes and day care centers, indicating the extent of potential reservoirs (3). Of clinical interest, influenza A virus appears to be transferred easily from hands to surfaces and vice versa (7). Influenza A virus can survive on a range of environmental surfaces, including stainless steel (2); thus, good hand hygiene is paramount to combat viral transmission. A recent study has suggested the application of copper-based surfaces to reduce the transmission of methicillin-resistant Staphylococcus aureus in health care facilities, due to copper's ability to rapidly kill this pathogen (9). Other studies have confirmed the antimicrobial properties of copper against several pathogenic bacteria, including Escherichia coli O157, Salmonella enterica, and Campylobacter jejuni (6, 10, 15), although any antiviral activity is yet to be tested. To test this hypothesis, we asked if copper surfaces could significantly reduce the number of infectious influenza A virus particles compared to the number found on surfaces made of the widely used material stainless steel.
To evaluate the effect of copper on influenza A virus (strain A/PR/8/34, H1N1; from NIBSC, London, United Kingdom), the virus was cultured in R-mix vials (Microgen Bioproducts, Camberley, United Kingdom) containing a monolayer of mink lung and human laryngeal carcinoma cells grown on glass coverslips (12), followed by inoculation onto sterile coupons of either copper or stainless steel. Coupons were aseptically inoculated with 20 μl of influenza A virus suspension (108 virus particles ml−1) and incubated at room temperature (22 ± 2°C) and 50 to 60% relative humidity for time periods ranging from 1 to 24 h. Postincubation, virus particles were resuspended in 5 ml sterile phosphate-buffered saline (PBS). The number of infectious units was determined by removing 100 μl PBS and inoculating three separate R-mix vials which were centrifuged (700 × g, 37°C, 1 h) and then incubated at 37°C for 24 h. To counteract any copper release into the PBS, 20 mM EDTA, which readily complexes free copper (13), was added.
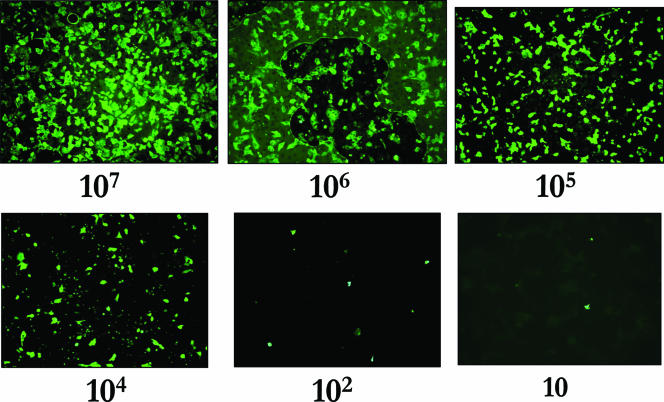
After incubation, the cell monolayers were fixed with 1 ml acetone for 5 min, washed with 0.5 ml PBS, and then stained using a respiratory virus detection kit (D3 kit; Microgen Bioproducts, United Kingdom) containing a fluorescein isothiocyanate-labeled antibody solution specific for influenza A viral antigens. Following staining, the coverslips were fixed onto standard glass microscope slides and examined using an episcopic differential interference contrast/epifluorescence microscope (8) equipped with 10× objectives and epifluorescent filters appropriate for fluorescein isothiocyanate fluorescence emission (excitation, 506 nm; emission, 529 nm). Images of R-mix cells infected by a range of virus particles (10 to 107) can be seen in Fig. 1, where the number of green fluorescing cells is proportional to the viral inoculum, providing a surrogate measure for viral titer. As is shown in Fig. 1, the sensitivity limit for this cell culture assay with influenza A virus is approximately 10 virus particles. By use of this calibration series of images, the numbers of infectious influenza A virus particles recovered from the sample experimental surfaces were determined.
FIG. 1.
Effect of influenza A virus particle number on R-mix cell fluorescence.
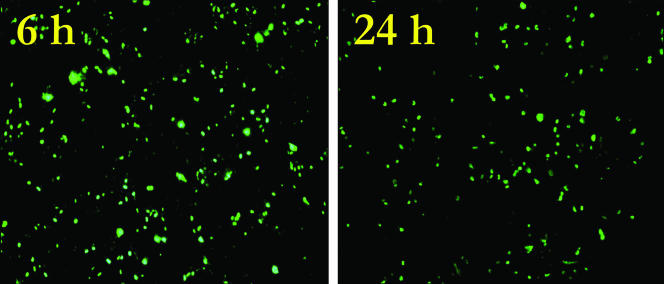
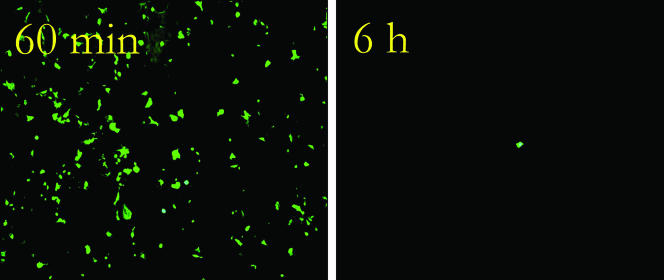
Figure 2 represents typical epifluorescent images of cell infection with influenza A virus recovered from stainless steel after an exposure period of either 6 h or 24 h in a desiccated state. After 6 h, 106 virus particles remained viable, and after 24 h, 5 × 105 virus particles were still capable of causing cell infection. The effect of exposure to copper surfaces can be seen in Fig. 3. In contrast to results observed with stainless steel, after 60 min on copper, the number of infectious virus particles was reduced to 5 × 105, the equivalent of 24 h of exposure on stainless steel, with this number reducing further to 5 × 102 after 6 h, representing nearly a 4-log decrease. Control experiments with copper or stainless steel surfaces inoculated with only sterile PBS showed no effect on the subsequent growth of the cell cultures, confirming that the reduced fluorescent emission from the R-mix cell line was due to inactivation of the influenza A virus and was not an effect on the cell line itself by copper.
FIG. 2.
Effect on influenza A virus infectivity after a 6-h or 24-h exposure to stainless steel.
FIG. 3.
Effect on influenza A virus infectivity after a 60-min or 6-h exposure to copper C11000.
Our results confirm previous findings of influenza A virus remaining infectious in large numbers on stainless steel (2), in contrast to results with copper surfaces, where rapid inactivation occurs after 6 h. It has been shown previously that once surfaces are contaminated, fingers can transfer virus particles to up to seven other clean surfaces (1), suggesting that materials which possess innate antiviral properties could act to prevent subsequent contamination. The mechanism for reduced infectivity after exposure to copper is not known at this point, although results from tests done with methicillin-resistant Staphylococcus aureus suggest that genomic material is degraded (J. O. Noyce, H. Michels, and C. W. Keevil, submitted for publication). Evidence to date has demonstrated that copper ions have the ability to disorder DNA by binding to and cross-linking between and within strands (11). If similar mechanisms occurred with the negative-sense RNA genome in influenza A virus, then viral replication could be inhibited by copper-linked RNA damage.
There is no single answer to controlling the spread of pathogenic microorganisms. The control of the influenza A virus, particularly with the emergence of potentially pandemic strains, demands the highest level of hygiene control, requiring multiple-barrier protection. Simply replacing steel fittings with copper will not prevent the transmission of influenza. However, the current study shows that copper surfaces may contribute to the number of control barriers able to reduce transmission of the virus, particularly in facilities, such as schools and health care units, where viral contamination has the ability to cause serious infection. As ever, the best approach to controlling influenza is to prevent infection itself through excellent standards of hygiene and vaccination programs.
Footnotes
Published ahead of print on 26 January 2007.
REFERENCES
- 1.Barker, J., I. B. Vipond, and S. F. Bloomfield. 2004. Effects of cleaning and disinfection in reducing the spread of norovirus contamination via environmental surfaces. J. Hosp. Infect. 58:42-49. [DOI] [PubMed] [Google Scholar]
- 2.Bean, B., B. M. Moore, B. Sterner, L. R. Peterson, D. N. Gerding, and H. H. Balfour, Jr. 1982. Survival of influenza viruses on environmental surfaces. J. Infect. Dis. 146:47-51. [DOI] [PubMed] [Google Scholar]
- 3.Boone, S. A., and C. P. Gerba. 2005. The occurrence of influenza A virus on household and day care center fomites. J. Infect. 51:103-109. [DOI] [PubMed] [Google Scholar]
- 4.CDC. 2005. Infection control measures for preventing and controlling influenza transmission in long-term care facilities. http://www.cdc.gov/flu/professionals/infectioncontrol/longtermcare.htm.
- 5.CDC. 2006. Update: influenza activity—United States and worldwide, 2005-06 season, and composition of the 2006-07 influenza vaccine. Morb. Mortal. Wkly. Rep. 55:645-648. [PubMed] [Google Scholar]
- 6.Faundez, G., M. Troncoso, P. Navarrete, and G. Figueroa. 2004. Antimicrobial activity of copper surfaces against suspensions of Salmonella enterica and Campylobacter jejuni. BMC Microbiol. 4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldmann, D. A. 2000. Transmission of viral respiratory infections in the home. Pediatr. Infect. Dis. J. 19:S97-S102. [DOI] [PubMed] [Google Scholar]
- 8.Keevil, C. W. 2003. Rapid detection of biofilms and adherent pathogens using scanning confocal laser microscopy and episcopic differential interference contrast microscopy. Water Sci. Technol. 47:105-116. [PubMed] [Google Scholar]
- 9.Noyce, J. O., H. Michels, and C. W. Keevil. 2006. Potential use of copper surfaces to reduce survival of epidemic methicillin-resistant Staphylococcus aureus in the healthcare environment. J. Hosp. Infect. 63:289-297. [DOI] [PubMed] [Google Scholar]
- 10.Noyce, J. O., H. Michels, and C. W. Keevil. 2006. Use of copper cast alloys to control Escherichia coli O157 cross-contamination during food processing. Appl. Environ. Microbiol. 72:4239-4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rifkind, J. M., Y. A. Shin, J. M. Heim, and G. L. Eichhorn. 1976. Cooperative disordering of single-stranded polynucleotides through copper crosslinking. Biopolymers 15:1879-1902. [DOI] [PubMed] [Google Scholar]
- 12.St. George, K., N. M. Patel, R. A. Hartwig, D. R. Scholl, J. A. Jollick, Jr., L. M. Kauffmann, M. R. Evans, and C. R. Rinaldo, Jr. 2002. Rapid and sensitive detection of respiratory virus infections for directed antiviral treatment using R-mix cultures. J. Clin. Virol. 24:107-115. [DOI] [PubMed] [Google Scholar]
- 13.Versteegh, J. F., A. H. Havelaar, A. C. Hoekstra, and A. Visser. 1989. Complexing of copper in drinking water samples to enhance recovery of Aeromonas and other bacteria. J. Appl. Bacteriol. 67:561-566. [DOI] [PubMed] [Google Scholar]
- 14.WHO. 2002. Influenza vaccines. WHO position paper. Wkly. Epidemiol. Rec. 28:229-240. [Google Scholar]
- 15.Wilks, S. A., H. Michels, and C. W. Keevil. 2005. The survival of E. coli O157 on a range of metal surfaces. Int. J. Food Microbiol. 105:445-454. [DOI] [PubMed] [Google Scholar]