Abstract
The prevalence of coral-associated fungi was four times higher in diseased Acropora formosa colonies than in healthy colonies. Since taxonomically related fungal species were isolated from diseased and healthy colonies, we suggest that their association with coral may be constitutive but that their abundance is dependent on coral health.
Reports of significant increases in the occurrence of coral diseases and disease-associated coral mortality are accumulating worldwide (18). Even though a variety of diseases and syndromes have been described, the causal agents have been unequivocally identified in only a few cases (22). Furthermore, it is currently difficult to distinguish between true pathogens, opportunists, and naturally associated biota. While significant efforts have been invested in the study of the interaction between corals and their associated bacterial communities, less attention has been given to the presence and role of fungi in coral health and disease. Despite their importance in terrestrial, freshwater, and marine ecosystems, little is known concerning the occurrence, identity, and nature of the fungal associates of both healthy and diseased corals (8). Some of the few examples of characterized fungus-coral associations include the presence of endolithic fungi observed within skeletal structures in a variety of coral species (2, 8, 15) and epilithic fungal communities associated with a mortality event in the Indian Ocean (11). In addition, a report on fungi isolated from Australian coral reefs indicates an increased presence of fungi in nearshore locations compared to that in offshore locations (13). In the Caribbean, the fungus Aspergillus sydowii was shown to be the major cause of a loss in Gorgonian sea fans (6), a close relative of reef-building corals. To date, actual identification of coral-associated fungi is limited. The presence of Cryptococcus spp. was detected, using 18S and 26S rRNA gene sequences, in the scleractinian coral Pocillopora damicornis maintained in aquaria (5). In addition, a range of thraustochytrid fungus isolates associated with the mucus of acroporid corals have been identified (16).
An initial step towards understanding the roles that fungi play in the biology of reef-building corals is to determine their presence/association in a given coral species. Bentis et al. (2) studied the presence of endolithic fungi in fixed samples of scleractinian corals, including Acropora cytherea and Acropora humulis. They suggested that direct coral-fungus interaction is geographically and taxonomically widespread and that fungal endoliths play a greater role in the ecology of coral reef systems than previously recognized. Although it has been suggested that endolithic fungi can be detrimental to the health of coral exposed to stress (8), possible linkages between the presence of fungi and the health of reef-building corals have not been explored systematically.
Corals from the family Acroporidae are widespread across the Indo-Pacific Ocean and the Caribbean Sea (21). While often being major reef-forming corals, members of the genus Acropora are highly susceptible to environmental stress (9, 10). Six distinct disease states have been observed to affect acroporid corals in the Indo-Pacific Ocean (18), including two novel coral diseases identified in Great Barrier Reef corals, termed brown band syndrome (24) and skeletal eroding band disease (1). While ciliated protozoa have been implicated as potential pathogens in both syndromes, their role in disease causation and tissue mortality is unclear (4), and little is known regarding the shifts in coral-associated microbial consortia following the onset of disease. Here we report, for the first time, the isolation of several ubiquitous fungal species from healthy Acropora formosa and demonstrate the increased incidence of coral-associated fungi in A. formosa exhibiting signs of brown band syndrome and skeletal eroding band disease.
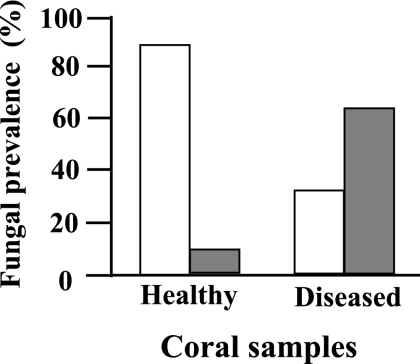
To first determine whether fungi could be readily isolated, samples (each approximately 10 cm long) of apparently healthy and diseased colonies of A. formosa were collected, in July 2006, by scuba diving (7 to 12 m) at Wistari Reef (23°27′S, 151°54′E) and Heron Reef (23°27′S, 151°54′E) on the southern Great Barrier Reef. Subsamples (1- to 2-cm branch fragments of 43 and 36 healthy and diseased colonies, respectively) were rinsed in sterile seawater and transferred to 14-ml snap-cap tubes containing 3 ml potato dextrose agar (Difco) amended with 250 mg liter−1 chloramphenicol (PDACl) and incubated at 25°C for 5 days (when no additional emergence of fungal growth was evident). The number of samples yielding fungal growth was significantly higher (P < 0.001, χ2 test) in diseased than in healthy specimens (Fig. 1). Thus, fungi were isolated from only about 12% of the healthy coral samples. In comparison, fungal growth was detected in more than 63% of the samples harvested from diseased animals. Similar differences in fungal prevalence in healthy and diseased coral samples were observed in samples collected after a 2-month interval at the same locations. Because more than one fungal colony (one or more species) emerged from a coral sample in some cases (see below), our numbers likely underestimate the fungal populations present. We also assume that other fungi, not capable of proliferating on PDACl, may be present in the animal tissue.
FIG. 1.
Prevelance of fungal association with healthy and diseased individual samples of Acropora formosa, as observed 5 days postsampling (samples were comprised of a total of 43 and 36 apparently healthy and diseased individuals, respectively). The absence or presence of fungi in all individuals is represented by empty or filled bars, respectively.
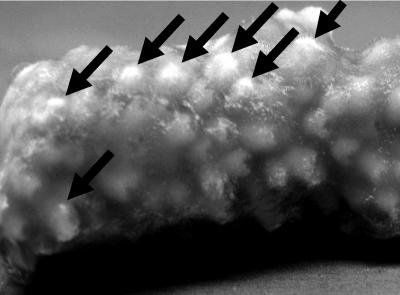
Most of the isolated fungi were dematiaceous in nature, and some morphological characteristics were common to a large number of the samples. Samples from pure cultures of mycelia (0.5 by 0.5 cm) of 27 representative isolates were subjected to two cycles of boiling/icing in 100 μl of water. A 1.5-μl volume of the extract was used as the PCR template (4 min at 94°C; followed by 32 cycles of 30 s at 94°C, 1 min at 62°C, and 45 s at 72°C; with a final extension step of 7 min at 70°C), using the ITS1-F and ITS4 primers (23). The amplicon sequences, along with the morphological features of the given cultures, were used to identify the isolated fungi. Fungi from the genera Alternaria and Phoma, as well as Aureobasidium pullulans and one unidentified species, were identified in the healthy coral samples (Table 1). Cultures of Phoma spp. (GenBank accession numbers EF120405 and EF120406) were also established from water and sediment samples. Similar fungi were identified in the diseased coral species along with Humicola fuscoatra and Penicillium citrinum (found on corals affected by brown band disease), a Fusarium sp. (found only on coral exhibiting signs of skeletal eroding band disease), and a Cladosporium sp. (found in coral exhibiting both brown band and skeletal eroding band diseases). Thus, alterations in fungal prevalence correlated with coral health. However, it remains to be determined whether the strains found only in samples of diseased coral are indicative of unique disease-related associations. Based on our observations, it appears that P. citrinum and an additional, unidentified, fungus (accession number EF127877) can inhabit the coral skeleton. This is based on the fact that after removal of the diseased coral tissue (with an airbrush) and subsequent immersion of the exposed skeleton into molten PDACl, colonies of these fungi can be seen emerging (within 3 days) from coral polyps (Fig. 2).
TABLE 1.
Fungal genera associated with Acropora formosa in the Great Barrier Reef
| Disease | Fungal strainsa |
|---|---|
| None | Phoma sp. (EF120404, EF120408, EF120410), Alternaria sp. (EF120409), Aureobasidium pullulans (EF127877), unidentified (EF127875) |
| Brown band | Phoma sp. (EF120411, EF120412, EF120413, EF127874, EF120407), Humicola fuscoatra (EF120414), Cladosporium sp. (EF120415) Penicillium citrinum (EF120416, EF127876), unidentified (EF120417) |
| Skeletal eroding | Phoma sp. (EF127878, EF120418, EF120419), Fusarium sp. (EF120420) |
| Aureobasidium pullulans (EF120421, EF120422), Alternaria sp. (EF120423) | |
| Brown band and skeletal eroding | Phoma sp. (EF120424), Cladosporium sp. (EF120425, EF120426) |
Numbers in parentheses indicate corresponding accession numbers.
FIG. 2.
Fungal colonies (marked with arrows) emerging from polyp skeleton structures of Acropora formosa exhibiting brown band syndrome. Coral tissue was removed prior to emersion into PDACl and incubation for 3 days.
Related fungi have been isolated from marine environments (including, in some instances, from undefined Cnidaria [14]). H. fuscoatra, a Phoma sp., and a Cladosporium sp. have been previously found in association with other sessile marine animals (an ascidian and sponges) (7, 14, 17), and there have been several reports on the association of Fusarium spp. with diseased crustaceans (20). Furthermore, we also isolated a Phoma sp. strain (accession number EF120405) from seawater (a single colony that grew from the 200 ml of filtered seawater that was collected separately at the same dive location). This strain has an internal transcribed spacer sequence identical to that of one of the strains isolated from healthy coral (EF120404) as well as from coral exhibiting symptoms of brown band syndrome (EF120407), further emphasizing the ubiquitous nature of some of these fungi. The majority of isolates described here belong to the genus Phoma. This genus is taxonomically problematic due to difficulties in distinguishing species from one another, and extensive additional sampling and thorough phylogenetic analyses are needed to develop placement strategies and an understanding of the evolutionary history of Phoma and related groups (19). The genetic relationship between aquatic and terrestrial Phoma-related species has yet to be determined. Evidence for the presence of a difference in the secondary metabolite contents of marine and terrestrial Phoma species has been demonstrated (14). This fact may be indicative of additional differences yet to be found and of the potential impact of metabolite production on the roles these (and other fungi) play in the marine environment, including the influence of these fungi on coral health.
This is the first description of fungal species associated with the reef-building coral A. formosa and the first report on the significant quantitative changes in fungal prevalence in diseased coral. Whether the fungi described are beneficial or detrimental or are opportunistic invaders of diseased or dead tissue is unclear. However, the fact that taxonomically related species were isolated from diseased and healthy coral suggests that their association with coral may be constitutive but that their proliferation is dependent on coral health. As fungal isolates representing most of the genera described here have been shown to produce a variety of antibacterial, -fungal, -viral, and -protozoan compounds (3, 12), it is tempting to speculate that they (as well as the isolates described here) produce such compounds in their natural environments. The implications of such possibilities on maintaining the ecological balance within the coral colony and its surroundings are vast and are perhaps further influenced in an age of anthropogenic ecological change.
Acknowledgments
This work was supported by the Israel Science Foundation (O.Y.) and the ARC Centre for Excellence of Coral Reef Studies (O.H.-G.).
Footnotes
Published ahead of print on 16 February 2007.
REFERENCES
- 1.Antonius, A., and D. Lipscomb. 2001. First protozoa coral-killer identified in the Indo-Pacific. Atoll Res. Bull. 481:1-21. [Google Scholar]
- 2.Bentis, C. J., L. Kaufman, and S. Golubic. 2000. Endolithic fungi in reef-building corals (Order: Scleractinia) are common, cosmopolitan, and potentially pathogenic. Biol. Bull. 198:254-260. [DOI] [PubMed] [Google Scholar]
- 3.Bhadury, P., B. T. Mohammad, and P. C. Wright. 2006. The current status of natural products from marine fungi and their potential as anti-infective agents. J. Ind. Microbiol. Biotechnol. 33:325-337. [DOI] [PubMed] [Google Scholar]
- 4.Croquer, A., C. Bastidas, D. Lipscomp, R. E. Rodrıguez-Martınez, E. Jordan-Dahlgren, and H. M. Guzman. 2006. First report of folliculinid ciliates affecting Caribbean scleractinian corals. Coral Reef. 25:187-191. [Google Scholar]
- 5.Domart-Coulon, I. J., C. S. Sinclair, R. T. Hill, S. Tambutté, S. Puverel, and G. K. Ostrander. 2004. A basidiomycete isolated from the skeleton of Pocillopora damicornis (Scleractinia) selectively stimulates short-term survival of coral skeletogenic cells. Mar. Biol. 144:583-592. [Google Scholar]
- 6.Geiser, D. M., J. W. Taylor, K. B. Ritchie, and G. W. Smith. 1998. Cause of sea fan death in the West Indies. Nature 394:137-138.9671296 [Google Scholar]
- 7.Gesner, S., N. Cohen, M. Ilan, O. Yarden, and S. Carmeli. 2005. Pandangolide 1a, a metabolite of the sponge-associated fungus Cladosporium sp., and the absolute stereochemistry of pandangolide 1 and iso-cladospolide B. J. Nat. Prod. 68:1350-1353. [DOI] [PubMed] [Google Scholar]
- 8.Golubic, S., G. Radtke, and T. Le Campion-Alsumard. 2005. Endolithic fungi in marine ecosystems. Trends Microbiol. 13:229-235. [DOI] [PubMed] [Google Scholar]
- 9.Loya, Y. 2004. The coral reefs of Eilat—past, present and future: three decades of coral community structure studies, p. 1-34. In E. Rosenberg and Y. Loya (ed.), Coral health and disease. Springer, Berlin, Germany.
- 10.Marshall, P. A., and A. H. Baird. 2000. Bleaching of corals on the Great Barrier Reef: differential susceptibilities amongst taxa. Coral Reefs 19:155-163. [Google Scholar]
- 11.McClanahan, T. R., S. M. McLaughlin, J. E. Davy, W. H. Wilson, E. C. Peters, K. L. Price, and J. Maina. 2004. Observations of a new source of coral mortality along the Kenyan coast. Hydrobiologia 530/531:469-479. [Google Scholar]
- 12.Miao, L., and P.-Y. Qian. 2005. Antagonistic antimicrobial activity of marine fungi and bacteria isolated from marine biofilm and seawaters of Hong Kong. Aquat. Microb. Ecol. 38:231-238. [Google Scholar]
- 13.Morrison-Gardiner, S. 2002. Dominant fungi from Australian coral reefs. Fung. Divers. 9:105-121. [Google Scholar]
- 14.Osterhage, C., M. Schwibibbe, G. M. Konig, and A. D. Wright. 2000. Differences between marine and terrestrial Phoma species as determined by HPLC-DAD and HPLC-MS. Phytochem. Anal. 11:288-294. [Google Scholar]
- 15.Priess, K., T. Le Campion-Alsumard, S. Golubic, F. Gadel, and B. A. Thomassin. 2000. Fungi in corals: black bands and density-banding of Porites lutea and P. lobataskeleton. Mar. Biol. 136:19-27. [Google Scholar]
- 16.Raghukumar, S., and R. Balasubram. 1991. Occurrence of Thraustochytrid fungi in corals and coral mucus. Indian J. Mar. Sci. 20:176-181. [Google Scholar]
- 17.Smetanina, O. F., T. A. Kuznetsova, A. V. Gerasimenko, A. I. Kalinovsky, M. V. Pivkin, P. C. Dmitrenok, and G. B. Elyakov. 2004. Metabolites of the marine fungus Humicola fuscoatra KMM 4629. Russ. Chem. Bull. 53:2643-2646. [Google Scholar]
- 18.Sutherland, K. P., J. W. Porter, and C. Torres. 2004. Disease and immunity in Caribbean and Indo-Pacific zooxanthellate corals. Mar. Ecol. Prog. Ser. 266:273-302. [Google Scholar]
- 19.Torres, M. S., J. F. White, Jr., G. Cazares, M. Bergen, J. F. Bischoff, and R. E. Sullivan. 2005. A new species and its phylogenetic placement in the Didymella/Phoma complex (Phaeosphaeriaceae, Pleosporales). Mycotaxon 93:297-308. [Google Scholar]
- 20.Van Khoa, L., K. Hatai, A. Yausa, and K. Sawada. 2005. Morphology and molecular phylogeny of Fusarium solani isolated from kuruma prawn Penaeus japonicus with black gills. Fish Pathol. 40:103-109. [Google Scholar]
- 21.Veron, J. E. N. 2000. Corals of the world. Australian Institute of Marine Science, Townsville, Australia.
- 22.Weil, E., G. Smith, and D. L. Gil-Agudelo. 2006. Status and progress in coral reef disease research. Dis. Aquat. Org. 69:1-7. [DOI] [PubMed] [Google Scholar]
- 23.White, T. J., T. D. Bruns, S. B. Lee, and J. W. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315-322. In N. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (ed.), PCR protocols: a guide to methods and applications. Academic Press, New York, NY.
- 24.Willis, B. L., C. A. Page, and E. A. Dinsdale. 2004. Coral disease on the Great Barrier Reef, p. 69-104. In E. Rosenberg and Y. Loya (ed.), Coral health and disease. Springer-Verlag, Berlin, Germany.