Abstract
Despite the routine application of therapeutic drug monitoring of cyclosporin (CsA) for two decades, there remain significant analytical issues. In addition, new developments have arisen in the delivery of this laboratory service as well as alternative clinical strategies for delivering optimal benefit to organ transplant recipients.
Sample collection strategies are evolving away from the traditional pre-dose/trough (C0) sample in favour of estimates of the absorption phase in the first 4–6 hours after the oral dose of CsA. This is based on the recognition of the relatively poor relationship between C0 and CsA exposure indices, such as area under the blood CsA concentration versus time curve (AUC), especially in the first few hours after the dose. By collecting serial blood samples over this limited period (4hr after the dose) and estimating the AUC0-4, one can gain insight into how well CsA has been absorbed for each transplant recipient, and individualise CsA dosage. However, a recent survey of Australasian CsA laboratories revealed that such AUC0-4 sampling strategies in the early post-dose period were poorly accepted in clinics across Australasia. The alternative that has proven to be more clinically acceptable is the use of a single sample 2-hours after the dose (C2). The C2 concentration has been demonstrated (particularly in kidney and liver transplant recipients) as correlating well with AUC0-4, allowing it to be used as a surrogate index of CsA absorption and exposure.
The laboratory survey also showed several areas of concern in the analytical sphere. The major one is that the majority of laboratories employ the two immunoassays that deliver the least specific result on C0 samples within the range of monoclonal methods, leading to high variability and clinically significant errors with patient samples. Laboratories have also adopted a range of dilution protocols for the significantly higher C2 concentrations, and this has proved a source of significant error. In addition, around 30% of laboratories were not involved in a proficiency-testing program. Thus there is clear opportunity to do much better analytically.
Hence, there remain significant challenges ahead to deliver better quality CsA assay services and dosage individualisation to further improve outcomes for the organ transplant recipients that we care for.
Background to CsA Monitoring
CsA is referred to as a "critical dosage drug", implying that it is most important to individualise the CsA dosage schedule for each patient to optimise pharmacological response (ie. inhibit rejection and avert toxicity). CsA also has a narrow therapeutic index, implying that there is not a large difference between the blood CsA concentrations required for therapeutic benefit and those associated with adverse effects; so there is only a narrow window available for treatment. We achieve dosage individualisation by measuring the CsA concentration in blood and adjusting the dosage for each recipient to meet target CsA concentrations associated with desirable clinical outcomes. Various ranges are used depending on such factors as the organ transplant type, the length of time after grafting and the drug combination.
In this way we can accommodate, at least partially, the generally poor and variable rate and extent of absorption (bioavailability) of CsA both inter- and intra-patient. On average, only about 40% of CsA in the oral dose survives the barriers to absorption and first-pass metabolism, in the gut-wall and liver to reach the systemic circulation, from where it reaches the tissues to exert its actions.1–3 Perhaps a better term for CsA would be "critical concentration drug", as the dosage schedule is typically adjusted up or down so as to attain the desired target blood CsA concentration, in the light of the patient's clinical indices. This variability with the dosage/concentration relationship of CsA is shared with other immunosuppressant drugs currently used in organ transplantation, including tacrolimus, sirolimus and mycophenolic acid, as well as newer drugs currently in clinical trials, (eg. FTY-720 and everolimus) as recently reviewed.4–6
CsA was introduced into clinical use for transplantation in Australia in the early 1980s and has been routinely monitored in clinical laboratories since that time. Initially the immunoassays available were rather crude by current standards. For example, the charcoal-separation polyclonal RIAs provided by the pharmaceutical manufacturer had minimal ability to distinguish the active parent CsA from some 30 CsA-metabolites that may be inactive or, in the case of AM1 and AM9, have up to 14–16% of parent CsA activity.7–9 As the diagnostic companies became involved in CsA measurement, we gradually obtained assays that were more or less able to distinguish parent CsA with less interference from its metabolites (more on this below). Metabolite AM1 poses the greatest concern, as it is present in the greatest concentration in patient samples, even exceeding parent CsA.10
Trough (C0) Monitoring
As is common practice with most therapeutic drug monitoring, the standard sample that was recommended through the first 15 years and in the various early international and Australian guidelines was the C0 sample drawn into an EDTA collection tube. The whole-blood CsA concentration was then measured following lysis and precipitation of the blood cells.11–18 The recent Australasian CsA survey indicated that the use of the recommended EDTA collection tubes and whole-blood assays were universally adopted in this region.19 Such universal usage is not the case internationally, where a minority of laboratories use plasma and/or heparinised-samples for CsA determination. EDTA-whole blood was used for the following reasons:
there was a significant temperature-dependent equilibrium between plasma and red blood cells such that each 1°C reduction in the temperature that plasma was separated from the cellular fraction caused a 14 μg/L reduction in plasma CsA concentration, making it difficult to obtain representative plasma CsA concentrations that reflected in vivo circulating concentrations (ie. at 37°C),
most (>95%) of the CsA in whole-blood resides inside blood cells making it difficult to attain assay sensitivity for the very low concentrations in plasma, 20 and
heparin anti-coagulated blood showed variable results due to the presence of micro-clots causing significant inconsistencies in the fraction sampled for assay in the laboratory.
Along this 20-year journey there were "voices in the wilderness" suggesting that C0 blood samples were less than optimal as an index of CsA exposure for dosage individualisation, and that we should be reviewing this choice.18,21–24 In more recent years, this appears to be coming to fruition, as several alternative approaches have been proposed for CsA clinical management. Much of the impetus for this has come from centres across Canada.25–40
AUC Strategies
If we presume that the 12hr dose-interval CsA concentration profile, and the area under the blood CsA concentration versus time curve, attained by drawing serial blood samples across this dosing interval (AUC0-12), provides the best index of CsA exposure, then we can consider where most of the variability within and between patients resides. Whilst there have been strategies to use the entire dosing interval to optimise dosage, it was observed that the greatest variability occurred in the absorption phase in the initial 4–6hr after the CsA dose in both adult and paediatric transplant recipients, including with CsA formulations with improved absorption properties, such as the microemulsion formulation, Neoral® (Novartis Pharmaceuticals, Basel, Switzerland).21,41,42 In most studies, it was noted that the traditional C0 sample provided limited or poor correlations with either the full AUC0-12, or the part where the most variability resides, AUC0-4. There are many examples of these relationships in the literature, particularly in renal and liver transplant recipients, but less in heart/lung or bone marrow transplantation.28,32,43–49 Depending on the CsA assay methodology adopted (discussed below), coefficients of determination (r2) for C0 with AUC0-4 were generally of the order <0.1 to 0.6. This rather unreliable relationship suggested other factors could impact on the use of C0 concentration as an index of CsA exposure and that C0 may provide a sub-optimal guide to dosage individualisation for patients.
One clinical monitoring option proposed was to quantify this variable AUC0-4 period. This was achieved by drawing a small number of accurately-timed blood samples (typically 2–3) in this period after the CsA dose, and then applying algorithms derived from the relevant transplant population to estimate the AUC0-4. This was called the "limited sampling strategy AUC" (AUClss), or "absorption profiling".18,26,27,36,44,50–52 Data from Canadian centres demonstrated that maintaining the CsA AUC0-4 between 4400 and 5500 μg.h/L delivered the lowest risk of rejection and minimised risk of adverse effects.28,36 Again, there are many examples in the literature of AUC0-4 approaches and these have been reviewed.53,54 However, as our Australasian CsA-lab survey in year 2000 has demonstrated, only one of forty-one laboratories responding to this survey was receiving AUC samples.19 Thus this approach has not been well accepted in our transplantation wards/clinics, presumably because it was too hard to implement (including the practical difficulty of drawing several well-timed blood samples), and/or possibly the AUC0-4 concept was too foreign to transplant physicians and surgeons.
Choice of Sample
The next measuring milestone evolved from AUC0-4. This was to seek an alternative single sample that, unlike C0, did correlate well with AUC0-4. Although other sample times following the dose have been suggested, the C2 sample has been most widely applied (and marketed by the Neoral® manufacturer, Novartis Pharmaceuticals, Basel, Switzerland) as showing the best correlation with AUC0-4.24,30 For example, one study reported coefficients of determination (r2) between each of C0, C1, C2, C3, C4, and AUC0-4, of 0.42, 0.75, 0.90, 0.85 and 0.61, respectively.30 Thus the C2 concentration was selected simply because it showed the best relationship with AUC0-4 among those times points tested. Most clinical studies have been performed in kidney and liver transplant recipients, and have shown r2 values between C2 and AUC0-4 ranging from approximately 0.80 to 0.93. Our study in renal patients showed a r2 of 0.85 and 0.87 respectively in patients taking CsA with and without diltiazem, a CsA-sparing agent.47 This will be further discussed below.
Whilst some authors have referred to the C2 sample as the 'peak' or Cmax concentration, it is probably inappropriate to link such terms with C2.55,56 Even with the more predictable Neoral® formulation, the CsA absorption profile is quite variable and so the actual peak concentration may frequently occur before or well after two hours for many reasons.41, 42 Rather, it is important to simply think of C2 as an index, or surrogate marker, of AUC0-4 which is in turn a marker of CsA absorption.
Pharmacodynamics of C2 Monitoring
The pharmacokinetic arguments which favour the use of AUC0-4 or C2 strategies are not the entire story, as there are also relationships at the tissue level where CsA exerts its action (ie. pharmacodynamics). It has been shown in vitro that CsA inhibition of calcineurin in activated T-cells (and hence inhibition of the immunological response to reject the transplanted organ) is directly related to CsA concentration, that this inhibition is reversible and that the maximum inhibition occurred in parallel to the CsA concentration at around 2hr after the dose.57,58 Thus, if "adequate" CsA concentrations are not achieved following CsA administration, then effective calcineurin inhibition may not follow, which has potential implications for graft rejection. Many of the recent studies have empirically evaluated the target CsA concentrations that need to be attained to optimise immunosuppression by averting rejection episodes, but not so high as to predispose the organ recipient to increased risk of acute adverse effects from CsA (including nephrotoxicity, hypertension, opportunistic infections, etc). With such a narrow therapeutic index, perhaps this conundrum may not be entirely soluble, and a relative risk/benefit rationale may need to be applied for calcineurin-inhibitor drugs like CsA.
Further arguments and recommendations favouring C2 monitoring are reviewed elsewhere, including the recent consensus papers from the CONCERT group, and our Australasian group, as well as other reviews and recommendations.5,33,38–40,48,59–63 They have all supported the use of C2 strategies as a more useful tool than C0 for CsA monitoring. However, like many issues regarding CsA over the years, there is also a (minority) counter argument, including one extensive review that concluded: "C0 is currently the standard method of monitoring CsA therapy and there is no evidence demonstrating the superiority of any other method of monitoring CsA over C0 in terms of patient outcomes”.54 Another lung transplant study concluded: “monitoring either the C2 or C6 concentration did not give a better indication of response to CsA therapy in patients with lung transplants compared with C0”.64
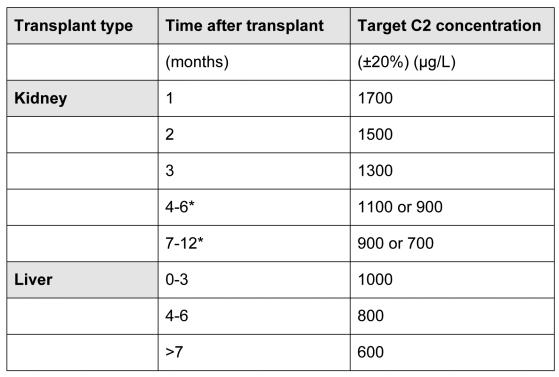
The decision whether to change to C2, or not, will typically be made by the transplant physicians and surgeons, and CsA laboratories need to be prepared to receive blood specimens based on a variety of sampling strategies for several more years until this is resolved. One of the key guides to these future directions could be the results of current international prospective clinical trials in kidney [MO2ART (Monitoring Of 2-hours Absorption in Renal Transplantation)] and liver [LIS2T (Liver International Study of 2-hour Neoral versus Tacrolimus-C0)] transplant recipients involving 30 centres in 10 countries. The target C2 concentrations being tested in these trials are shown in Table 1.
Table 1.
Target C2 CsA concentrations evaluated in MO2ART (renal) and LIS2T (hepatic) prospective, randomised, parallel-group, open-label international clinical trials.34
patients are divided 1:1 at 90 days post-transplantation in these 2 groups with different C2 targets.
Preliminary data from MO2ART in the first 277 patients enrolled indicated that those with immediate graft function (n=182) had a 9.7% incidence of biopsy-proven acute rejection (BPAR) 3-months after grafting, compared with 16.5% in those with delayed graft function (n=91, time to graft function was unknown in 4 patients).65 These data compared very favourably with other studies that explored C0 monitoring and/or lower CsA exposure. For example, the BPAR incidence was 45% in 55 de novo renal transplant recipients followed for 3 months where AUC0-4 was <4400 μg.h/L, and 58% in 38 de novo renal transplants with C2 concentrations below 1500 μg/L (compared with 0% of those >1500 μg/L).29,50 In this MO2ART interim report, 60% of immediate graft function patients had achieved the CsA C2 target concentration (1700 μg/L ±20%) by day 5, compared with only 20.2% of the delayed function group.65 The safety profile was considered comparable to that of a 'standard' transplant population, including no evidence of such CsA adverse effects as nephrotoxicity (median creatinine 132μmol/L, although the delayed function group took longer to recover their serum creatinine levels), and hypertension (mean±SD of 136±17mmHg systolic, 82±10mmHg diastolic).
CsA Assays
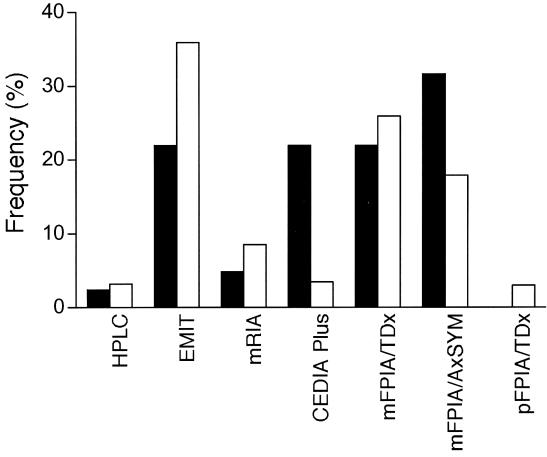
Whilst CsA can be measured using chromatographic approaches [HPLC/UV or LCMS(MS)], these have proved unpopular internationally, with <5% of laboratories in the International CsA Proficiency Testing Program (www.bioanalytics.co.uk) employing them. In Australasia, HPLC is used by only one laboratory which also has the largest CsA service in the region. The vast majority of laboratories use one of the commercial CsA immunoassays (Figure 1 and Table 2).19
Figure 1.
Shows the distribution of methods used in laboratories across Australasia in year 2000 in filled columns, as reported in a survey19 where 41 of 44 laboratories (93%) responded. The unfilled columns are shown for comparison from 431 laboratories reporting to the International Proficiency CsA Testing Scheme at this same time (December 2000) (www.bioanalytics.co.uk). This figure was adapted19 and reproduced with permission of Lippincott Williams & Wilkins, publishers of the journal Therapeutic Drug Monitoring.
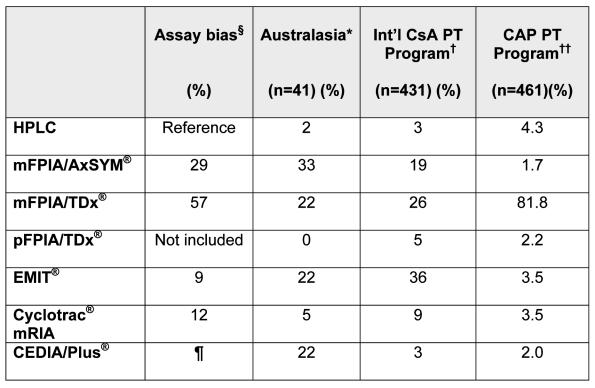
Table 2.
Shows the frequency (%) of CsA assay methods used in an Australasian survey19, compared with the International CsA Proficiency Testing Program†, and the CAP Program††, as related to the assay bias compared with a specific HPLC method 67.
as described by Steimer 67
total of 106% reflects that some labs had changed methods during year 2000 and so counted twice.
International Cyclosporin Proficiency Testing program, Analytical Services International, London, www.bioanalytics.co.uk.
Data both published10 and by personal communication from Dr Steven Soldin, Washington DC, USA.
The Steimer paper 67 described the original Cedia® method as having a bias of 18%; however, the current version, Cedia Plus®, compares more favourably with EMIT® and Cyclotrac® mRIA.
When developing immunoassays, the particular challenge to diagnostic manufacturers has been to develop antibodies that were selective for parent CsA, without significant cross-reactivity with CsA-metabolites which are inactive, or relatively so. Metabolites AM1 and AM9 have around 14–16% of the biological activity of parent CsA.7,8,66 Arguably, the area of greatest concern from the clinical perspective is the ability of these immunoassays to determine the concentration of the pharmacologically active parent CsA, without clinically significant cross-reactivity from CsA-metabolites causing a variable bias in the result generated. For, without a clear guide as to the active parent CsA concentration, dosage individualisation for the organ transplant recipient must be far from optimal. So if an interfering CsA-metabolite is present in significant concentrations in patient samples, then it poses a major source of potential bias in a given method. This explains at least one problem with the mFPIA® methods (discussed further below) as they do have greater cross-reactivity with AM1 than other methods tested.67 This CsA-metabolite has been shown to be present in significant concentrations in patient samples, even exceeding parent CsA.10 This issue of CsA immunoassays is so significant that some authors have expressed concern about the ability of several immunoassays to meet acceptable standards at all.68
In one study, using C0 samples from kidney and liver transplant recipients (n=145), the performance characteristics of immunoassays, including most of those currently available, were compared with a specific HPLC method.67 The least specific of those tested were the mFPIA® assays on the TDx® and the AxSYM® analysers (Abbott Diagnostics Division, Abbott Park, Illinois, USA). Thus, these methods are not accurate despite having the best precision of those tested. As shown in Table 2, these two mFPIA® assays were rated in that study as having a mean bias (range) of 57% (20 to 174%) and 29% (2 to 130), respectively. The other immunoassays tested showed the following mean biases (range): EMIT® (Dade Behring, Cupertino, California, USA) 12% (−7% to 53%), CEDIA® (Microgenics, Pleasanton, California, USA) 18% (−3% to 81%). It should be noted that an improved CEDIA Plus® method has subsequently become available from Microgenics that compares more favourably with EMIT® and Cyclotrac® mRIA (Diasorin, Stillwater, Minnesota, USA). Perhaps not surprisingly, the higher the mean bias for a method, the greater the range of variability observed. Steimer concluded that: “because assay bias cannot be predicted in individual samples, substantially erratic CsA dosing can result. The specificity of CsA assays for parent CsA remains a major concern”.67
As also shown in Table 2, another very recent study from over 600 laboratories in the College of American Pathologists' (CAP) Proficiency Testing program has shown that the dominant method among participants in that largely North American program was this problematic mFPIA/TDx® method (>80% of participating laboratories).10 This study suggested that the mFPIA® also appears to have a calibration problem, in addition to the metabolite cross-reactivity problem discussed above and demonstrated again in this study, as CsA-spiked samples (with no metabolites present) showed varying method-related biases. This study showed that, when referenced to mean LCMS data from several centres, results from HPLC laboratories were approximately 8–15% higher; EMIT® and Cyclotrac® mRIA 0–12% higher; mFPIA® (TDx®/AxSYM®) 22–28% higher; and pFPIA® (TDx®) 50–68% higher. These authors concluded that “there is a significant lack of specificity with the most commonly used immunoassays (viz, mFPIA®) for the measurement of CsA, which could significantly affect the correct interpretation of the patient's drug levels and lead to less than optimal outcome”; and, perhaps optimistically, that “the results of this study will better enable laboratory directors to understand CsA testing and the shortcomings of their particular assay.”
These two studies are consistent with others in the literature, including at least two early Australian studies, and this bias (at least with the mFPIA®/TDx® method) has been known for more than a decade.69,70 Ironically, however, one can also appreciate that there would be minimal incentive for this manufacturer to invest to improve their product when they enjoy such a significant market share, especially with >80% of the lucrative North American market. The mFPIA®/AxSYM® method that uses the same antibody as the mFPIA®/TDx® method (personal communication from Abbott Diagnostic Division) appears to have only partially resolved the bias. This limited improvement is reported by the manufacturer to be due to a change in the blood sample preparation.60,67,71 While data for this method has been questioned, it still has a clinically significant bias.60,67,71
Obviously, given the large variability associated with the bias of various methods, it is also inappropriate to adopt "correction factors" to normalise immunoassay mean bias to a chromatographic method, either in relation to the assay result, or in proposing alternative method-related target concentration ranges. One cannot simply apply constant 'fudge factors' to correct for CsA method discrepancies.
Consistent with a warning given not to presume that the CsA-metabolite fraction was constant across the CsA dosing interval (which obviously influences assay performance criteria), it has been recently suggested that the ratio of interfering CsA-metabolites compared with parent CsA at C2 appears to be much less than at C0.72 Two independent studies have demonstrated this using LCMSMS as the reference method.4,73 One of these, currently only available in abstract form, suggested that the fraction of the major interfering CsA-metabolite (AM1) at C2 was about one sixth that at C0.73 The implication is that even the less specific mFPIA® immunoassays compared more favourably with LCMSMS at C2 and so provided a more reliable index of circulating active CsA than they do with C0 samples. One might well speculate whether this improved assay performance with C2 samples has also contributed to the apparent improvement in clinical outcomes observed in transplant patients as a direct result of more accurate patient CsA dosage individualisation, in addition to the pharmacodynamic issues of calcineurin inhibition relationships discussed above.57,58 However, given that most centres are measuring combinations of C0 and C2 samples, there is still a clear imperative to use more specific assays.19 This observation has implications for AUC0-4 monitoring protocols, as the CsA-metabolite fraction would change from one time point to the next, and so the application of the various algorithms could also be method dependent. It would be highly desirable to see additional published data on this feature.
Laboratory Surveys
If laboratories had appropriate method selection criteria, and chose from the range of superior methods available, then there should not be a problem. However, the Australasian survey showed that 54% of the Australasian laboratories that responded (being 41 of 44 laboratories surveyed) were indeed using these two least selective mFPIA® assays (on TDx® and AxSYM® analysers) in year 2000 (Figure 1 and Table 1).19 Data from the International CsA Proficiency Testing Program (www.bioanalytics.co.uk), suggest that the Australasian situation with these two methods was comparable to the 431 mostly European CsA-laboratories in that Program at that time (Figure 1 and Table 1). Indeed these figures are still comparable (www.bioanalytics.co.uk), as 22.6 and 19.4% of centres were using the TDx® or AxSYM® mFPIA® methods respectively in December 2002 (Figure 2). This suggests that approximately half the laboratories in this PT-Program are not selecting their CsA method based on specificity for the intended analyte, (ie. parent CsA), which remains a major concern.
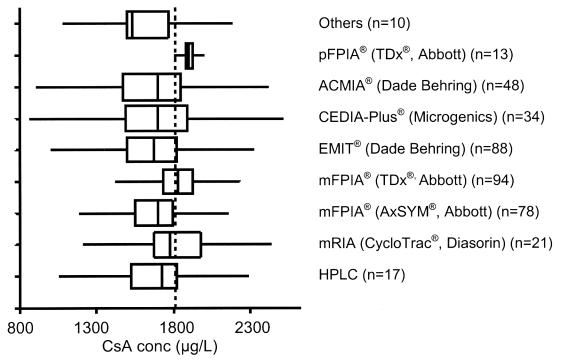
Figure 2.
Shows the results (as box-and-whisker plots) from one sample (225A) distributed in December 2002 from the International CsA Proficiency Testing Scheme where the CsA concentration exceeded the calibration range for most CsA immunoassay methods. The weighed-in concentration was 1800 μg/L (dashed vertical line). This illustrates the diversity of results returned from the 403 laboratories, regardless of assay method, and highlights the issues with performing accurate dilutions as recommended48 and discussed in the text. The line across the box represents the median, the lower and upper concentrations of the box represent the first and third quartile (25th and 75th percentile) respectively; and the lines extending beyond the box (the whiskers) represent the regions that are 1.5 times the interquartile range above and below the third and first quartiles.
This (unpublished) data is publicly available on the International CsA Proficiency Testing Scheme web-site (www.bioanalytics.co.uk).
Interestingly, the Australasian survey also showed that only 71% of the laboratories in this region participated in this International CsA Proficiency Testing (PT) program from London (www.bioanalytics.co.uk).19 The other 29% were not participating in PT programs at all.
Accreditation
The above data pose several questions, including, how regulatory authorities, such as FDA, approved such biased methods and why laboratory accreditation authorities aren't more insistent on requiring better method selection protocols and participation in PT-Programs. In relation to the latter issue, recent communication with the Australian accrediting authority (National Association of Testing Authorities/jointly with the Royal College of Pathologists of Australia, NATA/RCPA) has confirmed that they only require participation in a PT program where this program is available within Australia that they can assess and (preferably) accredit. Some might see this as a particular weakness requiring urgent attention. There is a need to recognise 'acceptable' international PT programs, possibly by undertaking memoranda of understanding with international accrediting authorities.
The second accreditation issue is that of the "expert peer review" system intrinsic to such NATA/RCPA accreditation. One can easily envisage a scenario where the technical assessors reviewing a general biochemistry laboratory that performs CsA testing may have limited expertise in pharmacological testing (such as with immunosuppressant drugs), possibly no more than the staff of the laboratory, and so not be in a strong position to comment on the laboratory's method selection criteria, or lack of PT program participation. Hence accreditation may be granted with no requirement or recommendation for improvement given.
Some centres (both public and privately operated), perhaps driven by cost pressures, operate by offering what may be the minimal service that would "survive" an accreditation assessment, rather than what may be most scientifically/clinically appropriate or valid in terms of patient outcomes. Obviously, poor pathology testing can result in poor clinical outcomes and so cost-cutting in pathology may result in significant and possibly greater cost increases in other areas, including; increased number and length of hospital admissions while drug dosages are optimised (possibly on arduous clinical grounds, due to the sub-optimal CsA concentration data reported), treatment of acute rejection episodes that may have been avoidable, increases in other morbidity and possibly loss of precious transplanted organs or even death. Although it is particularly difficult to prove such relationships in this complex area, the reader will understand the sentiments expressed.
Assay Dilution Protocols
The introduction of C2 monitoring over the past 5 years has also exposed other issues within clinical laboratories. Most of the commercial immunoassays have calibration ranges that were designed for the lower CsA concentrations associated with C0 monitoring. However, measuring the now frequent number of higher concentrations expected in the hours after the CsA dose, as with AUClss or C2 monitoring strategies, coupled with new clinical strategies of aiming for greater CsA exposure overall in the early post-transplant period, has meant that measured CsA concentrations now often exceed the upper calibration limit for some immunoassays, requiring the laboratory to perform a dilution. The data from the International CsA Proficiency Testing Program has repeatedly demonstrated that laboratories are generally not performing nearly well enough when dilutions are required. Even when laboratories were sent an unblinded sample with the stated concentration of 2000 μg/L, the data reported back varied from around 1000 to 3800 μg/L.72 This problem has been demonstrated again with a sample circulated in December 2002 (specimen 225A, www.bioanalytics.co.uk), with widely varying data being reported regardless of assay method (Figure 2).
The Australasian laboratory survey asked laboratories about their dilution protocol.19 Clearly many laboratories had introduced alternative protocols to those recommended by their respective reagent manufacturer. One can easily accept that this has led to a loss of assay control for many laboratories and is the basis of much of the error seen. For this reason the revised Australasian CsA monitoring guidelines have reproduced and recommended the (presumably) validated dilution method provided by the manufacturers.48 However, one CsA reagent package insert had an error in the multiplication factor following the recommended dilution!74 Manufacturers are adapting their methods to accommodate such higher concentrations. For example, the CEDIA Plus® method is currently configured to give two calibration ranges to accommodate "low" (25 to 450 μg/L) or "high" (450 to 2000 μg/L) concentrations. Notwithstanding this, the latter method did not perform better in the December 2002 proficiency testing program sample discussed above (specimen 225A, www.bioanalytics.co.uk) (Figure 2), possibly suggesting that some laboratories are not using both calibration ranges and may be still diluting the sample and assaying against the low range. Alternatively, other problems may exist. This is currently being surveyed by this PT-Program and so explanatory data will follow. Other manufacturers are also developing alternative strategies to avert the need for manual dilutions. Hence, in the future the laboratory should rarely need to perform a dilution regardless of the method used.
CsA-Sparing Agents
The intentional use of metabolic inhibitors such as diltiazem and/or ketoconazole to reduce CsA dosage (and hence cost of this expensive drug) is practiced in over 50% of Australian renal transplant recipients and other transplant groups including heart and lung.75–81 This CsA-sparing agent usage has the potential to distort CsA-metabolite patterns as illustrated in a preliminary study based on the quantitative difference between a specific and non-specific mRIA giving an index of CsA-metabolites.82–84 A recent report has suggested that correlation between C2 and AUC0-5 had improved as more potent CsA-sparing agents such as diltiazem and ketoconazole were included with CsA.81 Whilst the reason for this is not clear, it is consistent with a sequential reduction in the CYP3A and/or P-gp barriers to CsA absorption.1–3 More research is needed in this area.
The use of diltiazem as a CsA-sparing agent has been criticised as use of a pharmaceutical for a non-therapeutic purpose, (ie. economic reason, apart from possibly some clinical benefit in averting CsA-induced hypertension). However, a recent report from the extensive ANZDATA Registry has indicated that there was a significant (approximately 5%) improvement in graft and patient survival at 5 years in the 57% of patients who also received diltiazem treatment out of the 3,913 kidney recipients reviewed.78 This strongly suggests that here may well be therapeutic benefits in addition to the CsA-sparing agent usage. Whilst this fraction (5%) may not seem very large, it is this long-term graft survival that poses some of the greatest challenges for transplantation medicine, despite all the drug developments in recent years. The precise mechanism(s) by which diltiazem improves long-term graft survival is not fully understood, but they may potentially include improvement in perfusion of the transplanted kidney by protecting from the vaso-constrictive (hypertensive) properties of CsA.85
Consensus Documents
Given the complexity of the issues discussed herein, a team representing each Australian State and New Zealand (so as to also reflect regional issues) was assembled to develop a revised CsA consensus monitoring guideline document.48 Laboratories less versed in these matters could simply adopt these guidelines which were distributed to all known Australasian CsA laboratories.48 If adopted, they will hopefully improve the quality of CsA assay delivery and are commended to the reader. These guidelines include core recommendations such as:
Laboratories should review their method selection criteria as a matter of urgency,
Laboratory reports should clearly state the sample type (C0, C2, other),
Systems should be in place to confirm that C2 samples are drawn within the recommended ±10min of 2hr post-dose time point and comment on reports where this target is not achieved,4
Clinicians should be provided access to complex target range data, clearly too complicated to print on a laboratory report,
Laboratories should use only validated dilution protocols, preferably those recommended by the manufacturer,
Laboratory reports should indicate the CsA assay method used in generating the result, and state its bias.67
Generic Formulations
For all of this 20 year history of CsA usage in Australia, the choice of CsA formulation has been simplified by there being only one product available. The only exceptions were transition periods following the introduction of "improved" formulations, the most recent example of which followed the change from Sandimmum® to Neoral® (both Novartis Pharmaceuticals, Basel, Switzerland).64,86,87 However, this situation is about to change with the approval on May 1, 2002, by the Australian Federal Government's Therapeutic Goods Administration (TGA, Canberra, ACT, Australia) of the first generic CsA formulation in this country, a hydrocolloidal dispersion, Cysporin® (Hexal Pharmaceuticals, Germany, distributed by Mayne Pharma, Melbourne, Australia).
The issue with such alternatives is whether they can be directly substituted for the established product or not. The TGA rules for generic substitution are consistent with international guidelines, including the European Agency for the Evaluation of Medicinal Products, Committee for the Proprietary Medicinal Products, Note for Guidance on the Investigation of Bioavailability and Bioequivalence (www.emea.eu.int/pdfs/human/ewp/140198en.pdf) and the FDA equivalent (www.fda.gov/cder/guidance/index.htm). Simplistically, this requires the generic product to be bioequivalent to the established product. This is accepted if the 90% confidence interval for relative bioavailability (both in rate and extent of absorption) of the two products (generic and reference) lies between 80% and 125% in healthy volunteers. One can argue that this approach may be overly simplistic in many situations, including the complexities of organ transplantation. Should there be bioequivalence testing in patients who may have complicated absorption issues, such as liver transplant recipients and cystic fibrosis patients with a transplant, before two immunosuppressants are accepted as 'bioequivalent'? This matter is considered in greater detail elsewhere, and there are few published data to guide.88–90 Suffice to say that caution needs be exercised when considering such changes and it should not be presumed that 2 CsA formulations are necessarily readily interchangeable, even if 'approved' by regulatory authorities. Any switch should be followed by careful blood CsA concentration monitoring (ie. at a new steady-state a few days after the change, and subsequently confirmed, including as clinically indicated) to ensure that the patient is not disadvantaged.
Service Quality versus Costs
Some years ago, a general survey of therapeutic drug testing laboratories in Australasia was undertaken to identify changes that occurred through the early 1990's, a period of great change in many clinical laboratories and centres.91 This survey demonstrated what we would now presume to be 'common knowledge'; that there had been a significant shift away from therapeutic drug assays being performed in 'specialised laboratories', such as clinical pharmacology laboratories, or in biochemistry laboratories under the guidance of senior scientific or medical staff with specialised knowledge in pharmacology, toward more generic routine high volume service laboratories which were "cost competitive", with services concentrated onto fewer analysers. This survey showed that this change typically included significant professional job shedding, especially at more senior scientific levels, in favour of staff with lower skills (and therefore salary). As a direct result, method decisions may no longer be made by appropriately skilled medical pathologists or senior medical scientists with the patient's interest foremost. One even hears of centres that have abrogated such core professional responsibility to accountants or business managers. Thus, in many cases, clinical laboratories have tended to become a "number factory" generating data at the lowest cost and highest speed, without opportunity (or even desire in some cases) for result interpretation. As a result, the quality of the data generated and their clinical value have suffered.
One might ask whether the difficult experience described above for the provision of quality CsA assays illustrates the end result of such laboratory cost-cutting agenda. Are transplant physicians and surgeons now also required to exercise professional responsibility over the pathology testing that they request and only send their specimens to laboratories that can provide data that are most useful for their patients, rather than acting on the reasonable assumption, as in the past, that the laboratory would have the expertise and the will to provide the best quality data available? Anecdotally, the author recently asked transplant physicians/surgeons in a specialised audience whether they knew what method was used to assay their CsA samples. The indication was that less than 5% knew, suggesting that the majority still believe that their local laboratory is providing data based on well considered methods. Perhaps this faith is currently misplaced, and physicians and surgeons need to "vote with their feet" by directing their samples to laboratories using appropriate methods. In this way they may focus their laboratory's attention on the scientific quality of the assays they provide, albeit for commercial reasons.
Further international consensus guidelines will be reported for CsA in the near future from the joint Working Party of the International Federation of Clinical Chemists (IFCC), and the International Association of Therapeutic Drug Monitoring & Clinical Toxicology (IATDMCT). An interim report is available, which will be followed by further guidelines for the other major immunosuppressant drugs.92
Conclusion
As data shown here have demonstrated, CsA monitoring strategies are undergoing a major review at this time. CsA measurements are not done nearly well enough on an international scale, especially in North America. Many laboratories are not meeting achievable quality goals for CsA measurement because of poor method selection, inappropriate work practices and a failure to participate in appropriate quality assurance programs. There is a need to review our strategies in order to deliver a quality service that could easily be the best in the World. Do our organ transplant recipients deserve any less?
References
- 1.Wu C-Y, Benet LZ, Hebert MH, Gupta SK, Rowland M, Gomez DY, Wacher VJ. Differentiation of absorption and first-pass gut and hepatic metabolism in humans: Studies with cyclosporine. Clin Pharmacol Ther. 1995;58:492–7. doi: 10.1016/0009-9236(95)90168-X. [DOI] [PubMed] [Google Scholar]
- 2.Zhang YC, Benet L. The gut as a barrier to drug absorption. Clin Pharmacokinet. 2001;40:159–68. doi: 10.2165/00003088-200140030-00002. [DOI] [PubMed] [Google Scholar]
- 3.Lown KS, Mayo RR, Leichtman AB, Hsiao H-L, Turgeon DK, Schmiedlin-Ren P, Brown MB, Guo W, Rossi SJ, Benet LZ, Watkins PB. Role of intestinal P-glycoprotein (mdr1) in interpatient variation in the oral bioavailability of cyclosporine. Clin Pharmacol Ther. 1997;62:248–60. doi: 10.1016/S0009-9236(97)90027-8. [DOI] [PubMed] [Google Scholar]
- 4.Kahan BD, Keown P, Levy GA, Johnston A. Therapeutic drug monitoring of immunosuppressant drugs in clinical practice. Clin Ther. 2002;24:330–50. doi: 10.1016/s0149-2918(02)85038-x. [DOI] [PubMed] [Google Scholar]
- 5.Kahan BD. Update on pharmacokinetic/pharmacodynamic studies with FTY720 and sirolimus. Ther Drug Monit. 2002;24:47–52. doi: 10.1097/00007691-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Nashan B. Early clinical experience with a novel rapamycin derivative. Ther Drug Monit. 2002;24:53–8. doi: 10.1097/00007691-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Schlitt HJ, Christians U, Bleck J. Contribution of cyclosporin metabolites to immunosuppression in liver-transplanted patients with severe graft dysfunction. Transpl Int. 1991;4:38–44. doi: 10.1007/BF00335514. [DOI] [PubMed] [Google Scholar]
- 8.Rosano T, Freed B, Cerill J. Immunosuppressive metabolites of cyclosporine in blood of renal allograft recipients. Transplantation. 1986;42:262–7. doi: 10.1097/00007890-198609000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Copeland KR, Yatscoff RW, McKenna RM. Immunosuppressive activity of cyclosporine metabolites compared and characterized by mass spectrometry and nuclear magnetic resonance. Clin Chem. 1990;36:225–9. [PubMed] [Google Scholar]
- 10.Soldin SJ, Steele BW, Witte DL, Wand E, Elin RJ. Lack of specificity of cyclosporine immunoassays. Arch Pathol Lab Med. 2003;127:19–22. doi: 10.5858/2003-127-19-LOSOC. [DOI] [PubMed] [Google Scholar]
- 11.Holt DW, Johnston A. Cyclosporin microemulsion. A guide to use and monitoring. BioDrugs. 1997;7:175–97. doi: 10.2165/00063030-199707030-00002. [DOI] [PubMed] [Google Scholar]
- 12.Morris RG, Tett SE, Ray JE. Cyclosporin-A monitoring in Australia: Consensus recommendations. Ther Drug Monit. 1994;16:570–6. doi: 10.1097/00007691-199412000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Oellerich M, Armstrong VW, Kahan B, Shaw L, Holt DW, Yatscoff R, Lindholm A, Halloran P, Gallicano K, Wonigeit K, Scheutz E, Schran H, Annesley T. Lake Louise consensus meeting on cyclosporin monitoring in organ transplantation: Report of the consensus panel. Ther Drug Monit. 1995;17:642–54. doi: 10.1097/00007691-199512000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Oellerich M, Armstrong VW, Schutz E, Shaw LM. Therapeutic drug monitoring of cyclosporine and tacrolimus. Update on Lake Louise consensus conference on cyclosporin and tacrolimus. Clin Biochem. 1998;31:309–16. doi: 10.1016/s0009-9120(98)00049-6. [DOI] [PubMed] [Google Scholar]
- 15.Shaw LM, Bowers L, Demers L, Freeman D, Moyer T, Sanghvi A, Seltman H, Venkataramanan R. Critical issues in cyclosporin monitoring: Report of the task force on cyclosporin monitoring. Clin Chem. 1987;33:1269–88. [PubMed] [Google Scholar]
- 16.Shaw LM, Yatscoff RW, Bowers LD, Freeman DJ, Jeffery JR, Keown PA, McGilveray IL, Rosano TG, Wong PY. Canadian consensus meeting on cyclosporine monitoring: report of the consensus panel. Clin Chem. 1990;36:1841–6. [PubMed] [Google Scholar]
- 17.Kahan BD, Shaw LM, Holt D, Grevel J, Johnston A. Consensus Document: Hawk's Cay meeting on therapeutic drug monitoring of cyclosporine. Clin Chem. 1990;36:1510–6. [PubMed] [Google Scholar]
- 18.Johnston A, Sketris I, Marsden JT. A limited sampling strategy for the measurement of cyclosporine AUC. Transplant Proc. 1990;22:1345–6. [PubMed] [Google Scholar]
- 19.Morris RG, Lam AK. Cyclosporin monitoring in Australasia - survey of laboratory practices in 2000. Ther Drug Monit. 2002;24:471–8. doi: 10.1097/00007691-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Humbert H, Vernillet L, Cabiac MD, Barradas J, Billaud E. Influence of different parameters for the monitoring of cyclosporin. Transplant Proc. 1990;22:1210–5. [PubMed] [Google Scholar]
- 21.Kahan BD, Grevel J. Optimisation of cyclosporin therapy in renal transplantation by a pharmacokinetic strategy. Transplantation. 1988;46:631–44. doi: 10.1097/00007890-198811000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Grevel J, Kahan BD. Area under curve monitoring of cyclosporin therapy: the early post-transplant period. Ther Drug Monit. 1991;13:89–95. doi: 10.1097/00007691-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Grevel J, Welsh MS, Kahan BD. Cyclosporin monitoring in renal transplantation: area under the curve monitoring is superior to trough-level monitoring. Ther Drug Monit. 1989;11:252–66. [PubMed] [Google Scholar]
- 24.Cantarovich F, Bizollon CH, Cantarovich D, Lafrancois N, Dubenard JM, Traeger J. Cyclosporin plasma levels six hours after oral administration: A useful tool for monitoring therapy. Transplantation. 1988;45:389–94. doi: 10.1097/00007890-198802000-00029. [DOI] [PubMed] [Google Scholar]
- 25.Keown P, Kahan BD, Johnston A. Optimization of cyclosporin therapy with new therapeutic drug monitoring strategies. Transplant Proc. 1998;30:1645–9. doi: 10.1016/s0041-1345(98)00375-3. [DOI] [PubMed] [Google Scholar]
- 26.Belitsky P, Dunn S, Johnston A, Levy G. Impact of absorption profiling on efficacy and safety of cyclosporin therapy in transplant recipients. Clin Pharmacokinet. 2000;39 (2):117–25. doi: 10.2165/00003088-200039020-00003. [DOI] [PubMed] [Google Scholar]
- 27.Belitsky P, Levy GA, Johnston A. Neoral absorption profiling: An evolution in effectiveness. Transplant Proc. 2000;32:45S–52S. doi: 10.1016/s0041-1345(00)00863-0. [DOI] [PubMed] [Google Scholar]
- 28.Mahalati K, Belitsky P, Sketris I, West K, Panek R. Neoral monitoring by simplified sparse sampling area under the concentration-time curve. Transplantation. 1999;68:55–62. doi: 10.1097/00007890-199907150-00011. [DOI] [PubMed] [Google Scholar]
- 29.Mahalati K, Belitsky P, West K, Kieberd B, Fraser A, Sketris I, MacDonald AS, McAlister V, Lawen J. Approaching the therapeutic window for cyclosporine in kidney transplantation: A prospective study. J Am Soc Nephrol. 2001;12:828–33. doi: 10.1681/ASN.V124828. [DOI] [PubMed] [Google Scholar]
- 30.Mahalati K, Belitsky P, West K, MacDonald A, McAlister V, Lawen J. A 3-hour postdose cyclosporin level during the first week after kidney transplantation predicts acute rejection and cyclosporine nephrotoxicity more accurately than trough levels. Transplant Proc. 2000;32:786–7. doi: 10.1016/s0041-1345(00)00982-9. [DOI] [PubMed] [Google Scholar]
- 31.Levy G, Burra P, Cavallari A, Duvoux C, Lake J, Mayer AD, Mies S, Pollard SG, Varo E, Villamil F, Johnston A. Improved clinical outcomes for liver transplant recipients using cyclosporin monitoring based on 2-hr post-dose levels (C2) Transplantation. 2002;73:953–9. doi: 10.1097/00007890-200203270-00022. [DOI] [PubMed] [Google Scholar]
- 32.Levy GA, Lake JR, Beauregard-Zollinger L, Prestele H. Improved clinical outcomes for liver transplant recipients using cyclosporine blood level monitoring based on two-hour post-dose levels (abstract) Transplantation. 2000;69:S387. doi: 10.1097/00007890-200203270-00022. [DOI] [PubMed] [Google Scholar]
- 33.Levy GA, O'Grady C, Lilly LB, Grant D, Girgrah N, Greig PD. Conversion to C2 cyclosporine monitoring using Neoral immunosuppression in maintenance liver transplant patients improvement in renal function and hypertension (abstract). Proc Annual Congress Am Soc Transpl 2001;
- 34.Levy GA. C2 monitoring for optimising cyclosporin immunosuppression from the Neoral formulation. BioDrugs. 2001;15:279–90. doi: 10.2165/00063030-200115050-00001. [DOI] [PubMed] [Google Scholar]
- 35.Cole EH. Neoral Monitoring: Limitations of trough level monitoring and the potential role of limited sampling strategies. Transplant Proc. 2000;32:1556–58. doi: 10.1016/s0041-1345(00)01338-5. [DOI] [PubMed] [Google Scholar]
- 36.Barama A, Perner F, Beauregard-Zollinger L, Prestele H. Absorption profiling of cyclosporine therapy de novo kidney transplantation: A prospective, randomised study comparing sparse sampling to trough monitoring. Transplantation. 2000;69:S162–3. [Google Scholar]
- 37.Cole E, Keown P, Landsberg D, Halloran P, Shoker A, Rush D, Jeffrey J, Russell D, Stiller C, Calvin M, Norman P, Paul L, Zaltzman J, Loertscher R, Daloze P, Dandavino R, Boucher A, Handa P, Lawen J, Belitsky P, Parfrey P, Tan A, Hendricks L. Safety and tolerability of cyclosporin and cyclosporin micremulsion during 18 months of follow-up in stable renal transplant recipients: A report of the Canadian Neoral Study Group. Transplantation. 1998;65:505–10. doi: 10.1097/00007890-199802270-00009. [DOI] [PubMed] [Google Scholar]
- 38.Cole E, Midtvedt K, Johnston A, Pattison J, O'Grady C. Recommendations for the implementation of Neoral C2 monitoring in clinical practice. Transplantation. 2002;73:S19–S22. doi: 10.1097/00007890-200205151-00004. [DOI] [PubMed] [Google Scholar]
- 39.Levy G, Thervet E, Lake J, Uchida K. Patient management by Neoral C2 monitoring: An international consensus statement. Transplantation. 2002;73:S12–S18. doi: 10.1097/00007890-200205151-00003. [DOI] [PubMed] [Google Scholar]
- 40.Nashan B, Cole E, Levy G, Thervet E. Clinical validation studies of Neoral and C2 monitoring: A review. Transplantation. 2002;73:S3–S11. doi: 10.1097/00007890-200205151-00002. [DOI] [PubMed] [Google Scholar]
- 41.Johnston A, Kovarik JM, Mueller, Holt DW. Predicting patients' exposure to cyclosporin. Transplant Internat. 1996;9:S305–7. doi: 10.1007/978-3-662-00818-8_75. [DOI] [PubMed] [Google Scholar]
- 42.Melter M, Rodeck B, Kardoff R, Hoyer PF, Brodehl J. Pharmacokinetics of cyclosporine in pediatric long-term liver transplant recipients converted from Sandimmun to Neoral. Transplant Internat. 1997;10:419–25. doi: 10.1007/s001470050080. [DOI] [PubMed] [Google Scholar]
- 43.Marsh CL. Abbreviated pharmacokinetic profiles in area-under-the-curve monitoring of cyclosporine therapy in de novo renal transplant patients treated with Sandimmune or Neoral. Ther Drug Monit. 1999;21:27–34. doi: 10.1097/00007691-199902000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Johnston A, David OJ, Cooney GF. Pharmacokinetic validation of Neoral absorption profiling. Transplant Proc. 2000;32 (suppl 3A):53S–6S. doi: 10.1016/s0041-1345(00)00864-2. [DOI] [PubMed] [Google Scholar]
- 45.Cantarovich M, Besner JG, Barkun JS, Elstein E, Loertscher R. Two hour cyclosporin level determination is the appropriate tool to monitor Neoral therapy. Clin Transplant. 1998;12:243–9. [PubMed] [Google Scholar]
- 46.Cantarovich M, Elstein E, de-Varennes B, Barkun JS. Clinical benefit of Neoral dose monitoring with cyclosporine 2-hr post-dose levels compared with trough levels in a stable heart transplant patients. Transplantation. 1999;68:1839–42. doi: 10.1097/00007890-199912270-00003. [DOI] [PubMed] [Google Scholar]
- 47.Morris RG, Russ GR, Cervelli MJ, Juneja R, McDonald SP, Mathew TH. Comparison of trough, 2-hour, and limited AUC blood sampling for monitoring cyclosporin (Neoral®) at day 7 post-renal transplantation and incidence of rejection in the first month. Ther Drug Monit. 2002;24:479–86. doi: 10.1097/00007691-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 48.Morris RG, Ilett KF, Tett SE, Ray JE, Fullinfaw RO, Cooke R, Cook S. Cyclosporin monitoring in Australasia: 2002 update of consensus guidelines. Ther Drug Monit. 2002;24:677–88. doi: 10.1097/00007691-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 49.Dotti G, Gaspari F, Carusso R, Perico N, Remuzzi G, Barbui T, Rambaldi A. Pharmacokinetic study of the new cyclosporin-A formulation (Neoral™) in adult allogenic bone marrow transplant recipients. Haematologica. 2001;86:311–5. [PubMed] [Google Scholar]
- 50.CNRTSG. Absorption profiling of cyclosporine microemulsion (Neoral) during the first 2 weeks after renal transplantation. Transplantation. 2001;72:1024–32. doi: 10.1097/00007890-200109270-00008. [DOI] [PubMed] [Google Scholar]
- 51.INRTSG. Randomised, international study of cylosporine microemulsion absorption profiling in renal transplantation with Basiliximab immunoprophylaxis. Am J Transplant. 2002;2:157–66. doi: 10.1034/j.1600-6143.2002.020207.x. [DOI] [PubMed] [Google Scholar]
- 52.INRTSG. Cyclosporine microemulsion (Neoral®) absorption profiling and sparse-sample predictors during the first 3 months after renal transplantation. Am J Transplant. 2002;2:148–56. doi: 10.1034/j.1600-6143.2002.020206.x. [DOI] [PubMed] [Google Scholar]
- 53.David OJ, Johnston A. Limited sampling strategies for estimating cyclosporin area under the concentration-time curve: review of current algorithms. Ther Drug Monit. 2001;23:100–14. doi: 10.1097/00007691-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 54.Dumont RJ, Enson MHH. Methods for clinical monitoring of cyclosporin in transplant patients. Clin Pharmacokinet. 2000;38:427–47. doi: 10.2165/00003088-200038050-00004. [DOI] [PubMed] [Google Scholar]
- 55.Grant D, Kneteman N, Tchervenkov J. Peak cyclosporin levels (Cmax) correlate with freedom from liver graft rejection. Transplantation. 1999;67:1133–37. doi: 10.1097/00007890-199904270-00008. [DOI] [PubMed] [Google Scholar]
- 56.Cantarovich M, Barkun J, Besner J-G, Metrakos P, Alpert E, Deschenes M, Aalamain Z, Tchervenkov JI. Cyclosporine peak levels provide a better correlation with the are-under-the curve than trough levels in liver transplant patients treated with Neoral. Transplant Proc. 1998;30:1462–3. doi: 10.1016/s0041-1345(98)00316-9. [DOI] [PubMed] [Google Scholar]
- 57.Halloran PF, Helms LMH, Kung L, Noujaim J. The temporal profile of calcineurin inhibition by cyclosporine in vivo. Transplantation. 1999;68:1356–61. doi: 10.1097/00007890-199911150-00023. [DOI] [PubMed] [Google Scholar]
- 58.Batiuk TD, Pazderka F, Enns J, DeCastro L, Halloran PF. Cyclosporine inhibition of calcineurin activity in human lymphocytes in vivo is rapidly reversible. J Clin Invest. 1995;96:1254. doi: 10.1172/JCI118159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andrews DJ, Cramb R. Cyclosporin: revisions in monitoring guidelines and review of current analytical methods. Ann Clin Biochem. 2002;39:424–35. doi: 10.1258/000456302320314430. [DOI] [PubMed] [Google Scholar]
- 60.Morris RG. Cyclosporin assays, metabolite cross-reactivity, and pharmacokinetic monitoring. Ther Drug Monit. 2000;22:160–2. doi: 10.1097/00007691-200004000-00003. [DOI] [PubMed] [Google Scholar]
- 61.Oellerich M, Armstrong VW. Two-hour cyclosporine concentration determination: An appropriate tool to monitor Neoral therapy. Ther Drug Monit. 2002;24:40–6. doi: 10.1097/00007691-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 62.Armstrong VW, Oellerich M. New developments in the immunosuppressive drug monitoring of cyclosporine, tacrolimus, and azathioprine. Clin Biochem. 2001;34:9–16. doi: 10.1016/s0009-9120(00)00175-2. [DOI] [PubMed] [Google Scholar]
- 63.Keown PA. New concepts in cyclosporine monitoring. Curr Opin Nephrol Hypertens. 2002;11:619–26. doi: 10.1097/00041552-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 64.Trull A, Steel L, Sharples L, Stewart S, Parameshwar J, McNeil K, Wallwork J. Randomized, trough blood cyclosporine concentration-controlled trial to compare the pharmacodynamics of Sandimmune and Neoral in de novo lung transplant recipients. Ther Drug Monit. 1999;21:17–26. doi: 10.1097/00007691-199902000-00004. [DOI] [PubMed] [Google Scholar]
- 65.Toselli L, Pfeffer P, Stefoni S, Thervet E, Fornairon S, Keown P. Minimal rejection and excellent graft function by Neoral® C2 monitoring in renal transplantation: Interim results of MO2ART, a randomised prospective international study. XIX Internat Soc Organ Transplant 2002; poster presentation:
- 66.Yatscoff RW, Copeland KR, Faraci CJ. Abbott TDx monoclonal assay evaluated for measuring cyclosporine in whole blood. Clin Chem. 1990;36:1969–73. [PubMed] [Google Scholar]
- 67.Steimer W. Performance and specificity of monoclonal immunoassays for cyclosporin monitoring: How specific is specific? Clin Chem. 1999;45:371–81. [PubMed] [Google Scholar]
- 68.Scheutz E, Svinarov D, Shipkova M, Niedmann P-D, Armstrong VW, Wieland E, Oellerich M. Cyclosporin whole blood immunoassay (AxSYM, CEDIA, and EMIT); a critical overview of performance characteristics and comparison with HPLC. Clin Chem. 1998;44:2158–64. [PubMed] [Google Scholar]
- 69.Dusci LJ, Hackett LP, Chiswell GM, Ilett KF. Comparison of cyclosporine measurement in whole blood by high performance liquid chromatography, monoclonal fluorescence polarisation immunoassay and monoclonal enzyme multiplied immunoassay. Ther Drug Monit. 1992;14:327–32. doi: 10.1097/00007691-199208000-00012. [DOI] [PubMed] [Google Scholar]
- 70.Morris RG, Saccoia NC, Ryall RG, Peacock MK, Sallustio BC. Specific EMIT and FPIA assays for cyclosporin compared with Cyclotrac 125I-RIA. Ther Drug Monit. 1992;14:226–33. doi: 10.1097/00007691-199206000-00009. [DOI] [PubMed] [Google Scholar]
- 71.Wallemacq PE, Alexandre K. Evaluation of the new AxSYM cyclosporine assay: comparison with TDx monoclonal whole blood and EMIT cyclosporine assays. Clin Chem. 1999;45:432–35. [PubMed] [Google Scholar]
- 72.Holt DW, Johnston A, Kahan BD, Morris RG, Oellerich M, Shaw LM. New approaches to cyclosporine monitoring raise further concerns about analytical techniques. Clin Chem. 2000;46:872–4. [PubMed] [Google Scholar]
- 73.Scheutz E, Streit F, Dias VC. Immunoassays in comparison to LC-MS/MS, is there a difference between trough (C0) and C2 levels? Ther Drug Monit. 2001;23:471. [Google Scholar]
- 74.Abbott-Laboratories. Abbott AxSYM® system -cyclosporine. 1999; 69-1770/R3: 1–7.
- 75.Jones TE. The use of other drugs to allow a lower dosage of cyclosporin to be used. Clin Pharmacokinet. 1997;32:357–67. doi: 10.2165/00003088-199732050-00002. [DOI] [PubMed] [Google Scholar]
- 76.Jones TE, Morris RG. Survey of cyclosporin therapeutic ranges, assay methodology and use of 'sparing agents' in Australasian transplant centres. Ther Drug Monit. 1997;19:650–6. doi: 10.1097/00007691-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 77.Morris RG, Jones TE. Diltiazem disposition and metabolism in renal transplant recipients. Ther Drug Monit. 1998;21:365–70. doi: 10.1097/00007691-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 78.McDonald SP, Russ GR. Associations between use of cyclosporine-sparing agents and outcome in kidney transplant recipients. Kidney Internat. 2002;61:2259–65. doi: 10.1046/j.1523-1755.2002.00386.x. [DOI] [PubMed] [Google Scholar]
- 79.Chrysostomou A, Walker RG, Russ GR, d'Apice AJF, Kincaid-Smith P, Mathew TH. Use of diltiazem in cyclosporin-treated renal allograft recipients. Transplantation. 1993;55:300–4. doi: 10.1097/00007890-199302000-00014. [DOI] [PubMed] [Google Scholar]
- 80.Akhlaghi F, Keough AM, McLachlan AJ, Kaan A. Pharmacokinetics of cyclosporine in heart transplant recipients receiving metabolic inhibitors. J Heart Lung Transplant. 2001;20:431–8. doi: 10.1016/s1053-2498(00)00234-5. [DOI] [PubMed] [Google Scholar]
- 81.Ray JE, Keogh AM, McLachlan AJ, Akhlaghi F. Evaluation of cyclosporin concentration monitoring strategies in heart transplant recipients receiving metabolic inhibitors. J Heart Lung Transpl 2003; in press. [DOI] [PubMed]
- 82.Bleck JS, Thiesemann C, Kliem V, Christians U, Hecker H, Repp H, Frei U, Westhoff-Bleck M, Manns M, Sewing KF. Diltiazem increases the blood concentrations of cyclised cyclosporine metabolites resulting in different cyclosporine metabolite pattern in stable male and female renal allograft recipients. Br J Clin Pharmacol. 1996;41:551–6. doi: 10.1046/j.1365-2125.1996.34412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kunzedorf U, Walz G, Brockmoeller J, et al. Effects of diltiazem upon metabolism and immunosuppressive action of cyclosporine in kidney graft recipients. Transplantation. 1991;52:280–4. doi: 10.1097/00007890-199108000-00018. [DOI] [PubMed] [Google Scholar]
- 84.Foradori A, Mezzano S, Videla C, Pefaur J, Elberg A. Modification of the pharmacokinetics of cyclosporine A and metabolites by the concomitant use of Neoral and diltiazem or ketoconazole in the stable adult kidney transplants. Transplant Proc. 1998;30:1685–87. doi: 10.1016/s0041-1345(98)00393-5. [DOI] [PubMed] [Google Scholar]
- 85.Ruggenenti P, Perico N, Mosconi L, et al. Calcium channel blockers protect transplant patients from cyclosporine-induced daily renal hypoperfusion. Kidney Int. 1993;43:706–11. doi: 10.1038/ki.1993.101. [DOI] [PubMed] [Google Scholar]
- 86.Holt DW, Meuler EA, Kovarik JM, van-Bree JB, Richard F, Kutz K. Sandimmun Neoral pharmacokinetics: Impact of the new oral formulation. Transplant Proc. 1995;27:1434–7. [PubMed] [Google Scholar]
- 87.Rigotti P, Cadrocci R, Baldan N, Ferraresso M, Di-Landro D, Marchini M, Zaninotto G, Palatini P. Neoral versus Sandimmun in kidney-pancreas transplantation. Transplant Proc. 1997;29:2924–9. doi: 10.1016/s0041-1345(97)00731-8. [DOI] [PubMed] [Google Scholar]
- 88.Johnston A, Keown PA, Holt DW. Simple bioequivalence criteria: Are they relevant to critical dose drugs? Experience gained from cyclosporine. Ther Drug Monit. 1997;19:375–81. doi: 10.1097/00007691-199708000-00002. [DOI] [PubMed] [Google Scholar]
- 89.Christians U, First MR, Benet LZ. Recommendations for bioequivalence testing of cyclosporine generics revisited. Ther Drug Monit. 2000;22:330–45. doi: 10.1097/00007691-200006000-00017. [DOI] [PubMed] [Google Scholar]
- 90.Qazir YA, Forrest A, Tornatore KM, Schoenl M, Blas SD, Venuto RC. The clinical and economic impact of a 1:1 conversion from Neoral to Gengraf. 2002; P0505. [DOI] [PubMed]
- 91.Morris RG. Delivery of therapeutic drug monitoring services: survey of Australasian clinical pharmacology laboratories. Ther Drug Monit. 1998;20:598–601. doi: 10.1097/00007691-199812000-00002. [DOI] [PubMed] [Google Scholar]
- 92.Holt DW, Armstrong VW, Griesmacher A, Morris RG, Napoli KL, Shaw LM. International Federation of Clinical Chemistry/International Association of Therapeutic Drug Monitoring and Clinical Toxicology Working Group in immunosuppressive drug monitoring. Ther Drug Monit. 2002;24:59–67. doi: 10.1097/00007691-200202000-00011. [DOI] [PubMed] [Google Scholar]