Abstract
DNA microarrays of 86 genes from the yeasts Debaryomyces hansenii, Kluyveromyces marxianus, and Yarrowia lipolytica were developed to determine which genes were expressed in a medium mimicking a cheese-ripening environment. These genes were selected for potential involvement in lactose/lactate catabolism and the biosynthesis of sulfur-flavored compounds. Hybridization conditions to follow specifically the expression of homologous genes belonging to different species were set up. The microarray was first validated on pure cultures of each yeast; no interspecies cross-hybridization was observed. Expression patterns of targeted genes were studied in pure cultures of each yeast, as well as in coculture, and compared to biochemical data. As expected, a high expression of the LAC genes of K. marxianus was observed. This is a yeast that efficiently degrades lactose. Several lactate dehydrogenase-encoding genes were also expressed essentially in D. hansenii and K. marxianus, which are two efficient deacidifying yeasts in cheese ripening. A set of genes possibly involved in l-methionine catabolism was also used on the array. Y. lipolytica, which efficiently assimilates l-methionine, also exhibited a high expression of the Saccharomyces cerevisiae orthologs BAT2 and ARO8, which are involved in the l-methionine degradation pathway. Our data provide the first evidence that the use of a multispecies microarray could be a powerful tool to investigate targeted metabolism and possible metabolic interactions between species within microbial cocultures.
Cheese ripening is a complex phenomenon in which the curd undergoes a set of biochemical reactions during the ripening process. Such reactions are catalyzed by the microbial ecosystem living within the cheese matrix—among others, yeasts such as Debaryomyces hansenii, Kluyveromyces lactis, Kluyveromyces marxianus, and Yarrowia lipolytica, which play key roles in deacidification and the development of flavor compounds (3, 11).
The mechanisms by which yeast growth influences the maturation process are fermentation of lactose, utilization of lactic acid (with consequent pH increase), proteolytic and lipolytic activities, and release of autolysis products (13, 17). However, the contributions of yeasts to cheese flavor development during ripening are generally underestimated, and their roles are generally not well established. They develop at the early stages of ripening (16, 21, 22), when they participate in the deacidification of the curd through lactate/lactose consumption (11), and they could also be involved in flavor compound biosynthesis. Yeasts like K. lactis, K. marxianus, Y. lipolytica, and D. hansenii are found in a wide range of cheeses (11, 12, 16, 21, 31) and are expected to play an important role in the ripening. For instance, K. lactis, K. marxianus, and D. hansenii assimilate lactose, whereas a majority of yeast species isolated from cheese efficiently degrade lactate (11, 12). In smear soft cheese, the rise in pH at the cheese surface was found to be related to lactate degradation by D. hansenii (4, 21). The same situation prevails with K. lactis or K. marxianus, for which lactate degradation coincides with a dramatic increase in pH (4). The presence of yeasts during ripening is essential, since this rise in pH enables the acid-sensitive bacteria that are necessary for the cheese typicity to develop at the cheese surface. Due to the wide catabolic spectrum of Y. lipolytica, this yeast could be of interest in cheese making. A comparison of the technological characteristics of D. hansenii and Y. lipolytica has shown that Y. lipolytica was much more proteolytic and lipolytic than D. hansenii (30).
It has also been shown that cheese-ripening yeasts such as Y. lipolytica, D. hansenii, and K. lactis could produce volatile sulfur compounds (VSC) through l-methionine catabolism (3, 8, 19). The importance of such compounds in ripened cheese derives mainly from their reactivity and their high volatility at very low concentrations. Also, the involvement of a transamination step in l-methionine catabolism, as well as VSC production, has been demonstrated in Y. lipolytica (8) and K. lactis (19).
The recent publications of the genomes of some yeasts involved in cheese ripening (15, 28) opened new opportunities to investigate the metabolic capacities of such microorganisms during the making of dairy or other fermented food products. With the availability of whole-genome sequences, DNA microarray analysis offers the potential to monitor and compare the expression patterns of a wide range of mRNA species simultaneously (27). However, whole-genome DNA microarray analysis is generally used to examine differential expression patterns of genes of a single microorganism resulting, for example, from changes in the microbial environment, e.g., a stress effect, or in the microbial phenotype, e.g., biofilm formation (1, 23, 32). Analysis of gene expression is important, since changes in the physiology and metabolism of an organism are the consequences of changes in the pattern of gene expression. With the availability of an increasing number of genome sequences from microorganisms of food ecosystems (e.g., dairy products, fermented beverages, and meat), strategies using mixed genome microarrays can be now considered.
Until now, changes occurring during ripening were based essentially on global biochemical data, such as enzymatic activities, consumption of substrates, and biosynthesis of products, but could not fully describe the specific involvement of a given microorganism in the whole process. More precisely, we were not able to know which gene products, related to one protein and/or one function, were expressed in a given microorganism of the cheese ecosystem. The cheese-ripening yeasts D. hansenii, K. marxianus, and Y. lipolytica were used as model organisms of a cheese ecosystem.
The first part of this work was devoted to the design of species-specific oligonucleotide probes and to the validation of microarray data. In the second part, we identified which genes related to l-methionine and lactose/lactate catabolism were expressed in yeast pure cultures and which were expressed in yeast cocultures. The expression profiles of candidate genes were simultaneously followed for the three yeasts cultivated in coculture at three culturing times.
MATERIALS AND METHODS
Yeast strains and storage conditions.
Yeast strains used in this work were Debaryomyces hansenii 304, Kluyveromyces marxianus 44(8) (initially named K. lactis 44(8)) and Yarrowia lipolytica 370. These microorganisms were from our laboratory collection, having originally been isolated from French cheeses. They were selected on the basis of their efficiency in producing volatile sulfur compounds that contribute to the quality of cheeses (3). The microorganisms were stored in 5% glycerol/nonfat dried milk at −80°C.
Culture conditions and media.
The yeasts were cultivated in pure cultures or in coculture in 500-ml flasks containing 100 ml of medium. Precultures were cultivated for 2 days in potato dextrose broth (PDB) (Difco Laboratories, Detroit, MI) at 25°C with agitation (150 rpm). The PDB medium was inoculated with 1 ml of thawed stock suspension. These precultures served as inocula (1% vol/vol) for the subsequent cultures. The synthetic cheese medium (SCM) used for all strains was composed (per liter of distilled water) of 1 g yeast extract (Gibco, Heidelberg, Germany), 15 g Bacto Casamino Acids (Difco Laboratories), 38 ml of a 60% sodium lactate stock solution (Prolabo, Fontenay-sous-Bois, France), 0.1 g CaCl2 (Prolabo), 0.5 g MgSO4 7H2O (Prolabo), 6.8 g KH2PO4 (Sigma-Aldrich, St-Quentin Fallavier, France), 10 g NaCl (Prolabo). After adjusting the pH to 5.5 ± 0.1, the medium was autoclaved (120°C, 20 min) and then supplemented with 20 g · liter−1 lactose (Prolabo) and 6 g · liter−1 l-methionine (Sigma-Aldrich) prior to inoculation. Cultures were incubated at 25°C (150 rpm) for either 84 h or 120 h. The composition of the SCM was chosen since it corresponds to the amount of substrates (e.g., lactate and lactose) present in the curd of a soft cheese, like Camembert. The l-methionine concentration was chosen since it corresponds to the maximum amount of free l-methionine present in the curd of a soft cheese, like Camembert. This medium was shown to reproduce growth conditions found during ripening (21, 10).
Microbial analyses.
Viable cell counts were determined as CFU ml−1 following a standard aerobic plate count procedure with yeast extract glucose chloramphenicol agar (Biokar Diagnostics, Paris, France). Surface inoculation was carried out by using a spiral plater (Intersciences, St-Nom La Bretèche, France) on 120-mm-diameter petri dishes to ensure immediate differentiation of the colonies based on their size, appearance, and pigmentation and to detect any microbial contamination. The dishes were incubated at 25°C, and colonies were counted after 48 to 72 h.
Oligonucleotide probes design.
The appropriate design of oligonucleotide probes is crucial to ensure the success of transcriptional analyses. Therefore, one To-mer oligonucleotide probe was designed for each gene of interest, using ROSO software (26). D. hansenii, K. lactis, and Y. lipolytica sequence data were obtained from the Génolevures public database (http://cbi.labri.fr/Genolevures/). Oligonucleotide sequences were searched in the last 300 bases of each gene, with no stable secondary structure and several optimal thermodynamic properties as defined by ROSO (http://pbil.univ-lyon1.fr/roso/Home.php). As a final check, a low-homology search using BLASTN was performed against all genomes to ensure that each probe would not display any cross-hybridization. It should be noted that the K. lactis genome sequence was used to design the K. marxianus probes, because the two yeasts are closely related. The oligonucleotides used are listed in the supplemental material. They were synthesized by QIAGEN Operon (QIAGEN, Alameda, CA). In addition, six Arabidopsis thaliana probes were used as external positive controls and were provided by Stratagene (SpotReport Oligo array validation system; Stratagene, La Jolla, CA).
Oligonucleotide printing.
Printing onto Corning UltraGAPS coated slides (gamma amino propyl silane surface; Corning Life Sciences, Corning, NY) was performed at the Transcriptome Biochips Platform in Toulouse using a spotter (VersArray ChipWriter Pro; Bio-Rad) with 12 pins (SMP3; Telechem International, Sunnyvale, CA) in a 3-by-4 format. Each microarray comprises 86 oligonucleotides (30 for D. hansenii, 29 for K. lactis [K. marxianus], and 27 for Y. lipolytica) deposited in duplicate and 6 A. thaliana species-positive controls replicated four times to generate sufficient data points, giving a total of 196 elements per microarray. The diameter of each spot was approximately 100 μm. After printing, DNA elements were cross-linked to the slides by UV irradiation (Stratalinker UV cross-linker; Stratagene) and stored in a vacuum chamber until use.
Extraction and purification of total RNA. (i) Sample preparation.
Cells were centrifuged for 5 min at 8,200 × g and 4°C. The pellets were washed with Tris-EDTA (TE) buffer (1× TE; 10 mM Tris-HCl, 1 mM EDTA [pH 8.0]; Sigma-Aldrich) and then resuspended in 150 μl of 10% N-lauroylsarcosine (Sigma-Aldrich) and 1 ml of RNeasy lysis buffer (RLT; QIAGEN, Hilden, Germany)-β-mercaptoethanol (Sigma-Aldrich) (1:0.01). The suspension was mixed by vortex for approximately 3 min, poured into sterile 2-ml Eppendorf tubes (each aliquot containing 800 μl of suspension), and then stored at −80°C or used for the extraction.
(ii) Total RNA isolation.
For the RNA extraction, 200 mg of zirconium beads (diameter, 0.1 mm; BioSpec Products, Bartlesville, OK) and 800 μl of RLT-β-mercaptoethanol were added to an aliquot. The mixture was shaken in a FastPrep FP120 bead beating system (Bio101, Vista, CA) for 30 s at a machine speed setting of 6.0 m · s−1. Samples were cooled down on ice for 1 min, and the shaking procedure was repeated a second time. Phase separation was carried out after a centrifugation for 5 min at 1,700 × g and 4°C. The aqueous phase was transferred to a fresh tube, and an equal volume of 70% ethanol was added, after which the extraction was performed with an RNeasy Midi kit (QIAGEN), according to the manufacturer's instructions. Total RNA was eluted directly from the RNeasy silica-gel membrane into 500 μl of diethylpyrocarbonate-treated water and immediately precipitated by the addition of 50 μl of 3 M sodium acetate and 400 μl of absolute isopropanol (at 4°C). Tubes were mixed by inversion and placed at −20°C for at least 2 h. The RNA was collected by centrifugation (30 min, 20,800 × g, 4°C), and the pellets were washed twice with 250 μl of cold 70% ethanol, dried for 30 min at room temperature, and resuspended in 50 μl of 1× TE. Samples were then hydrated overnight at 4°C after the addition of 0.5 μl (20 U) of RNase inhibitor (RNasin; Promega, Madison, WI). The RNA integrity was visualized by electrophoresis at 6 V · cm−1 with a 1% agarose gel, which was stained with 0.3 μg · ml−1 ethidium bromide (Sigma-Aldrich) and photographed under UV light. Quantity and purity were assessed by measurement of the ratios A260:A230 and A260:A280 by using a spectrophotometer (Beckman DU640B; Beckman Instruments, Fullerton, CA).
Labeling of cDNA targets.
External RNA controls (A. thaliana mRNA spikes; SpotReport oligo array validation system, Stratagen) were added into total RNA samples (i) prior to cDNA synthesis, to control all of the downstream steps, and (ii) at different concentrations, to cover the entire range of expression levels of mRNAs of interest. mRNA of A. thaliana and mRNA of biological samples were then reverse transcribed and simultaneously cyanine 3 labeled with a CyScribe first-strand cDNA labeling kit (Amersham Biosciences, Piscataway, NJ), without any amplification. Reverse transcription-labeling reactions were performed at 42°C for 90 min in a thermocycler (GeneAmp PCR system 9700; Perkin-Elmer Applied Biosystems, Foster City, CA) by direct incorporation of dCTP-Cy3 (Amersham Biosciences) according to the manufacturer's instructions. RNA template and unincorporated fluorescent nucleotides were then eliminated by a chemical treatment (15 min at 37°C with 2 M NaOH). After neutralization with 2 M HEPES (pH 6.8) (Sigma-Aldrich), labeled cDNA was purified on GFX columns (CyScribe GFX purification kit; Amersham Biosciences) and then concentrated using a Microcon YM-30 filter (Millipore, Bedford, MA).
Microarray hybridization and washing.
To reduce the nonspecific adsorption of fluorescent probes to the surface, microarray slides were prehybridized by injecting 5 μl of 10 mg · ml−1 herring sperm DNA (Promega), previously heated at 95°C for 2 min, and 30 μl of DIGeasy hybridization buffer (Roche Diagnostics GmbH, Mannheim, Germany) to the slide covered with a 22-by-40-mm coverslip (LifterSlip premium printed cover glass; Erie Scientific Company, Portsmouth, NH). The slide was then put into an individual hybridization chamber (Corning, Avon, France) and immersed in a water bath for 1 h at 60°C. It was then washed in 0.1× SSC (0.15 M NaCl plus 0.015 M sodium citrate) and dried by centrifugation at 150 × g for 3 min at room temperature before hybridization. Labeled targets and 5 μl of herring sperm DNA were heated at 95°C for 5 min for denaturation and then snap-cooled on ice. Twenty microliters of the hybridization buffer was added to the mixture and then injected under a new coverslip. The hybridization chamber was incubated in a 60°C water bath overnight. The slide was then immersed in a solution of 2× SSC, 0.1% sodium dodecyl sulfate (SDS) to remove the coverslip and washed in 2× SSC, 0.1% SDS at 55°C for 5 min and then in 1× SSC at room temperature, followed by a rinse in 0.2× SSC before being dried by centrifugation.
Scanning, and quantification of microarray data.
Subsequently, slides were scanned at 532 nm (the wavelength for Cy3 fluorescence) using a robot ScanArray 4000 (Packard Biosciences, Boston, MA) with 5-μm-pixel resolution. Pictures were generated by using appropriate gains on the photomultiplier tube to obtain the highest signal intensity without saturation. Hybridization signals were analyzed with QuantArray software (Packard BioChip Technologies, Billerica, MA). The mean fluorescence intensity for each spot was quantified, and the expression level of each gene was calculated as the average of two individual hybridizations (duplicate probes for each gene of interest).
Statistical analysis of cDNA microarray experiments.
The glass plates contained few genes. The mean fluorescence intensity is, thus, biased by those genes that were most highly expressed and is therefore not a reliable value for the normalization of data. It should be noted that as a consequence, the variance cannot be relied upon either, as it is calculated with the mean value. We decided therefore to work with the median and minimum values under each experimental condition (24). The minimum value was taken as the zero (value). For normalization, the median was used instead of the mean value, and the standard deviation was calculated using the difference between the median and the minimum values. The new (normalized) value of a given gene, j, under a given experimental condition, c, was as follows: x′jc = (xjc − minc)/(medc − minc).
For statistical analyses, we applied the above procedure to the logged values. An analysis of variance (ANOVA) was performed using the GeneANOVA software (14). For each gene, the equation used was as follows: Yikj = μ + Ci + Bj + Rk + ɛijk, where Yikj is the gene intensity; μ is the mean of the intensities of expression measured for the gene; Ci, Bj, and Rk are, respectively, the effects of analyzed “culture time,” i (at early [36-h], mid- [72-h], or late [120-h] stationary phase); the biological repetition, j; and the spot replicate on microarray, k; and ɛijk is the residual error. The residual error, ɛijk, includes all the interactions. The threshold of 10% for the false discovery rate (FDR) was chosen to select genes whose expression changes in the “time course” are significant (the FDR is the expected proportion of erroneously rejected null hypotheses among the rejected ones) (6, 25).
High-performance liquid chromatography analyses.
Culture samples stored at −20°C were thawed at 4°C, centrifuged (2,060 × g; 15 min), and filtered using a polyethersulfone membrane filter (pore size, 0.22 μm; diameter, 33 mm; Dutscher, Brumath, France) before analysis. α-Keto-γ-methylthiobutyric acid (KMTBA) and α-hydroxy-γ-methylthiobutyric acid (HMTBA) contents of the filtrates were determined by high-performance liquid chromatography (HPLC Waters TCM; Waters, Saint Quentin en Yvelines, France) with a cation exchange column (diameter, 7.8 mm; length, 300 mm; Aminex HPX-87H; Bio-Rad, Ivry-Sur-Seine, France) thermostatted at 65°C. The mobile phase was sulfuric acid (0.01 N) dispensed at a 0.6-ml · min−1 flow rate. Detection of compounds of interest was performed with a Waters 486 tunable UV/visible detector regulated at 210 nm. Methionine was analyzed with a reversed-phase column (Symmetry C18; pore size, 100 Å; diameter, 4.6 mm; length, 100 mm; Waters). A gradient of H2O plus acetonitrile at a flow rate of 0.6 ml · min−1 was applied as follows: 100% H2O for 2.5 min, 100 to 90% for 0.5 min, 90 to 60% for 7 min, and 60 to 100% for 4 min. UV detection at 210 nm was used. All compounds were quantified from calibration curves established with pure chemicals.
Solid-phase microextraction gas chromatography-mass spectrometry analyses.
The VSC production was analyzed by an automatic solid-phase microextraction (SPME) method coupled to a gas chromatograph (Varian CP-3800; Varian, Inc., Walnut Creek, CA) and a single-quadrupole mass spectrophotometer equipped with an impact electronic source (model 1200, Varian, Inc.). Automation of the extraction and injection was achieved with a CombiPAL autosampler (CTC Analytics, Zwingen, Switzerland). Defrosted samples (5 ml) kept at 4°C were preincubated for 2 min at 40°C with agitation at 250 rpm. The extraction was carried out with 100-μm polydimethylsiloxane fiber (Supelco, Bellefonte, PA) for 40 min at 40°C and equal agitation conditions. The sample was injected by desorption at 250°C for 60 s in splitless mode using the standard Varian split/splitless injector (model 1177, Varian, Inc.). The volatiles were carried onto a nonpolar capillary column (HP-5 mass spectrometry; 30 m by 0.25 mm; 0.25-μm film thickness) swept by helium at a constant flow rate (1.2 ml/min). The compounds were then separated using the following temperature program. First, the temperature was maintained at 15°C for 8 min. Subsequently, the temperature reached 220°C with an increment of 5°C/min. Separated compounds were detected with a mass spectrometry detector. Data were collected in the range of 30 to 400 atomic mass units at a rate of 2 scans/s. Volatile compounds were identified by comparison of their ion chromatograms with those in the NIST/02 Mass Spectral Library (National Institute of Standards and Technology, Gaithersburg, MD). Data were analyzed using Statgraphics Plus software (Statistical Graphics Corp., Englewood Cliffs, NJ). Values are presented as the means ± standard deviations of three replicates.
RESULTS
Choice of candidate genes for the multispecies microarray construction.
Our first objective was to select genes possibly involved in the catabolism of lactose, lactate, and l-methionine. Taking Saccharomyces cerevisiae as the yeast reference, we therefore collected all the potential genes encoding such specific enzymes from the Saccharomyces Genome Database (http://www.yeastgenome.org/). Such genes were then searched for in the full genome of D. hansenii CBS767, K. lactis CLIB210, and Y. lipolytica CLIB122, using the Génolevures public database (http://cbi.labri.fr/Genolevures/). BLAST (2) was used to screen sequence databases for homology. Even distantly related homologs to S. cerevisiae genes were selected in order to ensure that potential genes of interest in D. hansenii, K. lactis, and Y. lipolytica were present on the microarray (Table 1). Possible related metabolic pathways were examined using the online service KEGG Pathway database (http://www.genome.ad.jp/kegg/pathway.html).
TABLE 1.
List of selected genes from S. cerevisiae and their respective homologues in D. hansenii, K. lactis, and Y. lipolytica
| Gene name | S. cerevisiae ORFs | SGD annotationa | Yeast type strain and accession no.b
|
||
|---|---|---|---|---|---|
| D. hansenii CBS767 | K. lactis CLIB210 | Y. lipolytica CLIB122 | |||
| LAC4 | No orthologue | β-Galactosidase | DEHA0G26345g | KLLA0B14883g | NMF |
| LAC12 | No orthologue | Lactose permease | DEHA0G26367g | KLLA0B14861g | NMF |
| JEN1 | YKL217w | Lactate transporter | DEHA0F18843g | KLLA0E16313g | YALI0D24607g |
| DEHA0D20427g* | KLLA0F10043g | YALI0D20108g | |||
| DEHA0F28809g* | YALI0C15488g | ||||
| YALI0E32901g | |||||
| YALI0C21406g | |||||
| YALI0B19470g | |||||
| CYB2 | YML054c | Lactate dehydrogenase cytochrome b2 | DEHA0E01166g | KLLA0D02640g | YALI0E21307g |
| KLLA0B14795g | |||||
| DLD1 | YDL174c | Lactate ferrocytochrome c oxidoreductase | DEHA0D09658g* | KLLA0E19789g | YALI0E03212g |
| KLLA0D03344g | |||||
| DLD2 | YDL178w | Lactate ferrocytochrome c oxidoreductase | DEHA0F07612g* | KLLA0B01397g* | NMF |
| ILV2 | YMR108w | Acetolactate synthase (catalytic subunit) | DEHA0G02574g* | KLLA0B12584g | YALI0C00253g |
| ILV6 | YCL009c | Acetolactate synthase (regulatory subunit) | DEHA0E22869g* | KLLA0F12364g | YALI0C09636g |
| PDA1 | YER178w | E1α subunit of the PDH complex | DEHA0G13728g* | KLLA0F12001g | YALI0F20702g |
| PDB1 | YBR221c | E1β subunit of the pyruvate dehydrogenase (PDH) complex | DEHA0C10065g | KLLA0F09603g | YALI0E27005g |
| YALI0F05038g* | |||||
| PDC1 | YLR044c | Major of three pyruvate decarboxylase isozymes | DEHA0G19525g* | KLLA0E16357g | YALI0D10131g |
| DEHA0B03784g | |||||
| DEHA0D18381g* | |||||
| PDC6 | YGR087c | Minor isoform of pyruvate decarboxylase | NMF | NMF | YALI0D06930g |
| THI3 | YDL080c | Probable decarboxylase | NMF | KLLA0F16962g | NMF |
| ARO10 | YDR380w | Phenylpyruvate decarboxylase | DEHA0D11066g* | KLLA0E02662g | NMF |
| BAT1 | YHR208w | Mitochondrial branched-chain amino acid aminotransferase | NMF | KLLA0A10307g | YALI0D01265g |
| BAT2 | YJR148w | Cytosolic branched-chain amino acid aminotransferase | DEHA0D07689g* | NMF | YALI0F19910g |
| ARO8 | YGL202w | Aromatic aminotransferase | DEHA0A06974g | KLLA0F10021g | YALI0E20977g |
| DEHA0A00253g* | KLLA0A04906g | ||||
| DEHA0A00231g* | |||||
| ARO9 | YHR137w | Aromatic aminotransferase | NMF | KLLA0D11110g | YALI0C05258g |
| MAP1 | YLR244c | Methionine aminopeptidase | DEHA0G13937g* | KLLA0D18436g | YALI0D17138g |
| MAP2 | YBL091c | Methionine aminopeptidase | DEHA0C04444g | KLLA0E06875g | YALI0D05159g |
| SAM2 | YDR502c | S-Adenosylmethionine synthase | DEHA0E14751g | KLLA0C01782g | YALI0B14509g* |
| SAM4 | YPL273w | S-Adenosylmethionine-homocysteine methyltransferase | DEHA0A00869g* | KLLA0D01551g | YALI0F25641g* |
| CYS3 | YAL012w | Cystathionine γ-lyase | DEHA0C16863g* | KLLA0F07909g | YALI0F05874g |
| CYS4 | YGR155w | Cystathionine β-synthase | DEHA0C15708g | KLLA0F09317g | YALI0E09108g |
| STR3 | YGL184c | Cystathionine β-lyase | DEHA0A06897g* | KLLA0C17028g | YALI0D00605g* |
| STR2 | YJR130c | Cystathionine γ-synthase | DEHA0D10285g* | KLLA0B04378g | NMF |
| YLL058w | Hypothetical cystathionine γ-synthase | NMF | NMF | YALI0D17402g* | |
| YHR112c | Cystathionine γ-synthase activity | DEHA0A01144g | KLLA0E21406g | NMF | |
| DEHA0A01529g | |||||
Annotations from the Saccharomyces Genome Database (SGD) (http://www.yeastgenome.org/).
Accession numbers of sequences were obtained from Génolevures (http://cbi.labri.fr/Genolevures/). NMF, no matches found; *, distantly related homolog of S. cerevisiae (see putative designations in the supplemental material).
Growth, substrate assimilation, and metabolite production by yeasts K. marxianus, D. hansenii, and Y. lipolytica cultivated as pure cultures or in cocultures. (i) Growth of microorganisms and pH over time.
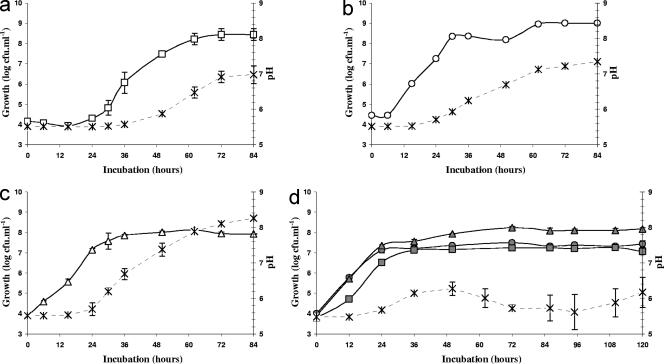
Growth of the three yeasts cultivated separately or in coculture are shown in Fig. 1. The development of K. marxianus started (Fig. 1a) after a 24-h lag phase during which the initial population remained unchanged (approximately 2.104 CFU ml−1). The K. marxianus population steadily increased between 30 h and 62 h of culture, reaching around 3.108 CFU ml−1 until the end of the experiment. In contrast, D. hansenii and Y. lipolytica displayed a continuous and rapid development in the first hours of the cultivation (Fig. 1b and c), increasing from an initial population of almost 2.104 CFU ml−1 to between 4.108 CFU ml−1 and 8.107 CFU ml−1 at 38 h, respectively. Thereafter, D. hansenii showed a stationary phase until 80 h, whereas the Y. lipolytica population stabilized at 108 CFU ml−1 after 58 h of cultivation. When the strains were cultivated in coculture (Fig. 1d), whereas the development of Y. lipolytica did not differ significantly, the maximal populations were significantly decreased (<1 to 1.5 log units) for D. hansenii and K. marxianus compared to those of pure cultures. However, the initial growth of all yeasts was accelerated when cultivated in coculture. This was particularly clear with K. marxianus, for which no lag phase was observed when it was cultivated in coculture with D. hansenii and Y. lipolytica (Fig. 1d). The initial growth acceleration observed in coculture, compared to that in pure cultures, suggests that there is a competition for growth among the yeasts.
FIG. 1.
pH and growth of three yeasts cultivated separately (open symbols) and in coculture (closed symbols) over time. (a) □, K. marxianus alone; (b) ○, D. hansenii alone; (c) ▵, Y. lipolytica alone; (d) ▪, K. marxianus; •, D. hansenii; and ▴, Y. lipolytica in coculture. pH levels. Error bars represent standard deviations calculated on the average values of six determinations.
pH levels. Error bars represent standard deviations calculated on the average values of six determinations.
The pH measurements showed that the three yeasts were able to neutralize the medium when cultivated as pure cultures but not when cultivated in coculture. We can reasonably suspect that most metabolites responsible for the neutralization of the medium in yeast pure cultures are catabolized by the yeast coculture. The nature of such metabolites remains to be identified. For K. marxianus cultures (Fig. 1a), a rise in pH was observed after 38 h of incubation and reached a plateau (pH 7.0 ± 0.2) after 72 h. D. hansenii and Y. lipolytica cultures followed similar variation patterns (Fig. 1b and c), although Y. lipolytica was the most efficient at increasing the pH (Fig. 1c). For both yeasts, the pH started to increase after 16 h and kept increasing thereafter until the end of the experiment. The rise in pH by D. hansenii cultures (Fig. 1b) was slightly slower and the final pH was lower than in Y. lipolytica cultures. In yeast cocultures, the pH started to increase after 16 h and reached a maximum (pH 6.3 ± 0.2) after 50 h (Fig. 1d), then steadily decreased thereafter, to increase again after 95 h of cultivation.
(ii) Lactose and lactate consumption.
The initial concentration of lactate was 22.9 ± 0.1 g · liter−1 and that of lactose was 18.3 ± 0.3 g · liter−1 (Table 2). Most lactose (over 92%) was consumed between 30 h and 62 h in K. marxianus cultures, while lactate was hardly consumed during the remaining time. During the stationary phase, 16% of the lactate was consumed, and lactose was completely exhausted at 72 h. In D. hansenii cultures, lactose consumption was progressive and continuous: 10% of the lactose was catabolized at 30 h, 34% at 55 h, and over 67% at 72 h. The degradation of the lactate paralleled lactose consumption after 30 h. In contrast to the other yeasts, Y. lipolytica consumed neither lactose nor lactate in the SCM, and initial concentrations remained unchanged during the 84 h of culture. In cocultures, lactose consumption was progressive, whereas lactate was hardly consumed. Also, the pH did not rise significantly in cocultures (Fig. 1d) compared to that of pure cultures (Fig. 1a, b, and c). Since all yeast species could develop in cocultures (Fig. 1d), this suggests a coculture effect among yeasts. It is possible that metabolites possibly involved in the neutralization process observed in yeast pure cultures are catabolized by the yeast coculture, resulting in no significant change in pH (Fig. 1d).
TABLE 2.
Lactose, lactate, and l-methionine consumption and KMTBA and HMTBA production by K. marxianus, D. hansenii, and Y. lipolytica cultivated in pure cultures and in coculturesa
| Yeast | Time (h) | % of consumption (g · liter−1) of:
|
% of production (g · liter−1) of:
|
|||
|---|---|---|---|---|---|---|
| Lactose | Lactate | l-Methionine | KMTBA | HMTBA | ||
| K. marxianus | 0 | 18.4 ± 0.1 | 22.9 ± 0.1 | 5.73 ± 0.05 | ND | ND |
| 30 | 18.3 ± 0.1 | 22.9 ± 0.1 | 5.77 ± 0.19 | ND | ND | |
| 36 | 18.2 ± 0.1 | 22.8 ± 0.2 | 5.82 ± 0.04 | 0.01 ± 0.00 | 0.03 ± 0.00 | |
| 55 | 8.9 ± 0.8 | 22.6 ± 0.7 | 5.39 ± 0.15 | 0.03 ± 0.00 | 0.09 ± 0.00 | |
| 62 | 1.4 ± 0.1 | 22.2 ± 0.3 | 5.05 ± 0.10 | 0.01 ± 0.00 | 0.12 ± 0.01 | |
| 72 | ND | 19.6 ± 0.6 | 4.79 ± 0.11 | 0.02 ± 0.00 | 0.16 ± 0.01 | |
| 84 | ND | 18.6 ± 0.7 | 4.69 ± 0.06 | 0.02 ± 0.00 | 0.22 ± 0.02 | |
| D. hansenii | 0 | 18.3 ± 0.1 | 22.9 ± 0.1 | 5.81 ± 0.02 | ND | ND |
| 30 | 16.5 ± 0.7 | 22.7 ± 0.1 | 5.93 ± 0.38 | ND | ND | |
| 36 | 14.1 ± 0.2 | 22.4 ± 0.2 | 5.95 ± 0.60 | 0.02 ± 0.00 | 0.04 ± 0.01 | |
| 55 | 12.0 ± 0.0 | 20.1 ± 0.7 | 5.86 ± 0.29 | 0.01 ± 0.00 | 0.05 ± 0.01 | |
| 62 | 9.6 ± 0.9 | 19.4 ± 0.4 | 5.75 ± 0.02 | 0.02 ± 0.00 | 0.14 ± 0.06 | |
| 72 | 6.1 ± 0.6 | 19.4 ± 0.9 | 5.59 ± 0.12 | 0.03 ± 0.01 | 0.32 ± 0.06 | |
| 84 | 4.7 ± 0.5 | 19.9 ± 0.4 | 5.26 ± 0.10 | 0.03 ± 0.01 | 0.55 ± 0.10 | |
| Y. lipolytica | 0 | 18.3 ± 0.1 | 22.9 ± 0.1 | 5.87 ± 0.03 | ND | ND |
| 30 | 18.5 ± 0.1 | 23.0 ± 0.1 | 5.75 ± 0.10 | 0.16 ± 0.01 | ND | |
| 36 | 18.6 ± 0.1 | 23.1 ± 0.1 | 5.29 ± 0.07 | 0.32 ± 0.01 | ND | |
| 55 | 18.6 ± 0.0 | 23.0 ± 0.1 | 2.95 ± 0.05 | 0.51 ± 0.02 | ND | |
| 62 | 18.5 ± 0.1 | 22.9 ± 0.1 | 2.10 ± 0.04 | 0.55 ± 0.01 | ND | |
| 72 | 18.7 ± 0.2 | 22.9 ± 0.1 | 1.37 ± 0.07 | 0.52 ± 0.02 | ND | |
| 84 | 18.6 ± 0.0 | 22.8 ± 0.1 | 0.72 ± 0.05 | 0.46 ± 0.02 | ND | |
| K. marxianus, D. hansenii, and Y. lipolytica in coculture | 0 | 18.3 ± 0.1 | 22.9 ± 0.1 | 5.72 ± 0.09 | ND | ND |
| 36 | 13.8 ± 0.3 | 23.0 ± 0.3 | 5.16 ± 0.00 | 0.13 ± 0.00 | ND | |
| 55 | 10.5 ± 0.3 | 23.0 ± 0.3 | 4.86 ± 0.06 | 0.20 ± 0.01 | 0.04 ± 0.01 | |
| 62 | 7.2 ± 0.4 | 22.9 ± 0.2 | 4.58 ± 0.03 | 0.27 ± 0.01 | 0.05 ± 0.01 | |
| 72 | 5.4 ± 0.2 | 22.9 ± 0.2 | 4.44 ± 0.06 | 0.32 ± 0.01 | 0.08 ± 0.01 | |
| 84 | 2.6 ± 0.1 | 23.0 ± 0.1 | 4.34 ± 0.03 | 0.34 ± 0.02 | 0.10 ± 0.02 | |
| 110 | 0.3 ± 0.1 | 22.8 ± 0.2 | 3.98 ± 0.07 | 0.44 ± 0.02 | 0.18 ± 0.03 | |
| 120 | 0.1 ± 0.0 | 21.8 ± 0.6 | 3.88 ± 0.03 | 0.45 ± 0.03 | 0.20 ± 0.02 | |
Results are the means ± standard deviations of six different experiments. ND, not detected.
(iii) l-Methionine consumption and KMTBA and HMTBA biosynthesis.
The results obtained showed that the consumption of l-methionine was much more important in Y. lipolytica cultures than in others (Table 2). Indeed, while Y. lipolytica consumed over 77% of l-methionine within 72 h, only 16% and almost 4% of the initial amount of this amino acid was utilized at this time by K. marxianus and D. hansenii, respectively (Table 2). In yeast cocultures, 22% of the l-methionine was consumed after 72 h and 32% after 120 h. In parallel to l-methionine degradation, a transient accumulation of the transamination product KMTBA was observed in the yeast coculture and in Y. lipolytica culture, although it was much more important in Y. lipolytica culture (Table 2). Moreover, HMTBA, which is the reduction product of KMTBA, was detected in K. marxianus and D. hansenii cultures, as well as in the yeast cocultures. The absence of HMTBA from Y. lipolytica cultures indicated that this strain is strongly oxidative.
(iv) Volatile sulfur compound biosynthesis.
The production of VSC was measured in the pure cultures of the three yeasts, as well as in yeast cocultures (Table 3). Y. lipolytica was by far the most efficient of the yeasts at producing VSC, with dimethyl disulfide being the major sulfur compound produced. This is in agreement with the fact that Y. lipolytica can degrade l-methionine most efficiently among the three yeasts (Table 2). The thioester methylthioacetate was produced only by D. hansenii and K. marxianus. In yeast cocultures, VSC production was lower than in Y. lipolytica cultures and surpassed the VSC biosynthesis of the two other yeasts, D. hansenii and K. marxianus. This suggests that the presence of Y. lipolytica promotes VSC production within the yeast coculture.
TABLE 3.
Production of VSC by K. marxianus, D. hansenii, and Y. lipolytica cultivated as pure cultures or coculturesa
| Yeast(s) | Time (h) | VSC production (peak area × 104 · CFU−1)
|
|||
|---|---|---|---|---|---|
| MTL | DMDS | DMTS | MTA | ||
| Cultivated as pure cultures | |||||
| K. marxianus | 72 | ND | 7 ± 2 | 2 ± 1 | 59 ± 8 |
| D. hansenii | 72 | ND | 37 ± 7 | 4 ± 0 | 11 ± 0 |
| Y. lipolytica | 72 | 1,905 ± 136 | 12,575 ± 726 | 11 ± 2 | ND |
| K. marxianus, D. | 72 | ND | 98 ± 17 | 8 ± 2 | ND |
| hansenii, and | 120 | ND | 523 ± 57 | 24 ± 9 | ND |
| Y. lipolytica cultivated in coculture | |||||
Results are shown as means ± standard deviations. MTL, methanethiol; DMDS, dimethyl disulfide; DMTS, dimethyl trisulfide; MTA, methylthioacetate; ND, not detected.
Gene expression analyses. (i) Multispecies microarray validation with pure cultures.
D. hansenii, K. marxianus, and Y. lipolytica were first cultivated separately in the SCM containing lactose, lactate, and l-methionine. Cells were grown to late exponential phase on the basis of kinetic studies performed as described above (Fig. 1), 36 h for D. hansenii and Y. lipolytica and 55 h for K. marxianus, and total RNA isolation was performed.
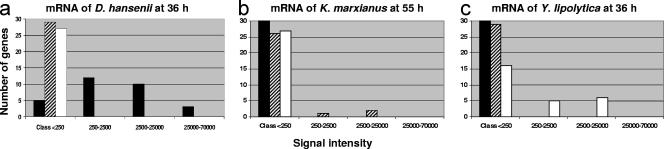
The presence of homologs on the same microarray increases the risk of cross-hybridizations. Effects of hybridization temperature on the hybridization efficiency and stringency were thus determined. First, assays were made at 42 and 52°C, and cross-hybridizations were observed between sequences of the three yeasts (data not shown). By increasing the hybridization temperature to 60°C, cross-hybridizations were avoided while keeping a high fluorescence signal (Fig. 2). For example, very low signal intensities were detected with K. marxianus strain- and Y. lipolytica strain-specific oligonucleotide probes when Cy3-labeled cDNA was prepared from D. hansenii mRNA (Fig. 2a).
FIG. 2.
Distribution of spot intensities (arbitrary units) under different experimental conditions: (a) Cy3-cDNA prepared from D. hansenii mRNA; (b) Cy3-cDNA prepared from K. marxianus mRNA; (c) Cy3-cDNA prepared from Y. lipolytica mRNA. The genes were ranked according to the intensities of the corresponding hybridization signals. The values were then arranged into four sets: Class <250; 250 to 2,500; 2,500 to 25,000; and 25,000 to 70,000, with increasing hybridization values. Black histograms, D. hansenii genes. Hatched histograms, K. marxianus genes. White histograms, Y. lipolytica genes.
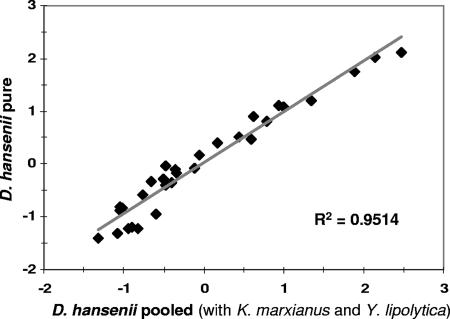
Parallel analyses were performed with the three pure cultures to test the reproducibility of the methodology (i.e., cDNA synthesis, purification, hybridization, and scanning parameters) when the transcripts of a given species were evaluated from a yeast coculture. As a representative example, signal intensities for D. hansenii-specific oligonucleotide probes were compared when Cy3-labeled cDNA was prepared from D. hansenii mRNA in pure culture versus signals from an artificial mixed culture with K. marxianus and Y. lipolytica mRNA. Prior to RNA extraction, the three pure cultures of D. hansenii, K. marxianus, and Y. lipolytica were pooled (1:1:1, vol/vol/vol). All RNAs were extracted, transcribed, and labeled by direct incorporation of dCTP-Cy3. The specific hybridization signals for each species were then compared to those obtained from pure cultures. For D. hansenii (Fig. 3), a good reproducibility between arrays was observed, with a correlation coefficient of 0.95. The same experiment was performed for K. marxianus and Y. lipolytica; in these two cases, a correlation coefficient of at least 0.96 was generated (data not shown).
FIG. 3.
Scatter plots of the signal intensities obtained from mRNA of D. hansenii in pure culture (pure) versus mRNA of D. hansenii in artificial mixed culture (pooled) with K. marxianus and Y. lipolytica.
(ii) Identification of expressed genes in yeast pure cultures.
In order to identify genes expressed for each yeast, expression profiling of those genes possibly involved in lactose/lactate and l-methionine catabolism was carried out. The reproducibility of the measurements was confirmed by duplicate biological experiments. Hybridization signals were quantified using Quantarray (Packard BioChip Technologies) software and normalized by the median (see Materials and Methods) value. Table 4 compares the expression level of each gene to the mean expression of all genes present on the array for a given species and, consequently, gives us biological information about the predominant biological processes in the yeast considered. Expression patterns varied considerably depending on the yeast, which suggests a complementary role for each given yeast during ripening. As expected, lactose catabolism was highly induced in K. marxianus as indicated by expression levels of both LAC4 (β-galactosidase) and LAC12 (lactose permease), while major genes involved in lactate and pyruvate catabolism were highly expressed in D. hansenii. In Y. lipolytica, the highest expression levels were reached by BAT2 and ARO8, two genes involved in amino acid catabolism, and PDC6, which encodes a pyruvate decarboxylase.
TABLE 4.
Transcript levels of D. hansenii, K. marxianus, or Y. lipolytica genes when yeasts were cultivated separately in SCM containing lactose, lactate, and l-methionine
| Gene | Putative functiona | Gene expression levels for yeast type strainb
|
|||||
|---|---|---|---|---|---|---|---|
|
D. hansenii 304
|
K. marxianus 44(8)
|
Y. lipolytica 370
|
|||||
| Accession no. | Transcript level | Accession no. | Transcript level | Accession no. | Transcript level | ||
| LAC4 | β-Galactosidase | DEHA0G26345g | −0.0 | KLLA0B14883g | +10.7 | ||
| LAC12 | Lactose permease | DEHA0G26367g | −1.1 | KLLA0B14861g | +10.3 | ||
| JEN1 | Lactate transporter | DEHA0F18843g | +0.5 | KLLA0E16313g | −0.0 | YALI0D24607g | −0.7 |
| DEHA0D20427g* | −1.2 | KLLA0F10043g | −0.2 | YALI0D20108g | −0.7 | ||
| DEHA0F28809g* | −1.3 | YALI0C15488g | −0.2 | ||||
| YALI0E32901g | −0.6 | ||||||
| YALI0C21406g | −0.5 | ||||||
| YALI0B19470g | −1.0 | ||||||
| CYB2 | Lactate dehydrogenase cytochrome b2 | DEHA0E01166g | +3.2 | KLLA0D02640g | −1.0 | YALI0E21307g | +1.1 |
| KLLA0B14795g | +0.5 | ||||||
| DLD1 | Lactate ferricytochrome c oxidoreductase | DEHA0D09658g* | +4.0 | KLLA0E19789g | −0.2 | YALI0E03212g | +5.0 |
| KLLA0D03344g | −0.4 | ||||||
| DLD2 | Lactate ferricytochrome c oxidoreductase | DEHA0F07612g* | +0.4 | KLLA0B01397g* | −0.2 | ||
| ILV2 | Acetolactate synthase (catalytic subunit) | DEHA0G02574g* | +1.0 | KLLA0B12584g | −0.7 | YALI0C00253g | +4.6 |
| ILV6 | Acetolactate synthase (regulatory subunit) | DEHA0E22869g* | +4.0 | KLLA0F12364g | −0.3 | YALI0C09636g | +5.3 |
| PDA1 | E1α subunit of the PDH complex | DEHA0G13728g* | +5.9 | KLLA0F12001g | +0.3 | YALI0F20702g | +4.9 |
| PDB1 | E1β subunit of the PDH complex | DEHA0C10065g | +6.1 | KLLA0F09603g | −0.0 | YALI0E27005g | +3.6 |
| YALI0F05038g* | +5.4 | ||||||
| PDC1 | Major pyruvate decarboxylase isozyme | DEHA0G19525g* | +7.3 | KLLA0E16357g | +7.9 | YALI0D10131g | +5.5 |
| DEHA0B03784g | +0.5 | ||||||
| DEHA0D18381g* | +4.3 | ||||||
| PDC6 | Minor isoform of pyruvate decarboxylase | YALI0D06930g | +6.8 | ||||
| THI3 | Probable decarboxylase | KLLA0F16962g | −0.4 | ||||
| ARO10 | Phenylpyruvate decarboxylase | DEHA0D11066g* | +4.4 | KLLA0E02662g | −0.1 | ||
| BAT1 | Branched-chain amino acid aminotransferase | KLLA0A10307g | −0.6 | YALI0D01265g | −0.9 | ||
| BAT2 | Branched-chain amino acid aminotransferase | DEHA0D07689g* | +4.7 | YALI0F19910g | +6.1 | ||
| ARO8 | Aromatic aminotransferase | DEHA0A06974g | +0.6 | KLLA0F10021g | −0.3 | YALI0E20977g | +7.3 |
| DEHA0A00253g* | +1.5 | KLLA0A04906g | −0.3 | ||||
| DEHA0A00231g* | −0.9 | ||||||
| ARO9 | Aromatic aminotransferase | KLLA0D11110g | −0.2 | YALI0C05258g | −0.2 | ||
| MAP1 | Methionine aminopeptidase | DEHA0G13937g* | +2.6 | KLLA0D18436g | −0.6 | YALI0D17138g | −0.5 |
| MAP2 | Methionine aminopeptidase | DEHA0C04444g | +2.9 | KLLA0E06875g | −0.9 | YALI0D05159g | −0.9 |
| SAM2 | S-Adenosylmethionine synthase | DEHA0E14751g | +4.4 | KLLA0C01782g | −0.6 | YALI0B14509g* | +0.3 |
| SAM4 | S-Adenosylmethionine-homocysteine methyltransferase | DEHA0A00869g* | −1.4 | KLLA0D01551g | −0.7 | YALI0F25641g* | −0.2 |
| CYS3 | Cystathionine γ-lyase | DEHA0C16863g* | +1.3 | KLLA0F07909g | −0.6 | YALI0F05874g | +0.3 |
| CYS4 | Cystathionine β-synthase | DEHA0C15708g | +1.8 | KLLA0F09317g | −0.2 | YALI0E09108g | +0.3 |
| STR3 | Cystathionine β-lyase | DEHA0A06897g* | +0.7 | KLLA0C17028g | −0.3 | YALI0D00605g* | −0.8 |
| STR2 | Cystathionine γ-synthase | DEHA0D10285g* | +1.0 | KLLA0B04378g | −0.3 | ||
| Hypothetical cystathionine γ-synthase | YALI0D17402g* | −0.4 | |||||
| Cystathionine γ-synthase activity | DEHA0A01144g | +0.6 | KLLA0E21406g | −0.2 | |||
| DEHA0A01529g | −0.9 | ||||||
Annotations are from Génolevures (http://natchaug.labri.u-bordeaux.fr/Genolevures/index.php).
Yeast cells were grown to late exponential phase (36 h for D. hansenii and Y. lipolytica and 55 h for K. marxianus). It should be noted that the transcript levels of two genes belonging to the same species can be compared but cannot be compared between homologous genes from two different species. *, distantly related homolog of S. cerevisiae (see putative designation in the supplemental material).
(iii) Gene expression analysis of the yeast coculture.
In order to give an insight into the relative importance of each yeast when cultivated in coculture, with respect to lactose/lactate and l-methionine catabolism, gene expression measurements were carried out at three different times, at early (36 h), mid- (72 h), and late (120 h) stationary phases of yeast coculture with the SCM (Table 5). The reproducibility of the measurements was confirmed by triplicate, independent experiments for each time point. Our experimental design included three experimental parameters (“culture time” analyzed, repetition of experiments, and replication of spots on microarray). Statistical analysis was performed with GeneANOVA software (see Materials and Methods). ANOVA showed that the biological factors “gene identity” and “culture time” were responsible for most of the variance. Only genes whose expression levels changed significantly (false discovery rate of <10%) over time are indicated in Table 5. Lactose metabolism was highly induced within 72 h as indicated by high expression levels of the LAC4 (β-galactosidase) and LAC12 (lactose permease) genes. This induction was followed by a dramatic decline thereafter (Table 5), which correlates with complete lactose consumption (Table 2). In Y. lipolytica, l-methionine catabolism was highly induced during the whole duration of the cultivation, as indicated by the expression levels of BAT2 and ARO8; this corresponds to a gradual accumulation of KMTBA observed in the yeast coculture (Table 2). In cocultures, lactate is less efficiently degraded compared to that in D. hansenii pure cultures (Table 2), although major genes involved in lactate/pyruvate catabolism were highly expressed (Table 5). Low expression levels of a D. hansenii lactate transporter at 36 to 72 h might explain this phenomenon.
TABLE 5.
Significant up- and down-regulated genes with an FDR of <10% over timea
| Gene | Accession no.b | FDR rate at:
|
Putative functionc | ||
|---|---|---|---|---|---|
| 36 h | 72 h | 120 h | |||
| LAC4 | KLLA0B14883g | +7.2 | +7.1 | +0.6 | β-Galactosidase |
| LAC12 | KLLA0B14861g | +3.6 | +2.4 | −1.6 | Lactose permease |
| JEN1 | DEHA0F18843g | +2.2 | −0.7 | +5.0 | Lactate transporter |
| ILV6 | DEHA0E22869g* | +5.2 | +4.7 | +3.4 | Acetolactate synthase (regulatory subunit) |
| PDA1 | DEHA0G13728g* | +8.2 | +7.2 | +5.8 | E1α subunit of the PDH complex |
| PDB1 | DEHA0C10065g | +10.1 | +8.6 | +7.9 | E1β subunit of the PDH complex |
| YALI0F05038g* | +2.6 | +1.9 | +6.0 | ||
| PDC1 | DEHA0D18381g* | +5.9 | +6.8 | +5.2 | Major of three pyruvate decarboxylase isozymes |
| DEHA0G19525g* | +8.8 | +10.0 | +7.0 | ||
| YALI0D10131g | −0.4 | +2.5 | +1.0 | ||
| ARO10 | DEHA0D11066g* | +6.0 | +5.8 | +8.6 | Phenylpyruvate decarboxylase |
| BAT2 | YALI0F19910g | +3.8 | +6.3 | +4.4 | Branched-chain amino acid aminotransferase |
| ARO8 | YALI0E20977g | +5.2 | +7.5 | +6.6 | Aromatic aminotransferase |
Samples were taken at early (36 h), mid- (72 h), and late (120 h) stationary phase of yeasts cocultured in synthetic cheese medium. The FDR was determined using GeneANOVA software.
*, distantly related homolog of S. cerevisiae (see putative designations in the supplemental material).
Annotations are from Génolevures (http://cbi.labri.fr/Genolevures/).
DISCUSSION
In this work, we were able to identify distinct and/or complementary roles of three cheese-ripening yeasts cultivated in pure cultures or in cocultures, with respect to lactose/lactate and l-methionine catabolism.
We have shown that lactose and lactate consumption profiles varied depending on the yeast species considered. Both lactose and lactate were consumed in K. marxianus cultures, which is in agreement with the results obtained from analyses of ewe's cheeses (12). Indeed, high mRNA signals of genes encoding β-galactosidase and lactose permease corroborate the high lactose consumption in K. marxianus cultures. In yeast cocultures, our results show that both genes were most highly expressed between 36 h and 72 h, which corresponds to a continuous lactose consumption. This is well in agreement with results obtained with K. lactis in which LAC4 and LAC12 genes were up-regulated in response to lactose addition (29 and references therein). Our data also revealed that the lactate consumption began concomitantly with the pH rise in K. marxianus cultures. In K. marxianus culture, microarray results showed low transcript levels of the open reading frames (ORFs) corresponding to lactate dehydrogenases and lactate transporters; this is in accordance with limited lactate catabolism, since around 16% of lactate was consumed after 72 h. When cultivated in coculture, in which lactate is not extensively degraded, none of the genes involved in lactate catabolism was significantly expressed from K. marxianus. In K. lactis, only one gene was found to express a pyruvate decarboxylase (Pdc) activity (7). Our microarray results showed that the K. marxianus PDC1 ortholog mRNA level was highly expressed when this strain was cultivated in pure culture. Also, the relative transcript levels of the K. marxianus PDA1 and PDB1 genes encoding pyruvate dehydrogenases (PDH) were very low.
In D. hansenii, although this yeast was able to grow efficiently on lactose (Table 2), the LAC4 and LAC12 genes were poorly expressed (Table 4). In contrast, lactate is poorly degraded by D. hansenii, but our results also showed that genes putatively involved in lactate and/or pyruvate (e.g., Ldh, Pdh, Pdc, and acetolactate synthase) catabolic pathways were up-regulated in D. hansenii under all culture conditions (pure culture or coculture). When yeasts were cultivated in coculture, in which lactate is poorly degraded, major genes involved in lactate and/or pyruvate catabolism were highly expressed, with the exception of the lactate permease gene JEN1. Since D. hansenii grows properly in coculture where lactose is efficiently degraded, we can suspect this yeast to degrade lactose by an as-yet-unknown pathway. Alternatively, we can suspect the yeast coculture (i) to produce an intermediate that induces the lactose degradation pathway in D. hansenii or (ii) to degrade lactose to an intermediate used by D. hansenii. Further work is required to clarify how lactose is degraded by this yeast.
In Y. lipolytica pure cultures, lactose and lactate concentrations remained unchanged. Neither LAC4 nor LAC12 genes were identified in the Y. lipolytica genome. This is in accordance with the natural inability of Y. lipolytica to use lactose as a carbon source (5). Moreover, none of the six JEN1 homolog genes was induced by lactate at the transcriptional level in Y. lipolytica cultures. However, the deacidification rate was highest with Y. lipolytica cultures. It is clear, therefore, that the pH increase does not depend on the assimilation of lactate in Y. lipolytica. From our study, we can propose that this pH increase is due to the release of ammonia during l-methionine catabolism, since this amino acid is efficiently degraded by this yeast. The involvement of aminotransferase(s) in l-methionine catabolism in the cheese-ripening yeasts Geotrichum candidum, D. hansenii, K. lactis, and Y. lipolytica has been suggested (3, 9). Two ORFs encoding aromatic aminotransferases were identified in the Y. lipolytica genome on the basis of sequence homology with ARO8 and ARO9 of S. cerevisiae. Moreover, Y. lipolytica has two branched-chain aminotransferase genes, one with a mitochondrial targeting signal and one which is cytoplasmic, like S. cerevisiae (8). The mitochondrial gene BAT1 is highly expressed during logarithmic phase and is repressed during stationary phase in S. cerevisiae, whereas the cytosolic isoenzyme BAT2 has the opposite pattern of expression (20). In other species, there is only one branched-chain aminotransferase (Bat), and it has either cytoplasmic features (as in D. hansenii) or mitochondrial features (as in K. lactis) (8). Our microarray experiments showed high transcriptional expressions of Y. lipolytica ARO8 (YlARO8) and BAT2 (YlBAT2), in pure cultures or in coculture with other yeasts, which correlates with the rapid production of KMTBA in Y. lipolytica concomitant with the degradation of l-methionine. Interestingly, l-methionine is specifically transported by one high-affinity and two low-affinity permeases in S. cerevisiae (18). A homology search against the three genomes of K. lactis, D. hansenii, and Y. lipolytica revealed that they contain several ORFs whose products show extensive sequence similarities to these l-methionine permeases. These ORFs show similarities to the high-affinity l-methionine permeases encoded by MUP1 (YGR055w). Two other genes carried by K. lactis and D. hansenii present high similarities with the low-affinity l-methionine permease gene MUP3 (YHL036w). As a high redundancy of high-affinity l-methionine permeases is observed in the Y. lipolytica genome, we can suspect that such l-methionine transporters may give a competitive advantage to Y. lipolytica, allowing it to grow better on l-methionine.
In conclusion, multispecies microarrays were successfully employed to identify major genes involved in lactose/lactate and l-methionine catabolism by three cheese-ripening yeasts cultivated in cocultures. We observed good agreement between expressed genes in the array experiments and biochemical data. It provides evidence of the reliability of our metabolic array data. Furthermore, we also found no interspecies cross-hybridization. Our data open up new prospects in the use of tailor-made microarrays to study the simultaneous expression of targeted metabolism processes in several species within a microbial association (e.g., the cheese ecosystem). Such an approach could therefore be developed to investigate functional redundancy and possible interspecies metabolic interactions within complex microbial associations.
Acknowledgments
Orianne Cholet is grateful to Ecole Doctorale ABIES for a Ph.D. scholarship.
We thank Nancie Reymond for helpful discussions and Audrey Suleau for providing excellent technical advice. We are grateful to Noémie Jacques for performing the strain identification.
Footnotes
Published ahead of print on 16 February 2007.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Alexandre, H., V. Ansanay-Galeote, S. Dequin, and B. Blondin. 2001. Global gene expression during short-term ethanol stress in Saccharomyces cerevisiae. FEBS Lett. 498:98-103. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arfi, K., H. E. Spinnler, R. Tâche, and P. Bonnarme. 2002. Production of volatile compounds by cheese-ripening yeasts: requirement for a methanethiol donor for S-methyl thioacetate synthesis by Kluyveromyces lactis. Appl. Microbiol. Biotechnol. 58:503-510. [DOI] [PubMed] [Google Scholar]
- 4.Arfi, K., M. N. Leclercq-Perlat, H. E. Spinnler, and P. Bonnarme. 2005. Importance of curd-neutralising yeasts on the aromatic potential of Brevibacterium linens during cheese ripening. Int. Dairy J. 15:883-891. [Google Scholar]
- 5.Barnett, J. A., R. W. Payne, and D. Yarrow. 2000. Yeasts: characteristics and identification, 3rd ed. Cambridge University Press, Cambridge, United Kingdom.
- 6.Benjamini, Y., and Y. Hochberg. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57:289-300. [Google Scholar]
- 7.Bianchi, M. M., L. Tizzani, M. Destruelle, L. Frontali, and M. Wésolowski-Louvel. 1996. The “petite negative” yeast Kluyveromyces lactis has a single gene expressing pyruvate decarboxylase activity. Mol. Microbiol. 19:27-36. [DOI] [PubMed] [Google Scholar]
- 8.Bondar, D. C., J. M. Beckerich, and P. Bonnarme. 2005. Involvement of a branched-chain aminotransferase in production of volatile sulfur compounds in Yarrowia lipolytica. Appl. Environ. Microbiol. 71:4585-4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonnarme, P., K. Arfi, C. Dury, S. Helinck, M. Yvon, and H. E. Spinnler. 2001. Sulfur compound production by Geotrichum candidum from l-methionine: importance of the transamination step. FEMS Microbiol. Lett. 205:247-252. [DOI] [PubMed] [Google Scholar]
- 10.Cholet, O., A. Hénaut, and P. Bonnarme. Transcriptional analysis of l-methionine catabolism in Brevibacterium linens ATCC9175. Appl. Microbiol. Biotechnol., in press. [DOI] [PubMed]
- 11.Corsetti, A., J. Rossi, and M. Gobbetti. 2001. Interactions between yeasts and bacteria in the smear surface ripened cheeses. Int. J. Food Microbiol. 69: 1-10. [DOI] [PubMed] [Google Scholar]
- 12.Cosentino, S., M. E. Fadda, M. Deplano, A. F. Mulargia, and F. Palmas. 2001. Yeasts associated with Sardinian ewe's dairy products. Int. J. Food Microbiol. 69:53-58. [DOI] [PubMed] [Google Scholar]
- 13.Devoyod, J. J. 1990. Yeasts in cheese-making, p. 229-240. In: J. F. T. Spencer and D. M. Spencer (ed.), Yeast technology. Springer Verlag, Berlin, Germany.
- 14.Didier, G., P. Brezellec, E. Rémy, and A. Hénaut. 2002. GeneANOVA: gene expression analysis of variance. Bioinformatics 18:490-491. [DOI] [PubMed] [Google Scholar]
- 15.Dujon, B., D. Sherman, G. Fischer, P. Durrens, S. Casaregola, I. Lafontaine, J. De Montigny, C. Marck, C. Neuveglise, E. Talla, N. Goffard, L. Frangeul, M. Aigle, V. Anthouard, A. Babour, V. Barbe, S. Barnay, S. Blanchin, J. M. Beckerich, E. Beyne, C. Bleykasten, A. Boisrame, J. Boyer, L. Cattolico, F. Confanioleri, A. De Daruvar, L. Despons, E. Fabre, C. Fairhead, H. Ferry-Dumazet, A. Groppi, F. Hantraye, C. Hennequin, N. Jauniaux, P. Joyet, R. Kachouri, A. Kerrest, R. Koszul, M. Lemaire, I. Lesur, L. Ma, H. Muller, J. M. Nicaud, M. Nikolski, S. Oztas, O. Ozier-Kalogeropoulos, S. Pellenz, S. Potier, G. F. Richard, M. L. Straub, A. Suleau, D. Swennen, F. Tekaia, M. Wesolowski-Louvel, E. Westhof, B. Wirth, M. Zeniou-Meyer, I. Zivanovic, M. Bolotin-Fukuhara, A. Thierry, C. Bouchier, B. Caudron, C. Scarpelli, C. Gaillardin, J. Weissenbach, P. Wincker, and J. L. Souciet. 2004. Genome evolution in yeasts. Nature 430:35-44.15229592 [Google Scholar]
- 16.Fadda, M. E., V. Mossa, M. B. Pisano, M. Deplano, and S. Cosentino. 2004. Occurrence and characterization of yeasts isolated from artisanal Fiore Sardo cheese. Int. J. Food Microbiol. 95:51-59. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira, A. D., and B. C. Viljoen. 2003. Yeasts as adjunct starters in matured cheddar cheese. Int. J. Food Microbiol. 86:131-140. [DOI] [PubMed] [Google Scholar]
- 18.Isnard, A. D., D. Thomas, and Y. Surdin-Kerjan. 1996. The study of methionine uptake in Saccharomyces cerevisiae reveals a new family of amino acid permeases. J. Mol. Biol. 262:473-484. [DOI] [PubMed] [Google Scholar]
- 19.Kagkli, D. M., P. Bonnarme, C. Neuvéglise, T. M. Cogan, and S. Casaregola. 2006. l-Methionine degradation pathway in Kluyveromyces lactis: identification and functional analysis of the genes encoding l-methionine aminotransferase. Appl. Environ. Microbiol. 72:3330-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kispal, G., H. Steiner, D. A. Court, B. Rolinski, and R. Lill. 1996. Mitochondrial and cytosolic branched-chain amino acid transaminases from yeast, homologs of the myc oncogene-regulated Eca39 protein. J. Biol. Chem. 271:24458-24464. [DOI] [PubMed] [Google Scholar]
- 21.Leclercq-Perlat, M. N., A. Oumer, J. L. Bergere, H. E. Spinnler, and G. Corrieu. 2000. Behavior of Brevibacterium linens and Debaryomyces hansenii as ripening flora in controlled production of smear soft cheese from reconstituted milk: growth and substrate consumption. J. Dairy Sci. 83:1665-1673. [DOI] [PubMed] [Google Scholar]
- 22.Leclercq-Perlat, M. N., F. Buono, D. Lambert, E. Latrille, H. E. Spinnler, and G. Corrieu. 2004. Controlled production of Camembert-type cheeses. I. Microbiological and physicochemical evolutions. J. Dairy Res. 71:346-354. [DOI] [PubMed] [Google Scholar]
- 23.Pysz, M. A., S. B. Conners, C. I. Montero, K. R. Shockley, M. R. Johnson, D. E. Ward, and R. M. Kelly. 2004. Transcriptional analysis of biofilm formation processes in the anaerobic, hyperthermophilic bacterium Thermotoga maritima. Appl. Environ. Microbiol. 70:6098-6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quackenbush, J. 2001. Computational analysis of microarray data. Nat. Rev. Genet. 2:418-427. [DOI] [PubMed] [Google Scholar]
- 25.Reiner, A., D. Yekutieli, and Y. Benjamini. 2003. Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics 19:368-375. [DOI] [PubMed] [Google Scholar]
- 26.Reymond, N., H. Charles, L. Duret, F. Calevro, G. Beslon, and J. M. Fayard. 2004. ROSO: optimizing oligonucleotide probes for microarrays. Bioinformatics 20:271-273. [DOI] [PubMed] [Google Scholar]
- 27.Schena, M., D. Shalon, R. W. Davis, and P. O. Brown. 1995. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science 270:467-470. [DOI] [PubMed] [Google Scholar]
- 28.Souciet, J., M. Aigle, F. Artiguenave, G. Blandin, M. Bolotin-Fukuhara, E. Bon, P. Brottier, S. Casaregola, J. de Montigny, B. Dujon, P. Durrens, C. Gaillardin, A. Lepingle, B. Llorente, A. Malpertuy, C. Neuveglise, O. Ozier-Kalogeropoulos, S. Potier, W. Saurin, F. Tekaia, C. Toffano-Nioche, M. Wesolowski-Louvel, P. Wincker, and J. Weissenbach. 2000. Genomic exploration of the hemiascomycetous yeasts: 1. A set of yeast species for molecular evolution studies. FEBS Lett. 487:3-12. [DOI] [PubMed] [Google Scholar]
- 29.Suleau, A., P. Gourdon, J. Reitz-Ausseur, and S. Casaregola. 2006. Transcriptomic analysis of extensive changes in metabolic regulation in Kluyveromyces lactis strains. Eukaryot. Cell 5:1360-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van den Tempel, T., and M. Jakobsen. 2000. The technological characteristics of Debaryomyces hansenii and Yarrowia lipolytica and their potential as starter cultures for production of danablu. Int. Dairy J. 10:263-270. [Google Scholar]
- 31.Welthagen, J. J., and B. C. Viljoen. 1998. Yeast profile in gouda cheese during processing and ripening. Int. Dairy J. 41:185-194. [DOI] [PubMed] [Google Scholar]
- 32.Xie, Y., L.-S. Chou, A. Cutler, and B. Weimer. 2004. DNA macroarray profiling of Lactococcus lactis subsp. lactis IL1403 gene expression during environmental stresses. Appl. Environ. Microbiol. 70:6738-6747. [DOI] [PMC free article] [PubMed] [Google Scholar]