Abstract
Magnesium is the fourth most abundant cation in the body and plays an important physiological role in many of its functions. Magnesium balance is maintained by renal regulation of magnesium reabsorption. The exact mechanism of the renal regulation is not fully understood. Magnesium deficiency is a common problem in hospital patients, with a prevalence of about 10%. There are no readily available and easy methods to assess magnesium status. Serum magnesium and the magnesium tolerance test are the most widely used. Measurement of ionised magnesium may become more widely available with the availability of ion selective electrodes.
Magnesium deficiency and hypomagnesaemia can result from a variety of causes including gastrointestinal and renal losses. Magnesium deficiency can cause a wide variety of features including hypocalcaemia, hypokalaemia and cardiac and neurological manifestations. Chronic low magnesium state has been associated with a number of chronic diseases including diabetes, hypertension, coronary heart disease, and osteoporosis. The use of magnesium as a therapeutic agent in asthma, myocardial infarction, and pre-eclampsia is also discussed.
Hypermagnesaemia is less frequent than hypomagnesaemia and results from failure of excretion or increased intake. Hypermagnesaemia can lead to hypotension and other cardiovascular effects as well as neuromuscular manifestations. Causes and management of hypermagnesaemia are discussed.
Introduction
Magnesium is the second most abundant intracellular cation and the fourth most abundant cation in the body. Magnesium plays an essential physiological role in many functions of the body (Table 1).1 This role is achieved through two important properties of magnesium; the ability to form chelates with important intracellular anionic-ligands, especially ATP, and its ability to compete with calcium for binding sites on proteins and membranes.2 Magnesium is essential for the synthesis of nucleic acids and proteins, for intermediary metabolism and for specific actions in different organs such as the neuromuscular and cardiovascular systems. Over 300 enzymes are dependent on magnesium. The Km for magnesium of many of these enzymes is near the intracellular free magnesium concentration. Magnesium influences the activity of enzymes by (i) binding to ligands such as ATP in ATP-requiring enzymes, (ii) binding to the active site of the enzyme, eg. enolase, pyruvate kinase, pyrophosphatase, (iii) causing a conformational change during the catalytic process, eg. Na+,K+-ATPase, (iv) promoting the aggregation of multi-enzyme complexes eg. aldehyde dehydrogenase, or (v) a mixture of the above mechanisms, eg. F1-ATPase.2
Table 1.
Physiological functions of magnesium
| Enzyme function | |
| Enzyme substrate (ATPmg, GTPmg) | |
| Kinases B | Hexokinase
Creatine kinase Protein kinase |
| ATPases or | |
| GTPases - | Na+,K+ -ATPase
Ca+, ATPase |
| Cyclases - | Adenylate cyclase
Guamylate cyclase |
| Direct enzyme activation | |
| Phosphofructokinase | |
| Creatine kinase | |
| 5-phosphoribosyl-pyrophosphate synthetase | |
| Adenylate cyclase | |
| Na+,K+-ATPase | |
| Membrane function | |
| Cell adhesion | |
| Transmembrane electrolyte flux | |
| Calcium antagonist | |
| Muscle contraction/relaxation | |
| Neurotransmitter release | |
| Action potential conduction in nodal tissue | |
| Structural function | |
| Protein | |
| Polyribosomes | |
| Nucleic acids | |
| Multiple enzyme complexes | |
| Mitochondria | |
By competing with calcium for membrane binding sites and by stimulating calcium sequestration by sarcoplasmic reticulum, magnesium helps to maintain a low resting intracellular free calcium ion concentration which is important in many cellular functions. The electrical properties of membranes and their permeability characteristics are also affected by magnesium.
Magnesium has important effects on the cardiovascular system. It affects myocardial contractility by influencing the intracellular calcium concentration and the electrical activity of myocardial cells and the specialised conducting system of the heart by its ability to influence movement of ions such as sodium, potassium and calcium across the sarcolemmal membrane.3 Magnesium may also affect the vascular smooth muscle tone. Magnesium has a key role in many other important biological processes such as cellular energy metabolism, cell replication, and protein synthesis.3
Magnesium Metabolism
Body content and distribution of magnesium
The normal adult human body contains approximately 1,000 mmols of magnesium (22–26 g).4 The distribution of magnesium within the body is shown in Table 2. About 60% of the magnesium is present in bone, of which 30% is exchangeable and functions as a reservoir to stabilise the serum concentration. About 20% is in skeletal muscle, 19% in other soft tissues and less than 1% in the extracellular fluid. Skeletal muscle and liver contain between 7–9 mmol/Kg wet tissue; between 20–30% of this is readily exchangeable. In normal adults, total serum magnesium ranges between 0.70 and 1.10 mmol/L. Approximately 20% of this is protein bound, 65% is ionised and the rest is complexed with various anions such as phosphate and citrate.4 Of the protein bound fraction, 60–70% is associated with albumin and the rest is bound to globulins.5 The reference range for serum ionised magnesium concentration (0.54–0.67 mmol/L) is narrower than that for calcium.4 Acid base disturbances (metabolic acidosis or alkalosis) have little effect on the distribution of serum magnesium. The concentration of magnesium in CSF is around 1.1 mmol/L, of which 55% is free and 45% is complexed with other compounds.6 The higher ultrafiltrable magnesium in CSF compared to serum is due to active transport of magnesium across the blood-brain barrier.
Table 2.
Distribution of magnesium in the adult human
| Tissue | Weight (Kg wet wt) | Concentration (mmol/Kg wet wt) | Content (mmol) | % of total body magnesium |
|---|---|---|---|---|
| Serum | 3.0 | 0.85 | 2.6 | 0.3 |
| Red blood cells | 2.0 | 2.5 | 5.0 | 0.5 |
| Soft tissue | 22.7 | 8.5 | 193.0 | 19.3 |
| Muscle | 30.0 | 9.0 | 270.0 | 27.0 |
| Bone | 12.3 | 43.2 | 530.1 | 52.9 |
| TOTAL | 70.0 | 1000.7 | 100 |
Intracellular magnesium is maintained within narrow concentration limits except in extreme situations such as hypoxia or prolonged magnesium depletion. Very little is known about the mechanisms involved in the regulation of intracellular magnesium.7 Free ionised magnesium constitutes only 0.5–5% of the total cellular magnesium; the remainder is bound to anionic compounds such as ATP, ADP, citrate, proteins, RNA and DNA or is sequestered within mitochondria and endoplasmic reticulum. The concentration of free magnesium within the cell measured using fluorescent dye is about 0.5 mmol/L.8 However the measured concentration of free magnesium varies with the cell type and technique used. The distribution of magnesium within the cell is heterogenous, with lower concentrations in the peripheral regions of the cytoplasm than in the perinuclear region.7
The rate of magnesium transport across cell membranes varies in different cell types, being higher in heart, liver and kidney and lower in skeletal muscle, red cells and brain. Intracellular magnesium is also higher in rapidly proliferating cells indicating that cellular magnesium transport is linked to metabolic activity of the cell.9
Magnesium balance
The recommended daily allowance (RDA) for magnesium in adults is 4.5 mg/Kg/day, lower than the previous recommendation of 6–10 mg/Kg/day.4 The daily requirement is higher in pregnancy, lactation and following debilitating illness. Recent dietary surveys show that the average intake in many western countries is less than the RDA.4
Magnesium intake depends on the magnesium concentration in drinking water, and food composition. Magnesium is plentiful in green leafy vegetables (which are rich in magnesium-containing chlorophyll) cereal, grain, nuts and legumes. Chocolates, vegetables, fruits, meats and fish have intermediate values, and dairy products are poor in magnesium. Drinking water can be an important source of magnesium, especially ‘hard water’ which contains up to 30 mg/L of magnesium. In general the intake of magnesium is directly related to energy intake except when the majority of the energy comes from refined sugars or alcohol. Refining or processing of food may deplete magnesium content by nearly 85%. Furthermore, cooking, especially boiling of magnesium-rich foods, will result in significant loss of magnesium.10 The processing and cooking of food may therefore explain the apparently high prevalence of low magnesium intake in many populations.
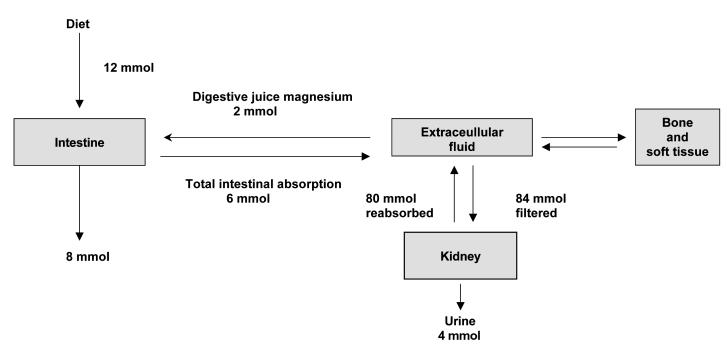
Although plasma magnesium concentration is kept within narrow limits, the exact physiological mechanisms which regulate this are not fully understood.4,11 Figure 1 illustrates the metabolism of magnesium in a healthy adult. The average magnesium intake of a normal adult is approximately 12 mmol/day. In addition to this, approximately 2 mmol/day of magnesium is secreted into the intestinal tract in bile and pancreatic and intestinal juices. From this pool 6 mmol (about 30%) is absorbed giving a net absorption of 4 mmol/day.
Figure 1.
Distribution of Magnesium in the body
Fractional intestinal absorption of magnesium is inversely related to intake; 65% at low intake and 11% at high intake. Most of the absorption occurs in the ileum and colon. At normal intakes absorption is primarily passive. During low magnesium intake a saturable component of magnesium absorption can be demonstrated.12 Factors controlling magnesium absorption are not well understood. Studies suggest a role for parathyroid hormone (PTH) in regulating magnesium absorption, but the role of vitamin D and its active metabolite 1,25 dihydroxyvitamin D is more controversial. Phytates in the diet bind to magnesium and impair its absorption. However the quantities present in normal diet do not affect magnesium absorption. Other dietary factors that are thought to affect magnesium absorption are oxalate, phosphate, proteins, potassium and zinc.
The kidney plays a major role in magnesium homeostasis and the maintenance of plasma magnesium concentration. Under normal circumstances, when 80% of the total plasma magnesium is ultrafiltrable, 84 mmol of magnesium is filtered daily and 95% of this reabsorbed leaving about 3–5 mmol to appear in the urine. Approximately 15–20% of filtered magnesium is reabsorbed in the proximal tubular segments, 65–75% in TALH and the rest in the distal segments.8,13 Magnesium transport in the proximal tubule appears to be primarily a uni-directional passive process depending on sodium/water reabsorption and the luminal magnesium concentration. Magnesium transport in TALH is directly related to sodium chloride reabsorption and the positive luminal voltage in the segment.7 In TALH, approximately 25% of the filtered sodium chloride is reabsorbed through the active transcellular transport, (sodium-chloride-potassium transport) and passive paracellular diffusion.14 This creates a favourable luminal positive potential at TALH where most of the magnesium is reabsorbed. Magnesium reabsorption is also inversely related to the rate of fluid flow in the tubular lumen. Reabsorption in the distal convoluted tubule is active and transcellular.15
Factors influencing renal magnesium excretion are listed in Table 3. The plasma magnesium concentration is a major determinant of urinary magnesium excretion. Hypermagnesaemia is associated with an increase in magnesium excretion due to an increase in the filtered load and reduced reabsorption in TALH.
Table 3.
Factors affecting tubular reabsorption of magnesium
| Plasma magnesium concentration/magnesium status |
| Glomerular filtration rate |
| Volume status |
| Hormones |
| PTH |
| Calcitonin |
| Antidiuretic hormone |
| Glucagon |
| Insulin |
| Phosphate depletion |
| Acid base status |
| Hypercalcaemia |
| Diuretics |
| Miscellaneous factors |
When dietary intake of magnesium is reduced, fractional excretion may decrease to <0.5% of the filtered load mainly due to increased reabsorption in TALH. The mechanism of this adaptation is not well understood.3 An increase in the filtered load of magnesium due to an increase in GFR, without change in the plasma concentration, may also affect tubular handling of magnesium. Chronic renal failure is associated with reduced fractional reabsorption in the remaining functional nephrons, and plasma magnesium concentration is maintained until the end-stage. The mechanism underlying this adaptive response is uncertain. Extracellular fluid volume expansion increases magnesium excretion due to increased delivery of sodium and water to the TAHL causing a decrease in magnesium reabsorption.
No single hormone has been shown to be specifically related to magnesium homeostasis. Several hormones including PTH, antidiuretic hormone (ADH), calcitonin, glucagon and insulin have been shown to affect magnesium reabsorption. Of these, PTH is the most important. PTH increases reabsorption in the distal tubules by a cyclic AMP mediated process.15,16
Phosphate depletion is associated with a significant increase in urinary magnesium excretion and may cause hypomagnesaemia. Hypercalcaemia is associated with an increased urinary excretion of both calcium and magnesium. The increase in magnesium excretion in hypercalcaemia is greater than the increase in calcium excretion and is due to diminished reabsorption in the Loop of Henle. Hypercalcaemia causes a reduction in isotonic reabsorption in the proximal tubule causing greater delivery of sodium, water, calcium and magnesium to the loop of Henle. As a result of this increased flow to TALH, calcium and magnesium transport may be inhibited. In addition, the high peritubular concentration of calcium directly inhibits the transport of both ions in this segment.
Osmotic diuretics such as mannitol and glucose cause a marked increase in magnesium excretion. Loop diuretics induce hypermagnesuria, and the increase in magnesium excretion is greater than that of sodium or calcium suggesting that loop diuretics may directly inhibit magnesium transport.
Assessment of Magnesium Status
At present, there is no simple, rapid and accurate laboratory test to indicate the total body magnesium status. The most commonly used method for assessing magnesium status is the serum magnesium concentration. Other methods available for assessing magnesium status are listed in Table 4.
Table 4.
Tests used in assessing magnesium status
| Serum Magnesium Concentration |
| Total magnesium |
| Ultrafiltrable magnesium |
| Ionised magnesium |
| Intracellular Magnesium content |
| Red cells |
| Mononuclear blood cells |
| Skeletal muscle |
| Physiological tests |
| Metabolic balance studies |
| 24 h urinary excretion of magnesium |
| Magnesium loading test |
| Intracellular free magnesium ion concentration |
| Fluorescent dye |
| Nuclear magnetic resonance spectroscopy |
| Others |
| Magnesium balance |
| Isotope studies |
| Hair or tooth magnesium |
| Functional assays |
Serum total magnesium can be measured by a variety of techniques.4,17 Serum is preferable to plasma as the anticoagulant could be contaminated with magnesium or affect the assay. For instance, citrate binds not only calcium but also magnesium and affects fluorometric and colorimetric procedures. Haemolysis, bilirubin, lipaemia, high phosphate concentration and delay in separating serum can affect the measurement.18 In adults serum magnesium concentration is not influenced by sex or age except in the very elderly where it may be slightly higher.18 Serum magnesium concentration increases after a short period of maximal exercise but decreases after endurance exercises.18 It is lower during the third trimester of pregnancy and is higher in subjects on a vegetarian diet.18,19 Intra-individual variation in serum magnesium ranges between 3.4% and 4.7%.20
The total serum magnesium concentration is not the best method to evaluate magnesium status as changes in serum protein concentrations may affect the total concentration without necessarily affecting the ionised fraction or total body magnesium status. The correlation between serum total magnesium and total body magnesium status is poor.4,21
Measurement of ultrafiltrable magnesium may be more meaningful than the total magnesium as it is likely to reflect ionised magnesium concentration, but methods are not available for routine use.
In the last few years, ion selective electrodes for magnesium have been developed and several commercial analysers are now available for the measurement of ionised magnesium concentration.3,4 Measurement of ionised magnesium has been found to be useful in several clinical situations.3,4 However, results from different instruments do not agree as the electrodes are not entirely selective for ionised magnesium concentration and a correction is applied based on the ionised calcium concentration.4
Red cell magnesium concentration can be determined easily but does not seem to correlate well with total body magnesium status or with other measures of magnesium status.22 The magnesium content of mononuclear cells may be a better predictor of skeletal and cardiac muscle magnesium content. However, this method is technically more difficult and intraindividual variation is high at about 12–22%.23 As muscle contains nearly 30% of the total body magnesium it is an appropriate tissue for the assessment of magnesium status and studies in patients undergoing heart surgery showed that skeletal muscle magnesium was a better predictor of heart magnesium than lymphocyte or serum magnesium concentration.24 However, this is an invasive and expensive procedure requiring special expertise.
In the steady state, a 24-hour urine excretion of magnesium reflects intestinal absorption and is also of value in determining whether magnesium wasting is occurring by the renal route. In the presence of hypomagnesaemia, magnesium excretion > 1 mmol/day is suggestive of renal magnesium wasting. On the other hand, magnesium excretion < 0.5 mmol/day is suggestive of magnesium deficiency.10
The magnesium tolerance test has been used for many years and it appears to be an accurate means of assessing magnesium status. In this test, the percentage of magnesium retained after parenteral administration of magnesium is determined. The percentage of magnesium retained is increased in magnesium deficiency and is inversely correlated with the concentration of magnesium in bone.25 In a study of 23 healthy subjects, 13 hypomagnesaemic patients and 24 normomagnesaemic patients at high risk of magnesium deficiency, the percentage retention was 14±4% (mean ± SEM) in normals, 85±3% in hypomagnesaemic patients and 51±5% in patients at risk of developing magnesium deficiency. These data suggest that this test is a very sensitive method to detect magnesium deficiency.9,11 The test, however, depends on normal renal function and is of limited value in patients with renal magnesium loss.
Intracellular free magnesium concentration can be determined using fluorescent probes such as fura-2 or by nuclear magnetic resonance (NMR).4 Magnesium balance studies and studies using isotopes of magnesium are mainly used in research. Hair and tooth have also been used to assess magnesium status. Activation of enzymes such as creatine kinase and alkaline phosphatase by magnesium has also been examined as a measure of magnesium status. In experimental studies, however, it was not shown to be as good as serum or red cell magnesium concentration.26
In summary, no single method is satisfactory to assess magnesium status. The simplest, most useful and readily available tests are the measurement of serum total magnesium and the magnesium tolerance test.3,4 Ionised magnesium measurement may become more readily available with the development of reliable analysers.
Magnesium Deficiency and Hypomagnesaemia
The terms hypomagnesaemia and magnesium deficiency are commonly used interchangeably. However, total body magnesium depletion can be present with normal serum magnesium concentrations and there can be significant hypomagnesaemia without total body deficit.
Hypomagnesaemia is more prevalent than previously appreciated.11 Prevalence of hypomagnesaemia varies from 7% to 11% in hospital patients.27,28,29 In patients with other electrolyte abnormalities hypomagnesaemia is more frequent, 40% in hypokalaemic patients, 30% in hypophosphataemic patients, 23% in hyponatraemic patients and 22–32% in hypocalcaemia patients.27,30,31 The prevalence of hypomagnesaemia in critically ill patients is even higher, ranging from 20% to 65%.3,32,33 Hypomagnesaemia in intensive care patients is associated with increased mortality.34
Hypomagnesaemia is frequently undetected. Measurement of serum magnesium concentration in 1,000 samples received for electrolyte determination showed that only 10% of the hypomagnesaemic patients had magnesium requested.35 Thus it has been suggested that magnesium should be determined routinely in all acutely ill patients especially in those with conditions, diseases or treatment that may predispose to magnesium deficiency.36
Aetiology and pathogenesis
Hypomagnesaemia may result from one or more of the following mechanisms: redistribution, reduced intake, reduced intestinal absorption, increased gastrointestinal loss and increased renal loss. Causes of hypomagnesaemia and magnesium deficiency are listed in the Table 5.
Table 5.
Causes of hypomagnesaemia
| Redistribution of magnesium |
| Refeeding and insulin therapy |
| Hungry bone syndrome |
| Correction of acidosis |
| Catecholamine excess |
| Massive blood transfusion |
| Gastrointestinal causes |
| Reduced intake |
| Mg free intravenous fluids |
| Dietary deficiency |
| low oxalate diet |
| cellulose phosphate |
| Reduced absorption |
| Malabsorption syndrome |
| Chronic diarrhoea |
| Intestinal resection |
| Primary infantile hypomagnesaemia |
| Renal loss |
| Reduced sodium reabsorption |
| Saline infusion |
| Diuretics |
| Renal disease |
| Post obstructive nephropathy |
| Post renal transplantation |
| Dialysis |
| Diuretic phase of acute renal failure |
| Inherited disorders |
| Bartter's syndrome |
| Gitelman's syndrome |
| Endocrine causes |
| Hypercalcaemia |
| Primary hyperparathyroidism |
| Malignant hypercalcaemia |
| Hyperthyroidism |
| Hyperaldosteronism |
| Diabetes mellitus |
| Alcoholism |
| Drugs |
| Diuretics |
| Cytotoxic drugs: Cisplatin, Carboplatin, Gallium nitrate |
| Antimicrobial agents |
| Aminoglycosides: Gentamicin, Tobramycin, Amikacin |
| Antituberculous drugs: Viomycin, Capreomycin |
| Immunosuppressants: Cyclosporin, Ritodrine |
| Beta adrenergic agonists: Theophylline, Salbutamol, Riniterol |
| Other drugs |
| Amphotericin B |
| Pentamidine |
| Foscarnet |
| Pamidronate |
| Anascrine |
| Miscellaneous |
Hypomagnesaemia due to redistribution
Hypomagnesaemia due to the shift of magnesium from extracellular fluid into cells or bone is seen in refeeding of starved patients (refeeding syndrome), during treatment of metabolic acidosis, and in hungry bone syndrome which is seen after parathyroidectomy or in patients with diffuse osteoblastic metastases. Hypomagnesaemia is seen in up to 20% of patients with acute pancreatitis, probably due to deposition of magnesium in areas of necrosis. However, magnesium deficiency may also contribute to the hypomagnesaemia in pancreatitis.37 High concentrations of catecholamines cause an intracellular shift and may be one of the factors contributing to the hypomagnesaemia seen during and after cardiac surgery and in congestive heart failure.10,38
Gastrointestinal causes
Magnesium deficiency entirely due to reduced dietary intake in otherwise healthy subjects is very uncommon. Hypomagnesaemia may be seen in patients who are maintained on magnesium-free intravenous fluids or total parenteral nutrition, especially in those patients who have a marginal or reduced serum magnesium to start off with.36
Magnesium deficiency is frequently observed in conditions causing steatorrhoea or severe chronic diarrhoea such as Crohn's disease, ulcerative colitis, coeliac disease, Whipple's disease and short bowel syndrome.36 In general, the degree of magnesium depletion correlates with the severity of diarrhoea, stool fat content and faecal magnesium concentration. The malabsorption of magnesium is secondary to the formation of insoluble magnesium soaps, and a low-fat diet improves magnesium balance in these patients.
An inherited disorder of isolated magnesium malabsorption associated with hypocalcaemia, tetany and seizures has been described in infants as well as in older individuals.39 Children with this condition usually present at 4–5 weeks of age with generalised convulsions associated with protein losing enteropathy, hypoalbuminaemia and anasarca. The disorder is caused by a mutation in the TRPM 6 gene which codes for an ion channel, resulting in defective carrier mediated transport in the small intestine.14 The fractional intestinal magnesium absorption in this condition is reduced to about 35% compared to 70% in normal infants.40 This defect can be overcome by increasing the oral intake of magnesium to approximately 5 times the normal daily requirement.14
Renal Causes
Proximal tubular magnesium reabsorption is proportional to sodium reabsorption, and a reduction in sodium reabsorption during long-term intravenous fluid therapy may result in magnesium deficiency.
a) Renal Disease
Hypomagnesaemia is occasionally observed in chronic renal failure due to an obligatory renal magnesium loss.41 It is also seen during the diuretic phase of acute renal failure, in post-obstructive diuresis and after renal transplantation.40 Patients on continuous ambulatory peritoneal dialysis develop hypomagnesaemia when low magnesium dialysis fluid is used.42
b) Inherited Disorders
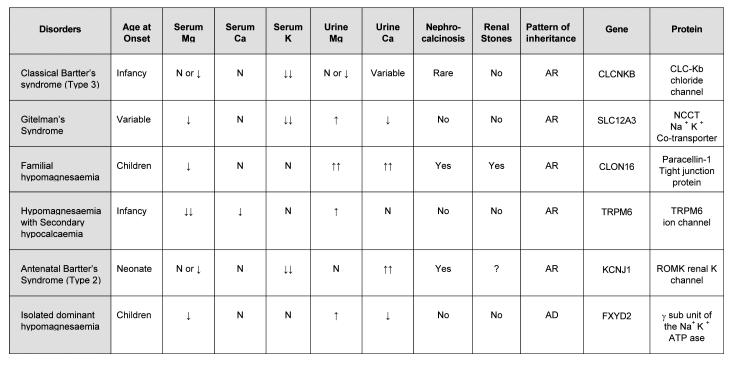
Several different congenital disorders of renal tubular reabsorption of magnesium have been described but there is no consensus on their classification.13,14 The classification, features and molecular defects in some of the syndromes are listed in Table 6. Classical Bartter's syndrome, which presents in infancy, is associated with hypokalaemia, metabolic alkalosis, hypereninaemia, and secondary hyperaldosteronism. It is caused by a mutation in CLCNKB gene, which encodes for the chloride channel CLC-Kb in TALH and distal tubules.14 Hypomagnesaemia is seen in up to 50% of these patients but hypocalciuria is not present. Other forms of Bartter's syndrome (antenatal) are associated with mutations in ROMK or NKCC2 transporter genes.43 Gitelman syndrome, which presents in childhood or adolescence, is associated with hypomagnesaemia and hypocalciuria. The defect is in the gene coding for the NaCl co-transporter (NCCT) of the distal tubule.14 More than 100 mutations have been described.
Table 6.
AR - Autosomal recessive, AD - Autosomal dominant
Drugs
A variety of drugs including antibiotics and chemotherapeutic agents cause magnesium wasting (Table 5).44 Loop diuretics inhibit magnesium transport in TALH and cause magnesium depletion, especially during long-term use.44,45 Short-term administration of thiazide diuretics which act on the distal convoluted tubules where less that 5% of magnesium is absorbed, does not produce magnesium wasting. However, long term administration may produce substantial magnesium depletion due to secondary hyperaldosteronism, increased sodium load and interaction with calcium metabolism.45
Hypomagnesaemia is a frequent complication of cisplatin treatment and may be acute or chronic. The incidence of hypomagnesaemia increases with cumulative dose.44 In the acute phase, poor dietary intake and the use of diuretics are contributing factors. Chronic hypomagnesaemia starts to develop 3 weeks after the initial chemotherapy and usually persists for several months.46 Occasionally hypomagnesaemia may persist for several years after completion of treatment.47 In chronic magnesium wasting hypercalciuria, renal magnesium wasting and hypokalaemic metabolic alkalosis, a picture similar to Gitelman's syndrome, is seen consistent with a distal tubular defect.48 Acute toxicity is suggested to be due to an effect on the proximal tubule.49 Cisplatin accumulates in the renal cortex and degenerative changes are seen in the proximal tubules. The excretion of proximal tubular protein ß2 microglobulin and the enzyme N-acetyl-ß-glucosaminidase (NAG) are increased in patients treated with cisplatin and there is good correlation between the excretion of NAG and of magnesium.50 Carboplatin, an analogue of cisplatin, causes nephrotoxicity and hypomagnesaemia less frequently.51
Hypomagnesaemia is a recongnised complication of aminoglycoside treatment. It has been associated with a variety of aminoglycosides including gentamicin, tobramycin and amikacin, as well with the anti-tuberculous agents viomycin and capreomycin.52 In healthy subjects the standard dose of gentamicin causes increased excretion of magnesium.53 Symptomatic hypomagnesaemia is seen with high dose treatment especially in the elderly or if there are other associated conditions causing magnesium loss.54 Aminoglycosides preferentially accumulate in the proximal tubule leading to cell damage and increased excretion of proximal tubular enzymes such as alanine aminopeptidase.55 Juxta-glomerular hyperplasia with hyperreninaemia and secondary hyperaldosteronism has also been reported.55
Cyclosporin, a commonly used immunosuppressive agent, causes hypomagnesaemia which is usually mild and asymptomatic and does not necessitate stopping the medication.52 Occasionally severe symptomatic magnesium deficiency is seen. Ionised magnesium concentration is low in renal transplant patients and shows a correlation with blood cyclosporin concentration.56 Short-term treatment causes intracellular shift of magnesium whereas long-term treatment causes renal magnesium wasting. Tacrolimus, another immunosuppressive agent, also causes renal magnesium loss and hypomagnesaemia.57
Theophylline, especially in toxic doses, is reported to cause hypomagnesaemia.58 Intravenous administration of theophylline to asthmatic subjects causes increased magnesium excretion, and patients on theophylline have an increased risk of developing hypomagnesaemia.59,60 The hypomagnesaemia is accompanied by other metabolic abnormalities including hypokalaemia, hyponatraemia, hypophosphataemia and hyperglycaemia. A linear relationship has been shown between plasma drug concentration over a range of 5.5 to 110 μmol/L and the above metabolic abnormalities. Adrenaline and the β-2 agonists salbutamol and ritodrine cause hypomagnesaemia due to shift of magnesium into the cells.61
Amphotericin B, a highly nephrotoxic agent, can lead to severe hypokalaemia and hypomagnesaemia during chronic administration, but this can be prevented by amiloride therapy.62 Administration of pentamidine may cause severe symptomatic hypomagnesaemia due to renal magnesium wasting.63,64 This drug accumulates in the kidney and can be detected up to one year following administration.64 Up to 70% of patients treated with foscarnet have been shown to have hypomagnesaemia.65 Other drugs causing hypomagnesaemia include pamidronate and anascrine.52
Endocrine Causes
Although PTH has been shown to increase the reabsorption of magnesium, reduced serum as well as red cell and mononuclear cell magnesium have been described in primary hyperparathyroidism.66 This is due to the effect of hypercalcaemia on renal tubular magnesium reabsorption. Hypomagnesaemia may develop after parathyroidectomy due to the entry of magnesium into cells as part of the ‘hungry bone syndrome’. Hypercalcaemia of malignancy may also cause hypomagnesaemia.9 Hypomagnesaemia has also been reported in primary and secondary hyperaldosteronism.36
Diabetes Mellitus
Diabetes mellitus, both type I and type II, are said to be the commonest causes of magnesium deficiency, with 25–39% of patients being affected.67 A decrease in total, ultrafiltrable, ionised, red cell and white cell magnesium have been found.68,69 Studies using the magnesium loading test have further confirmed magnesium deficiency in diabetes.70 Magnesium depletion is due to increased magnesium excretion brought about by osmotic diuresis; there may additionally be a specific tubular defect.71 The decrease in serum magnesium concentration is correlated with fasting blood glucose, glycated haemoglobin, albumin excretion and the duration of diabetes.69 Magnesium depletion, via its effect on inositol transport, has been suggested to be of pathogenic significance in the development of diabetic complications.72
Alcoholism
Hypomagnesaemia is a common finding in acute and chronic alcoholism with an incidence of up to 30%.73 Low serum total, ionised and intracellular magnesium, negative magnesium balance and greater retention of magnesium after parenteral administration have been reported.70 Mechanisms contributing to magnesium depletion include poor nutritional status, magnesium loss through vomiting and diarrhoea, malabsorption resulting from steatorrhoea due to chronic pancreatitis or liver disease, phosphate depletion, vitamin D deficiency, acute alcoholic ketoacidosis, hyperaldosteronism secondary to liver disease and renal tubular dysfunction.74 Magnesium deficiency in alcoholism may also contribute to complications such as osteoporosis, cardiovascular disease, stroke and hypertension.52
Manifestations of hypomagnesaemia and magnesium deficiency
Many patients with magnesium deficiency and hypomagnesaemia remain asymptomatic. As magnesium deficiency is usually secondary to other disease processes or drugs, the features of the primary disease process may complicate or mask magnesium deficiency. Signs and symptoms of magnesium deficiency are usually not seen until serum magnesium decreases to 0.5 mmol/L or lower.
Manifestations may depend more on the rate of development of magnesium deficiency and/or on the total body deficit rather than the actual serum magnesium concentration.40 Clinical manifestations of severe or moderate magnesium deficiency are listed in Table 7.
Table 7.
Clinical Features of Hypomagnesaemia and magnesium deficiency
| Electrolyte disturbance |
| Hypokalaemia |
| Hypocalcaemia |
| Neuromuscular and central nervous system |
| Carpopedal spasm |
| Convulsations |
| Muscle cramps |
| Muscle weakness, fasciculations, tremors |
| Vertigo |
| Nystagmus |
| Depression, psychosis |
| Athetoid movements & choreform movements |
| Cardiovascular |
| Atrial tachycardias, fibrillation |
| Supraventricular arrhythmias |
| Ventricular arrhythmias |
| Torsade de pointes |
| Digoxin sensitivity |
| Complications of magnesium deficiency |
| Altered glucose homeostasis |
| Atherosclerotic vascular disease |
| Hypertension |
| Myocardial infarction |
| Osteoporosis |
| Miscellaneous |
| Migraine |
| Asthma |
| Chronic fatigue syndrome |
| Impaired athletic performance |
Biochemical Manifestations
a) Hypokalaemia
Magnesium and potassium homeostases are closely related.75 Hypokalaemia is a frequent finding in patients with hypomagnesaemia, and hypokalaemia predicts the presence of hypomagnesaemia. Potassium depletion cannot be corrected until magnesium depletion is corrected. The exact mechanism for the development of hypokalaemia in magnesium deficiency is not clear, but may be related to the dependence of Na+,K+-ATPase, Na,K-Cl co-transport, potassium channels and other transport processes on magnesium.52 Hypokalaemia of magnesium deficiency contributes to the cardiac manifestations of hypomagnesaemia, but may delay the onset of tetany.
b) Hypocalcaemia
Hypocalcaemia is a common manifestation in hypomagnesaemia. Up to one third of patients with hypomagnesaemia in intensive care units may have hypocalcaemia. Symptomatic hypocalcaemia is usually seen in moderate to severe magnesium deficiency and there is a positive correlation between serum magnesium and calcium concentrations. Even mild degrees of magnesium depletion can cause a significant decrease in serum calcium concentration.76 Hypocalcaemia of magnesium deficiency cannot be corrected by treatment with calcium, vitamin D or both. Magnesium therapy alone will restore serum calcium concentration to normal.
Several factors contribute to the hypocalcaemia of magnesium deficiency.77 One of the important factors is impaired secretion of PTH. Although the acute effect of extracellular magnesium on PTH secretion is similar to that of calcium, in magnesium deficiency there is impaired PTH release. In addition to impaired PTH secretion there is evidence for an increase in metabolism of PTH and end-organ resistance for PTH.78 End-organ resistance is suggested by the presence of normal or elevated serum concentration of PTH in the face of hypocalcaemia, and decreased osteocalcin concentration.78 Administration of exogenous PTH to patients with hypocalcaemic hypomagnesaemia has little effect on serum calcium concentrations or on urinary excretion of cyclic AMP and phosphate. In magnesium deficiency, vitamin D metabolism is altered with a decrease in serum 1,25 dihydroxyvitamin D due to impairment in conversion of 25 hydroxyvitamin D to 1,25 dihydroxyvitamin D.76,79 There is also evidence for increased clearance of 1,25 dihydroxyvitamin D and end- organ resistance.79
Neuromuscular and Central Nervous System Manifestations
The earliest manifestations of magnesium deficiency are usually neuromuscular and neuropsychiatric disturbances. The most common clinical manifestations are hyperexcitability, including positive Chvostek's and Trousseau's signs, tremor, fasciculations and tetany. Frank tetany in magnesium deficiency is usually associated with hypocalcaemia. There may be several mechanisms contributing to the wide variety of neuromuscular problems in magnesium deficiency. Magnesium is required for stabilisation of the axon. The threshold for axon stimulation is decreased and nerve conduction velocity increased when serum magnesium concentration is low. By competitively inhibiting the entry of calcium into pre-synaptic nerve terminals, magnesium influences the release of neurotransmitters at the neuromuscular junction and causes hyper-responsive neuromuscular activity.52 Magnesium also influences muscle contraction and relaxation by its effect on calcium handling by the muscle cell. In the muscle cell, the sarcoplasmic reticulum regulates the contraction/relaxation cycle by releasing and reactivating calcium. In magnesium deficiency, the release of calcium from the sarcoplasmic reticulum is increased. In addition, magnesium is required for the re-uptake of calcium. The net effect on the muscle of a low intracellular magnesium concentration is increased contractility to a given stimulus and reduced ability to recover from the contraction, making it prone to tetany.
The effect of magnesium deficiency on the central nervous system is even more complicated and less well understood. Magnesium deficiency seems to cause an intracellular calcium overload and disturbances in its subcellular distribution. Magnesium deficiency is associated with stimulation of excitatory neurotransmitters such as serotonin and acetylcholine, non-competitive blockade of the N-methyl-D-aspartate receptor and possibly inhibition of action of the inhibitory amino acid γ-amino butyric acid.3
Cardiovascular Manifestations
Magnesium deficiency can affect cardiac electrical activity, myocardial contractility and vascular tone. It also potentiates digoxin toxicity.
Although cardiac arrhythmias are well known to be associated with hypomagnesaemia, the contribution of hypomagnesaemia to its pathogenesis is not fully known due to coexisting hypokalaemia and other electrolyte disturbances. The relationship between magnesium deficiency and arrhythmias associated with acute myocardial infarction seems to be complex. The frequency of cardiac arrhythmias occurring after myocardial infarction is higher in hypomagnesaemic patients and is reduced by magnesium administration.10 ECG changes are non-specific and include a slight prolongation of conduction and the depression of the ST segment. Magnesium depletion increases susceptibility to arrhythmogenic effects of drugs such as isoproterenol and cardiac glycosides. The spectrum includes supraventricular and ventricular arrhythmias.80 Torsade de pointes, a repetitive polymorphous ventricular tachycardia with prolongation of QT interval, has been reported in cases of hypomagnesaemia, and this and other arrhythmias have been successfully treated with magnesium.21 However, this may be a pharmacological effect, independent of underlying magnesium deficiency.81
The role of hypomagnesaemia and magnesium deficiency in congestive heart failure is not clear. Hypomagnesaemia is present in <10% of patients with mild to moderate heart failure but is more common in severe congestive heart failure. The effects of hypomagnesaemia are complicated by concomitant abnormalities in potassium. Hypomagnesaemia has been shown to increase the frequency of arrhythmias in heart failure, but is not associated with increased mortality.82
a) Magnesium and Digoxin Toxicity
Hypomagnesaemia and magnesium depletion may contribute to digoxin toxicity even in the presence of apparently therapeutic concentration of serum digoxin.83 As 16–19% of patients on digitalis have been shown to have hypomagnesaemia, routine monitoring of serum magnesium concentration in digitalised patients may be important.84 Digoxin is thought to act via its inhibition of Na+,K+-ATPase, a magnesium dependent enzyme. In low magnesium states intracellular potassium is reduced, enhancing the inhibitory effect of digoxin.75
Magnesium and Glucose Homeostasis
Magnesium is involved in many of the enzyme systems regulating glucose homeostasis. Magnesium deficiency, therefore, may give rise to alterations in glucose metabolism.11 Magnesium affects glucose homeostasis by influencing insulin secretion as well as glucose uptake by cells. Magnesium deficiency inhibits the acute phase of insulin release in response to a glucose challenge. Magnesium also plays a role in glucose disposal and/or insulin sensitivity, and magnesium deficiency is associated with insulin resistance.11 Conversely, insulin resistance is associated with a lower serum magnesium concentration. Magnesium supplementation has been shown to reduce insulin requirement and improve glucose disposal in diabetic patients. It also improves glucose handling in the elderly and glucose oxidation in thiazide-treated hypertensive patients.85
Magnesium Deficiency and Atherosclerosis
There is a substantial body of epidemiological and experimental evidence linking magnesium deficiency and atherosclerotic cardiovascular disease.21 Experimental studies suggest that magnesium deficiency may play a role in the pathogenesis of atherosclerosis.11 Magnesium deficiency may contribute to the progression of atherosclerosis by its effects on lipid metabolism, platelet aggregation and blood pressure (see below). Experimental magnesium deficiency is characterised by increased triglycerides, cholesterol, VLDL, LDL, apolipoprotein B and triglyceride-rich lipoproteins and a reduced HDL, apolipoprotein A1 and plasma lecithin-cholesterol acyltransferase activity.86 Peroxidation of lipoproteins due to free radical production and increased platelet aggregation may also contribute to the development of atherosclerosis. Magnesium supplementation of hyperlipidaemic subjects has been shown to cause a reduction in total and LDL cholesterol and apolipoprotein B, and an increase in HDL cholesterol and triglycerides.21
Magnesium Deficiency, Hypertension and Vascular Tone
There is an inverse relationship between magnesium intake and blood pressure and epidemiological studies show an increased incidence of hypertension in areas where the magnesium content of water is low.11 Furthermore, magnesium supplementation was associated with a significant decrease in blood pressure in 10 out of 15 studies.21 In-vitro and in-vivo studies show that magnesium can influence vascular tone and reactivity. Magnesium deficiency increases angiotensin II induced plasma aldosterone concentration and production of thromboxane and vasoconstrictor prostaglandins.87 Insulin resistance caused by magnesium deficiency also increases vascular tone. Changes in cytosolic free calcium produced by magnesium deficiency may increase vascular reactivity even further. Magnesium supplementation can reduce the pressor effect of angiotensin II and stimulate the production of the vasodilator prostaglandin I2.11 Magnesium may also influence the release of nitric oxide and its effects on vascular tone.83
Magnesium and Myocardial Infarction
Epidemiological studies have shown a correlation between hardness of water and the incidence of myocardial infarction and of sudden death.21 Patients with acute myocardial infarction also have lower exchangeable magnesium content and retain abnormally high amounts of magnesium after a magnesium load than control subjects.88 Because of these relationships and because of the fact that magnesium depletion may worsen or precipitate acute myocardial infarction, precipitate arrhythmias and enhance reperfusion injury, intravenous magnesium therapy has been advocated in the management of acute myocardial infarction (see section on therapeutic uses).
Magnesium and Bone
Magnesium deficiency has been implicated in osteoporosis.89 Magnesium content of trabecular bone is significantly lower in subjects with osteoporosis, and magnesium tolerance studies show increased retention of magnesium. Serum and red blood cell magnesium also appear to be lower in these subjects. Recent studies also suggest magnesium supplementation increases bone density or arrests bone loss in 80% of osteoporotic subjects.89 Magnesium intake is frequently below the recommended dietary intake in the elderly. Postmenopausal women are encouraged to consume at least 1000 mg of calcium per day. This can lead to a dietary calcium/magnesium ratio of 4:1, rather than the recommended 2:1 ratio, and reduce the efficiency of magnesium absorption. The mechanism whereby reduced magnesium status exacerbates osteoporosis is not clear but is probably multifactorial. As the hydrogen/potassium-ATPase pump in the cells of periosteum and endosteum are magnesium dependent, the pH of bone extracellular fluid may fall in magnesium deficiency, resulting in demineralisation. In addition, the formation of 1,25 dihydroxyvitamin D involves a magnesium-dependent hydroxylase enzyme, and serum 1,25 dihydroxyvitamin D concentrations have been shown to be lower in magnesium deficiency.76,79
Magnesium and Renal Calculi
In-vitro studies of crystal growth show that magnesium is an inhibitor of crystal growth. Administration of magnesium orally to stone formers causes a reduction in urine saturation index. Urinary excretion of magnesium was reported to be lower in stone formers in some studies but not in others.90 A study of 1,270 patients with recurrent nephrolithiasis showed that 6.8% of the subjects had hypomagnesuria suggesting that in some stone formers decreased magnesium excretion may contribute to the pathogenesis of renal calculi.91
Magnesium and Asthma
Magnesium has also been implicated in asthma. Ionised magnesium concentration was 20% lower in asthmatics. In an epidemiological study a reduced magnesium intake, as determined by a dietary questionnaire, was associated with hyperreactivity of the airways to metacholine. The authors concluded that dietary magnesium intake was independently related to lung function, and low magnesium intake may therefore be involved in the aetiology of asthma.92 In a recent multicentre trial magnesium sulphate was shown to improve pulmonary function in severe asthma.93
Miscellaneous Conditions
Chronic fatigue syndrome, characterised by flu-like symptoms that are followed by months or years of disabling lethargy as well as mood alterations, has been linked with low magnesium status. However, not all studies have shown evidence of low magnesium status in this syndrome.52 Sudden deaths in athletes have been attributed to magnesium deficiency. As magnesium is important for the supply and utilisation of energy rich compounds, concern has been expressed that exercise-induced magnesium deficiency may impair athletic performance.52 Magnesium deficiency is also thought to contribute to increased susceptibility to infections and migraine.52
Management
Prophylaxis
In patients likely to develop magnesium deficiency, prophylactic measures should be taken to prevent the development or progression of magnesium deficiency and hypomagnesaemia. High-risk subjects such as chronic alcoholics, patients receiving total parenteral nutrition, long term diuretic therapy or other drugs causing magnesium loss and those with chronic diarrhoeal and steatorrhoeal states should have serum magnesium monitored regularly and, if necessary, supplemented with magnesium. Patients on parenteral nutrition should receive prophylactic doses of 4–8 mmol/day of magnesium. Higher doses may be required in malnourished individuals and in those with continued magnesium loss.
Treatment
It is important to note that the extent of magnesium deficiency is impossible to predict. Patients who present with signs and symptoms of magnesium deficiency should be treated promptly with magnesium. As oral magnesium is poorly absorbed and, in large doses, causes gastrointestinal side effects, replacement by the oral route is not practicable in symptomatic patients. In critically ill patients with ventricular tachycardia or convulsions, it has been recommended that 8 mmol of magnesium should be given as magnesium sulphate over a minute, followed by 40 mmol of magnesium over the next 5 hours.10 If necessary, another 40 mmol may be administered over the next 10 hours. In less urgent situations, 0.5 mmol/Kg/24 hr may be given by continuous intravenous infusion or 4 mmol (2 mls of 50% magnesium sulphate) by intramuscular injection every 3 or 4 hours for the first day. Intramuscular injections are painful and intravenous infusion is preferable. It should be noted that up to 50% of magnesium infused may be lost in the urine. Serum magnesium concentration should be monitored frequently. However, restoration of serum magnesium concentration to normal does not necessarily indicate repletion of body magnesium stores and therapy should be continued for approximately 3–7 days. If patients continue to lose magnesium from the intestines or kidneys, therapy may have to be continued for a longer duration. Once repletion has been accomplished, patients can usually maintain a normal magnesium status on a regular diet provided the cause of the magnesium loss has been corrected. If the patient cannot eat, a daily maintenance dose of 4 mmol of magnesium should be given parenterally.11
Mild asymptomatic hypomagnesaemia can be treated by a diet rich in magnesium. In chronic magnesium loss, oral magnesium supplementation may be required. Although magnesium is available in the form of sulphates, lactate, hydroxide, oxide and chloride, only magnesium gluconate is recommended for magnesium supplementation as it appears to be better absorbed and causes less diarrhoea.36 An initial dose of 12 mmol per day increasing to 48 mmol a day can be given. Magnesium salts should be given in divided doses (3 or 4 times a day) to avoid diarrhoea. Administration of potassium and calcium together with magnesium may be necessary since associated loss of these cations is common in severe magnesium deficiency.
Assessment of renal function before replacement therapy is important, and magnesium therapy in patients with any renal failure should be undertaken cautiously. During intravenous replacement therapy it is important to monitor serum concentrations of magnesium, potassium and other major cations as well as deep tendon reflexes. If there is deterioration in renal function, the dose of magnesium should be halved and serum magnesium monitored more frequently. If hypermagnesaemia or signs of magnesium intoxication (hypotension, bradycardia or depression of tendon reflexes) develop, therapy should be stopped.
Therapeutic uses of Magnesium
Magnesium has been used as a therapeutic agent for a very long time. Magnesium salts as a cathartic agent was its earliest therapeutic application and is still used for this purpose. Magnesium has been used therapeutically in acute myocardial infarction based on the observation that it causes vasodilatation, improves myocardial contractility, limits infarct size (in animal models of AMI) and modifies coagulation. In early studies magnesium therapy was associated with reduced mortality in AMI due to its anti-arrhythmic effect. The second Leicester intravenous magnesium intervention trial (LIMIT.2) was the first large-scale randomised placebo-controlled trial and involved 2316 patients. This study showed a 24% reduction in short-term mortality and a 25% reduction in the incidence of left ventricular failure as well as a reduction in long-term mortality.94,95 However, the second large scale study involving 58,500 subjects, ISIS-4 (Fourth International Study of Infarct Survival), showed no benefit from magnesium therapy.96 This difference has been attributed to differences in study design. It has been suggested that magnesium therapy may be beneficial only in those not receiving or are unsuitable for thrombolytic treatment. In the recent MAGIC (magnesium in coronaries) trial of over 6000 high-risk patients, magnesium therapy had no benefit.97 It was concluded that there was no indication for routine magnesium therapy in high-risk patients.
The association between magnesium deficiency and cardiac arrhythmia has been discussed before. The therapeutic role of magnesium in cardiac arrhythmias has been studied extensively. Magnesium has a well-established role in the management of torsade de pointes (long QT syndrome).98 However, its role in other arrhythmias is not clear. Nevertheless, magnesium therapy should be considered in those with refractory arrhythmias.10 Magnesium is also used in cardiac surgery in order to reduce perioperative arrhythmias although there is debate still on its effectiveness. Magnesium is included in cardioplegic solutions during cardiopulmonary bypass as a cardioprotective agent. Although this practice is extensive, it is not universally accepted.10,21
Magnesium has been used as a therapeutic agent in the treatment of asthma for many years without any convincing evidence.21
Magnesium has been used in the treatment of pre-eclampsia and eclampsia. There is now strong evidence that magnesium sulphate is an effective agent in eclampsia. In a large multicentre trial, it was clearly shown that magnesium sulphate was superior to other anticonvulsants in the treatment of eclampsia.99 More recently, the placebo-controlled Magnesium Sulphate for Prevention of Eclampsia (MAGPIE) trial clearly demonstrated that magnesium sulphate reduced the risk of eclampsia in pre-eclamptic women by 50%.100 The mechanism of action is not clear, but magnesium may act by improving cerebral vascular perfusion.101 However, nimodipine, a selective cerebral vasodilator, was not found to be as effective as magnesium.102 Magnesium has also been used to treat premature labour for many years although its effectiveness has not been convincingly demonstrated.103
Hypermagnesaemia
Prevalence of Hypermagnesaemia varies from 5.7% to 9.3%.35,104 The highest serum magnesium concentrations reported so far are 18 mmol/L in a 33 week old premature infant and 13.4 mmol/L in a 78 year old woman who swallowed water from the Dead Sea.104,105 Severe hypermagnesaemia in fact seems to be a feature in patients who drown in the Dead Sea.105
Causes of Hypermagnesaemia (Table 8)
Table 8.
Causes of Hypermagnesaemia
| Redistribution |
| Acute acidosis |
| Excessive intake |
| Oral |
| Antacids |
| Cathartics |
| Swallowing salt water |
| Rectal |
| Purgation |
| Parenteral |
| Urethral irrigation |
| Renal failure |
| Chronic renal failure |
| Acute renal failure |
| Rhabdomyolysis |
| Others |
| Lithium therapy |
| Familial hypocalciuric hypercalcaemia |
| Hypothyroidism |
| Addison's disease |
| Milk alkali syndrome |
| Depression |
Hypermagnesaemia commonly occurs due to the excessive administration of magnesium salts or magnesium-containing drugs, especially in patients with reduced renal function. Hypermagnesaemia may rarely be due to redistribution from cells.52
Excessive Intake
Magnesium containing medications are commonly used as laxatives, antacids and as rectal enemas. Hypermagnesaemia has often been described with the use of magnesium containing cathartics for treatment of drug overdose, in patients taking magnesium-containing cathartics and antacids for therapeutic purposes and following rectal administration of magnesium, even in the presence of normal renal function.106 In 75% of these cases hypermagnesaemia was clinically unsuspected, and the total amount of magnesium ingested was not excessive but bowel disorders may have enhanced the absorption.107 This, and other studies highlight the importance of monitoring serum magnesium concentration in patients taking magnesium-containing medications. A serum magnesium concentration as high as 9.5 mmol/L has been reported after an overdose of magnesium containing cathartics, with the patient presenting in a coma.52 Fatal hypermagnesaemia following magnesium sulphate gargles has also been reported.108 Urethral irrigation with hemiacidrin has been reported to cause symptomatic hypermagnesaemia.
Renal Failure
Hypermagnesaemia is common in patients with end stage renal disease, in those undergoing dialysis and in acute renal failure.52 In chronic renal failure, serum magnesium concentration is usually maintained until the GFR falls below 30 ml/min. However, severe hypermagnesaemia may result, especially if magnesium-containing medications are used. In patients undergoing regular dialysis, the serum magnesium concentration is directly related to the dialysate magnesium concentration. Hypermagnesaemia is also seen in patients undergoing continuous ambulatory peripheral dialysis (CAPD).109
Miscellaneous Causes
Lithium therapy causes mild hypermagnesaemia as well as hypercalcaemia.44 Modest elevations in serum magnesium concentration have been reported in familial hypocalciuric hypercalcaemia. Mild hypermagnesaemia has also been seen in hypothyroidism, Addison's disease and milk alkali syndrome.52
Effects of Hypermagnesaemia (Table 9)
Table 9.
Clinical Manifestations of Hypermagnesaemia
| Neuromuscular |
| Confusion |
| Lethargy |
| Respiratory depression |
| Absent tendon reflexes |
| Paralytic ileus |
| Bladder paralysis |
| Muscle weakness/paralysis |
| Cardiovascular |
| Hypotension |
| Bradycardia |
| Inhibition of AV and inteventricular conduction |
| Heart block |
| Cardiac arrest |
| Others |
| Nausea, vomiting |
Signs and symptoms of hypermagnesaemia are not usually apparent until serum magnesium is in excess of 2 mmol/L; however, the serum concentration at which signs and symptoms appear varies widely.
Neuromuscular Manifestations
Neuromuscular symptoms are the most common presenting problem of hypermagnesaemia and magnesium intoxication. Magnesium prevents the release of pre-synaptic acetylcholine from both sympathetic and neuromuscular junctions.105 Hypermagnesaemia causes blockage of neuromuscular transmission and depresses the conduction system of the heart and the sympathetic ganglia. Clinically, one of the earliest effects of magnesium intoxication is the disappearance of deep tendon reflexes. This is often seen at magnesium concentrations of 2.0–4.5 mmol/L. Somnolence may be observed at serum concentrations of 2 mmol/L or above. Other manifestations include muscle weakness proceeding to flaccid paralysis of voluntary and/or respiratory muscles, and depressed respiration at concentrations in excess of 5 mmol/L. The effects of magnesium on the neuromuscular junction are antagonised by calcium and the effects of hypermagnesaemia are therefore exaggerated in the presence of hypocalcaemia.
Cardiovascular Manifestations
A moderate increase in serum magnesium concentrations to 2–3 mmol/L results in mild reduction in supine as well as erect blood pressure. Higher concentration of magnesium may cause severe symptomatic hypotension.107 Magnesium causes vascular relaxation as well as antagonising the effect of vasopressor agents.10 This effect is mediated via prostacycline and calcium flux, as cyclo-oxygenase inhibitors and calcium channel blockers prevent the hypotensive effect of magnesium.11 The negative inotropic effect of hypermagnesaemia may contribute to the hypotension. Other potential factors contributing to the hypotension include central nervous system effects, skeletal muscle paralysis and depression of the carotid baro-receptor.
Magnesium is also cardiotoxic. At serum magnesium concentrations greater than 3 mmol/L, prolonged PR intervals, increased QRS duration and prolonged QT intervals are seen. Mild bradycardia is observed at concentrations greater than 7 mmol/L, and complete heart block as well as cardiac arrest may occur at concentrations greater than 7 mmol/L. Electrocardiographic changes in hypermagnesaemia are not specific and cannot be relied upon for the diagnosis of magnesium intoxication.40,52
Hypocalcaemia
Magnesium intoxication causes a reduction in serum calcium concentration. This has most commonly been reported in patients receiving magnesium therapy for pregnancy-induced hypertension.110 Serum parathyroid hormone concentration falls rapidly in response to magnesium infusion showing that hypocalcaemia may be partly due to the suppressive effects of acute hypermagnesaemia on PTH secretion.110
Other Effects
Hypermagnesaemia may cause smooth muscle paralysis resulting in paralytic ileus. Other non-specific manifestations of magnesium intoxication include nausea, vomiting and cutaneous flushing. Hypermagnesaemia may also interfere with blood clotting due to interference with platelet adhesiveness, thrombin generation time and clotting time.40
Management of Hypermagnesaemia
Most cases of hypermagnesaemia can be prevented. The possibility of hypermagnesaemia should be anticipated in any patient receiving magnesium treatment, especially if the patient has reduced renal function, and serum magnesium concentration should be monitored daily.
When hypermagnesaemia is found, magnesium therapy should be withdrawn and this is all that is needed in most patients with mild to moderate hypermagnesaemia. In patients with symptomatic hypermagnesaemia, serum magnesium should be lowered and the effects of hypermagnesaemia antagonised. Calcium antagonises the toxic effects of magnesium and therefore patients with severe magnesium intoxication should be given 1 gm of intravenous calcium gluconate. This should be followed by the infusion of 150–100 mg of calcium over 5–10 minutes, which usually causes a dramatic improvement in the patient's clinical condition. Administration of glucose and insulin may also help to promote magnesium entry into cells. If the patient is in renal failure, peritoneal or haemodialysis against a low dialysis magnesium fluid will rapidly and effectively lower the serum magnesium concentration. Occasionally exchange transfusion has been used in severe neonatal hypermagnesaemia.
Conclusion
Disorders of magnesium metabolism are common in hospital patients and are frequently unrecognised. Low magnesium intake may be a contributor to many diseases including diabetes, cardiovascular disease and osteoporosis. Common complications of hypomagnesaemia include cardiac arrhythmias, and hypocalcaemia. Hypermagnesaemia, though less frequent, can also lead to cardiovascular and neuromuscular manifestations. Early recognition of disordered magnesium metabolism and correction of the electrolyte imbalance is necessary to avoid these complications.
References
- 1.Altura BM. Basic biochemistry and physiology of magnesium: a brief review. Mag Tr Ele. 1991;10:167–171. [PubMed] [Google Scholar]
- 2.Ryan MF. The role of magnesium in clinical biochemistry: an overview. Ann Clin Biochem. 1991;28:19–26. doi: 10.1177/000456329102800103. [DOI] [PubMed] [Google Scholar]
- 3.Noronha JL, Matuschak GM. Magnesium in critical illness: metabolism, assessment, and treatment. Intensive Care Med. 2002;28:667–679. doi: 10.1007/s00134-002-1281-y. [DOI] [PubMed] [Google Scholar]
- 4.Saris NE, Mervaala E, Karppanen H, Khawaja JA, Lewenstam A. Magnesium. An update on physiological, clinical and analytical aspects. Clin Chim Acta. 2000;294:1–26. doi: 10.1016/s0009-8981(99)00258-2. [DOI] [PubMed] [Google Scholar]
- 5.Kroll MH, Elin RJ. Relationships between magnesium and protein concentrations in serum. Clin Chem. 1985;31:244–246. [PubMed] [Google Scholar]
- 6.Morris ME. Brain and CSF magnesium concentrations during magnesium deficit in animals and humans: neurological symptoms. Magnes Res. 1992;5:303–313. [PubMed] [Google Scholar]
- 7.de Rouffignac C, Quamme G. Renal magnesium handling and its hormonal control. Physiol Rev. 1994;74:305–322. doi: 10.1152/physrev.1994.74.2.305. [DOI] [PubMed] [Google Scholar]
- 8.Quamme GA, Rabkin SW. Cytosolic free magnesium in cardiac myocytes: identification of a Mg2+ influx pathway. Biochem Biophys Res Commun. 1990;167:1406–1412. doi: 10.1016/0006-291x(90)90679-h. [DOI] [PubMed] [Google Scholar]
- 9.Rude RK. Magnesium metabolism and deficiency. Endocrinol Metab Clin North Am. 1993;22:377–395. [PubMed] [Google Scholar]
- 10.Fawcett WJ, Haxby EJ, Male DA. Magnesium: physiology and pharmacology. Br J Anaesth. 1999;83:302–320. doi: 10.1093/bja/83.2.302. [DOI] [PubMed] [Google Scholar]
- 11.Nadler JL, Rude RK. Disorders of magnesium metabolism. Endocrinol Metab Clin North Am. 1995;24:623–641. [PubMed] [Google Scholar]
- 12.Kayne LH, Lee DB. Intestinal magnesium absorption. Miner Electrolyte Metab. 1993;19:210–217. [PubMed] [Google Scholar]
- 13.Yu AS. Evolving concepts in epithelial magnesium transport. Curr Opin Nephrol Hypertens. 2001;10:649–53. doi: 10.1097/00041552-200109000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Konrad M, Weber S. Recent advances in molecular genetics of hereditary magnesium-losing disorders. J Am Soc Nephrol. 2003;14:249–260. doi: 10.1097/01.asn.0000049161.60740.ce. [DOI] [PubMed] [Google Scholar]
- 15.Dai LJ, Ritchie G, Kerstan D, Kang HS, Cole DE, Quamme GA. Magnesium transport in the renal distal convoluted tubule. Physiol Rev. 2001;81:51–84. doi: 10.1152/physrev.2001.81.1.51. [DOI] [PubMed] [Google Scholar]
- 16.Vetter T, Lohse MJ. Magnesium and the parathyroid. Curr Opin Nephrol Hypertens. 2002;11:403–10. doi: 10.1097/00041552-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Ryan MF, Barbour H. Magnesium measurement in routine clinical practice. Ann Clin Biochem. 1998;35:449–459. doi: 10.1177/000456329803500401. [DOI] [PubMed] [Google Scholar]
- 18.Young DS. Effects of preanalytical variables on clinical laboratory tests. 2nd Ed. Washington, AACC 1997.
- 19.Bardicef M, Bardicef O, Sorokin Y, Altura BM, Altura BT, Cotton DB, Resnick LM. Extracellular and intra-cellular magnesium depletion in pregnancy and gestational diabetes. Am J Obstet Gynecol. 1995;172:1009–1013. doi: 10.1016/0002-9378(95)90035-7. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Revalderia J, Garcia-Bermejo S, Menchen-Herreros A, Fernandez-Rodriguez E. Biological variation of Zn, Cu, and Mg in serum of healthy subjects. Clin Chem. 1990;36:2140–2141. [PubMed] [Google Scholar]
- 21.Fox C, Ramsoomair D, Carter C. Magnesium: its proven and potential clinical significance. South Med J. 2001;94:1195–1201. [PubMed] [Google Scholar]
- 22.Elin RJ, Hosseini JM, Gill JR., Jr Erythrocyte and mononuclear blood cell magnesium concentrations are normal in hypomagnesemic patients with chronic renal magnesium wasting. J Am Coll Nutr. 1994;13:463–466. doi: 10.1080/07315724.1994.10718435. [DOI] [PubMed] [Google Scholar]
- 23.Martin BJ, Lyon TD, Walker W, Fell GS. Mononuclear blood cell magnesium in older subjects: evaluation of its use in clinical practice. Ann Clin Biochem. 1993;30:23–27. doi: 10.1177/000456329303000104. [DOI] [PubMed] [Google Scholar]
- 24.Moller Jensen B, Klaaborg KE, Alstrup P, Arendrup H, Klitgard NA, Pedersen KE. Magnesium content of the human heart. Scand J Thorac Cardiovasc Surg. 1991;25:155–158. doi: 10.3109/14017439109098102. [DOI] [PubMed] [Google Scholar]
- 25.Goto K, Yasue H, Okumura K, Matsuyama K, Kugiyama K, Miyagi H, Higashi T. Magnesium deficiency detected by intravenous loading test in variant angina pectoris. Am J Cardiol. 1990;65:709–712. doi: 10.1016/0002-9149(90)91375-g. [DOI] [PubMed] [Google Scholar]
- 26.Fischer PW, Giroux A. An evaluation of plasma and erythrocyte magnesium concentration and the activities of alkaline phosphatase and creatine kinase as indicators of magnesium status. Clin Biochem. 1991;24:215–218. doi: 10.1016/0009-9120(91)90616-m. [DOI] [PubMed] [Google Scholar]
- 27.Whang R, Oei TO, Aikawa JK, Watanabe A, Vannatta J, Fryer A, Markanich M. Predictors of clinical hypo-magnesemia. Hypokalemia, hypophosphatemia, hyponatremia, and hypocalcemia. Arch Intern Med. 1984;144:1794–1796. [PubMed] [Google Scholar]
- 28.Wong ET, Rude RK, Singer FR, Shaw ST., Jr A high prevalence of hypomagnesemia and hpermagnesemia in hospitalised patients. Am J Clin Pathol. 1983;79:348–352. doi: 10.1093/ajcp/79.3.348. [DOI] [PubMed] [Google Scholar]
- 29.Hayes JP, Ryan MF, Brazil N, Riordan TO, Walsh JB, Coakley D. Serum hypomagnesaemia in an elderly day-hospital population. Ir Med J. 1989;82:117–11. [PubMed] [Google Scholar]
- 30.Kingston ME, Al-Siba'i MB, Skooge WC. Clinical manifestations of hypomagnesemia. Crit Care Med. 1986;14:950–954. doi: 10.1097/00003246-198611000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Boyd JC, Bruns DE, Wills MR. Frequency of hypo-magnesemia in hypokalemic states. Clin Chem. 1983;29:178–179. [PubMed] [Google Scholar]
- 32.Chernow B, Bamberger S, Stoiko M, et al. Hypomagnesemia in patients in postoperative intensive care. Chest. 1989;95:391–397. doi: 10.1378/chest.95.2.391. [DOI] [PubMed] [Google Scholar]
- 33.Ryzen E. Magnesium homeostasis in critically ill patients. Magnesium. 1989;8:201–212. [PubMed] [Google Scholar]
- 34.Rubeiz GJ, Thill-Baharozian M, Hardie D, Carlson RW. Association of hypomagnesemia and mortality in acutely ill medical patients. Crit Care Med. 1993;21:203–209. doi: 10.1097/00003246-199302000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Whang R, Ryder KW. Frequency of hypomagnesemia and hypermagnesemia. Requested vs routine. JAMA. 1990;263:3063–3064. [PubMed] [Google Scholar]
- 36.al-Ghamdi SM, Cameron EC, Sutton RA. Magnesium deficiency: pathophysiologic and clinical overview. Am J Kidney Dis. 1994;24:737–752. doi: 10.1016/s0272-6386(12)80667-6. [DOI] [PubMed] [Google Scholar]
- 37.Ryzen E, Rude RK. Low intracellular magnesium in patients with acute pancreatitis and hypocalcemia. West J Med. 1990;152:145–148. [PMC free article] [PubMed] [Google Scholar]
- 38.Satur CM, Stubington SR, Jennings A, Newton K, Martin PG, Gebitekin C, Walker DR. Magnesium flux during and after open heart operations in children. Ann Thorac Surg. 1995;59:921–927. doi: 10.1016/0003-4975(95)00049-q. [DOI] [PubMed] [Google Scholar]
- 39.Prebble JJ. Primary infantile hypomagnesaemia: report of two cases. J Paediatr Child Health. 1995;31:54–56. doi: 10.1111/j.1440-1754.1995.tb02915.x. [DOI] [PubMed] [Google Scholar]
- 40.Yu ASL. Disturbances of magnesium metabolism. In: The Kidney. Ed: Brenner BM. WB Saunders 6th Ed, Philadelphia, 1999; 1055–1070.
- 41.Torralbo A, Portoles J, Perez Perez AJ, Barrientos A. Hypomagnesemic hypocalcemia in chronic renal failure. Am J Kidney Dis. 1993;21:167–171. doi: 10.1016/s0272-6386(12)81088-2. [DOI] [PubMed] [Google Scholar]
- 42.Tattersall JE, Dick C, Doyle S, Greenwood RN, Farrington K. Alkalosis and hypomagnesaemia: unwanted effects of a low-calcium CAPD solution. Nephrol Dial Transplant. 1995;10:258–262. [PubMed] [Google Scholar]
- 43.Warnock DG. Genetic forms of renal potassium and magnesium wasting. Am J Med. 2002;112:235–6. doi: 10.1016/s0002-9343(01)01136-6. [DOI] [PubMed] [Google Scholar]
- 44.Swaminathan R. Disorders of metabolism 2. In: Textbook of Adverse Drug Reactions, Eds: Davies DM, RE Ferner, H de Glanville. 5th ed. Chapman & Hall Medical, London 1998; 442–540.
- 45.Dorup I. Magnesium and potassium deficiency. Its diagnosis, occurrence and treatment in diuretic therapy and its consequences for growth, protein synthesis and growth factors. Acta Physiol Scand Suppl. 1994;618:1–55. [PubMed] [Google Scholar]
- 46.Markmann M, Rothman R, Reichman B, Hakes T, Lewis JL, Jr, Rubin S, Jones W, Almadrones L, Hoskins W. Persistent hypomagnesemia following cisplatin chemotherapy in patients with ovarian cancer. J Cancer Res Clin Oncol. 1991;117:89–90. doi: 10.1007/BF01613129. [DOI] [PubMed] [Google Scholar]
- 47.Koch NP, Hadj-Aissa A, Schell M, Dubourg L, Brunat-Mentigny M, Cochat P. Long-term nephrotoxicity of cisplatin, ifosfamide, and methotrexate in osteosarcoma. Pediatr Nephrol. 1998;12:572–575. doi: 10.1007/s004670050507. [DOI] [PubMed] [Google Scholar]
- 48.Bianchetti MG, Kanaka C, Ridolfi-Luthy A, Hirt A, Wagner HP, Oetliker OH. Persisting renotubular sequelae after cisplatin in children and adolescents. Am J Nephrol. 1991;11:127–130. doi: 10.1159/000168288. [DOI] [PubMed] [Google Scholar]
- 49.Townsend DM, Deng M, Zhang L, Lapus MG, Hanigan MH. Metabolism of Cisplatin to a nephro-toxin in proximal tubule cells. J Am Soc Nephrol. 2003;14:1–10. doi: 10.1097/01.asn.0000042803.28024.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daugaard G, Abildgaard U, Holstein-Rathlou NH, Bruunshuus I, Bucher D, Leyssac PP. Renal tubular function in patients treated with high-dose cisplatin. Clin Pharmacol Ther. 1988;44:164–172. doi: 10.1038/clpt.1988.132. [DOI] [PubMed] [Google Scholar]
- 51.English MW, Skinner R, Pearson AD, Price L, Wyllie R, Craft AW. Dose-related nephrotoxicity of carboplatin in children. Br J Cancer. 1999;81:336–341. doi: 10.1038/sj.bjc.6690697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swaminathan R, Hypo - hypermagnesaemia. In: Oxford Textbook of Nephrology. Eds: Davison AM, Cameron JS, Gunfield J-P, Kerr DNS, Ritz E, Winearls. 2nd ed. Oxford University Press, Oxford. 1998; 271–310.
- 53.Elliott C, Newman N, Madan A. Gentamicin effects on urinary electrolyte excretion in healthy subjects. Clin Pharmacol Ther. 2000;67:16–21. doi: 10.1067/mcp.2000.103864. [DOI] [PubMed] [Google Scholar]
- 54.Shiah CJ, Tsai DM, Liao ST, Siauw CP, Lee LS. Acute muscular paralysis in an adult with subclinical Bartter's syndrome associated with gentamicin administration. Am J Kidney Dis. 1994;24:932–935. doi: 10.1016/s0272-6386(12)81064-x. [DOI] [PubMed] [Google Scholar]
- 55.Ali BH, Abdel Gayoum AA, Bashir AA. Gentamicin nephrotoxicity in rat: some biochemical correlates. Pharmacol Toxicol. 1992;70:419–423. doi: 10.1111/j.1600-0773.1992.tb00500.x. [DOI] [PubMed] [Google Scholar]
- 56.Mazzaferro S, Barberi S, Scarda A, Pasquali M, Rubino F, D'Erasmo E. Ionised and total serum magnesium in renal transplant patients. J Nephrol. 2002;15:275–80. [PubMed] [Google Scholar]
- 57.Lote CJ, Thewles A, Wood JA, Zafar T. The hypo-magnesaemic action of FK506: urinary excretion of magnesium and calcium and the role of parathyroid hormone. Clin Sci (Lond) 2000;99:285–292. [PubMed] [Google Scholar]
- 58.Hagley MT, Traeger SM, Schuckman H. Pronounced metabolic response to modest theophylline overdose. Ann Pharmacother. 1994;28:195–196. doi: 10.1177/106002809402800207. [DOI] [PubMed] [Google Scholar]
- 59.Knutsen R, Bohmer T, Falch J. Intravenous theophylline-induced excretion of calcium, magnesium and sodium in patients with recurrent asthmatic attacks. Scand J Clin Lab Inves. 1994;54:119–125. doi: 10.3109/00365519409086518. [DOI] [PubMed] [Google Scholar]
- 60.Flack JM, Ryder KW, Strickland D, Whang R. Metabolic correlates of theophylline therapy: a concentration-related phenomenon. Ann Pharmacother. 1994;28:175–179. doi: 10.1177/106002809402800202. [DOI] [PubMed] [Google Scholar]
- 61.Haffner CA, Kendall MJ. Metabolic effects of beta 2-agonists. J Clin Pharm Ther. 1992;17:155–164. doi: 10.1111/j.1365-2710.1992.tb01285.x. [DOI] [PubMed] [Google Scholar]
- 62.Wazny LD, Brophy DF. Amiloride for the prevention of amphotericin B-induced hypokalemia and hypo-magnesemia. Ann Pharmacother. 2000;34:94–7. doi: 10.1345/aph.19127. [DOI] [PubMed] [Google Scholar]
- 63.Otsuka M, Kanamori H, Sasaki S, et al. Torsades de pointes complicating pentamidine therapy of Pneumocystis carinii pneumonia in acute myelogenous leukemia. Intern Med. 1997;36:705–708. doi: 10.2169/internalmedicine.36.705. [DOI] [PubMed] [Google Scholar]
- 64.Shah GM, Alvarado P, Kirschenbaum MA. Symptomatic hypocalcemia and hypomagnesemia with renal magnesium wasting associated with pentamidine therapy in a patient with AIDS. Am J Med. 1990;89:380–382. doi: 10.1016/0002-9343(90)90354-g. [DOI] [PubMed] [Google Scholar]
- 65.Noormohamed FH, Youle MS, Tang B, Martin-Munley S, Gazzard BG, Lant AF. Foscarnet-induced changes in plasma concentrations of total and ionized calcium and magnesium in HIV-positive patients. Antivir Ther. 1996;1:172–179. [PubMed] [Google Scholar]
- 66.Elin RJ, Hosseini JM, Fitzpatrick L, Blizitoes MM, Marx SJ. Blood magnesium status of patients with parathyroid disease. Magnes Tr Ele. 1990;9:119–123. [PubMed] [Google Scholar]
- 67.de Valk VH. Magnesium in diabetes mellitus. Neth J Med. 1999;54:139–146. doi: 10.1016/s0300-2977(99)00005-4. [DOI] [PubMed] [Google Scholar]
- 68.Crook M, Couchman S, Tutt P, Amiel S, Swaminathan R. Erythrocyte, plasma total, ultrafiltrable and platelet magnesium in type 2 (non-insulin dependent) diabetes mellitus. Diabetes Res. 1994;27:73–79. [PubMed] [Google Scholar]
- 69.Corsonello A, Ientile R, Buemi M, et al. Serum ionized magnesium levels in type 2 diabetic patients with microalbuminuria or clinical proteinuria. Am J Nephrol. 2000;20:187–192. doi: 10.1159/000013582. [DOI] [PubMed] [Google Scholar]
- 70.Gullestad L, Dolva LO, Waage A, Falch D, Fagerthun H, Kjekshus J. Magnesium deficiency diagnosed by an intravenous loading test. Scand J Clin Lab Invest. 1992;52:245–253. doi: 10.3109/00365519209088355. [DOI] [PubMed] [Google Scholar]
- 71.Garland HO. New experimental data on the relationship between diabetes mellitus and magnesium. Magnes Res. 1992;5:193–202. [PubMed] [Google Scholar]
- 72.Grafton G, Bunce CM, Sheppard MC, Brown G, Baxter MA. Effect of Mg2+ on Na(+)-dependent inositol transport. Role for Mg2+ in etiology of diabetic complications. Diabetes. 1992;41:35–39. doi: 10.2337/diab.41.1.35. [DOI] [PubMed] [Google Scholar]
- 73.Vamvakas S, Teschner M, Bahner U, Heidland A. Alcohol abuse: potential role in electrolyte disturbances and kidney diseases. Clin Nephrol. 1998;49:205–213. [PubMed] [Google Scholar]
- 74.Rivlin RS. Magnesium deficiency and alcohol intake: mechanisms, clinical significance and possible relation to cancer development (a review) J Am Coll Nutr. 1994;13:416–423. doi: 10.1080/07315724.1994.10718430. [DOI] [PubMed] [Google Scholar]
- 75.Ryan MP. Interrelationships of magnesium and potassium homeostasis. Miner Electrolyte Metab. 1993;19:290–295. [PubMed] [Google Scholar]
- 76.Fatemi S, Ryzen E, Flores J, Endres DB, Rude RK. Effect of experimental human magnesium depletion on parathyroid hormone secretion and 1,25-dihydroxyvitamin D metabolism. J Clin Endocrinol Metab. 1991;73:1067–1072. doi: 10.1210/jcem-73-5-1067. [DOI] [PubMed] [Google Scholar]
- 77.Zofkova I, Kancheva RL. The relationship between magnesium and calciotropic hormones. Magnes Res. 1995;8:77–84. [PubMed] [Google Scholar]
- 78.Mori S, Harada S, Okazaki R, Inoue D, Matsumoto T, Ogata E. Hypomagnesemia with increased metabolism of parathyroid hormone and reduced responsiveness to calcitropic hormones. Intern Med. 1992;31:820–824. doi: 10.2169/internalmedicine.31.820. [DOI] [PubMed] [Google Scholar]
- 79.Risco F, Traba ML, de la Piedra C. Possible alterations of the in vivo 1,25(OH)2D3 synthesis and its tissue distribution in magnesium-deficient rats. Magnes Res. 1995;8:27–35. [PubMed] [Google Scholar]
- 80.Varon ME, Sherer DM, Abramowicz JS, Akiyama T. Maternal ventricular tachycardia associated with hypomagnesemia. Am J Obstet Gynecol. 1992;167:1352–1355. doi: 10.1016/s0002-9378(11)91715-3. [DOI] [PubMed] [Google Scholar]
- 81.Millane TA, Ward DE, Camm AJ. Is hypomagnesemia arrhythmogenic? Clin Cardiol. 1992;15:103–108. doi: 10.1002/clc.4960150210. [DOI] [PubMed] [Google Scholar]
- 82.Eichhorn EJ, Tandon PK, DiBianco R, Timmis GC, Fenster PE, Shannon J, Packer M. Clinical and prognostic significance of serum magnesium concentration in patients with severe chronic congestive heart failure: the PROMISE Study. J Am Coll Cardiol. 1993;21:634–640. doi: 10.1016/0735-1097(93)90095-i. [DOI] [PubMed] [Google Scholar]
- 83.Reinhart RA. Clinical correlates of the molecular and cellular actions of magnesium on the cardiovascular system. Am Heart J. 1991;121:1513–1521. doi: 10.1016/0002-8703(91)90160-j. [DOI] [PubMed] [Google Scholar]
- 84.Martin BJ, McAlpine JK, Devine BL. Hypomagnesaemia in elderly digitalised patients. Scott Med J. 1988;33:273–274. doi: 10.1177/003693308803300309. [DOI] [PubMed] [Google Scholar]
- 85.Paolisso G, Scheen A, Cozzolino D, Di Maro G, Varricchio M, D'Onofrio F, Lefebrvre PJ. Changes in glucose turnover parameters and improvement of glucose oxidation after 4-week magnesium administration in elderly noninsulin-dependent (type II) diabetic patients. J Clin Endocrinol Metab. 1994;78:1510–1514. doi: 10.1210/jcem.78.6.8200955. [DOI] [PubMed] [Google Scholar]
- 86.Nozue T, Kobayashi A, Uemasu F, Takagi Y, Sako A, Endoh H. Magnesium status, serum HDL cholesterol, and apolipoprotein A-1 levels. J Pediatr Gastroenterol Nutr. 1995;20:316–318. doi: 10.1097/00005176-199504000-00009. [DOI] [PubMed] [Google Scholar]
- 87.Nadler JL, Buchanan T, Natarajan R, Antonipillai I, Bergman R, Rude R. Magnesium deficiency produces insulin resistance and increased thromboxane synthesis. Hypertension. 1993;21:1024–1029. doi: 10.1161/01.hyp.21.6.1024. [DOI] [PubMed] [Google Scholar]
- 88.Urdal P, Landmark K, Basmo GM. Mononuclear cell magnesium and retention of magnesium after intravenous loading in patients with acute myocardial infarction. Scand J Clin Lab Invest. 1992;52:763–766. doi: 10.3109/00365519209115523. [DOI] [PubMed] [Google Scholar]
- 89.Sojka JE, Weaver CM. Magnesium supplementation and osteoporosis. Nutr Rev. 1995;53:71–74. doi: 10.1111/j.1753-4887.1995.tb01505.x. [DOI] [PubMed] [Google Scholar]
- 90.al-Qadreh A, Athanasopoulou H, Voskaki I. Inhibitors of stone formation in hypercalciuric children with and without stone disease. Eur Urol. 1992;21:227–230. doi: 10.1159/000474843. [DOI] [PubMed] [Google Scholar]
- 91.Levy FL, Adams-Huet B, Pak CY. Ambulatory evaluation of nephrolithiasis: an update of a 1980 protocol. Am J Med. 1995;98:50–59. doi: 10.1016/S0002-9343(99)80080-1. [DOI] [PubMed] [Google Scholar]
- 92.Britton J, Pavord I, Richards K, Wisniewski A, Knox A, Lewis S, Tattersfield A, Weiss S. Dietary magnesium, lung function, wheezing, and airway hyperreactivity in a random adult population sample. Lancet. 1994;344:357–362. doi: 10.1016/s0140-6736(94)91399-4. [DOI] [PubMed] [Google Scholar]
- 93.Silverman RA, Osborn H, Runge J, et al. IV magnesium sulfate in the treatment of acute severe asthma: a multicenter randomized controlled trial. Chest. 2002;122:489–97. doi: 10.1378/chest.122.2.489. [DOI] [PubMed] [Google Scholar]
- 94.Woods KL, Flegher S, Roffe C, Haider Y. Intravenous magnesium sulphate in suspected acute myocardial infarction: results of the second Leicester Intravenous Magnesium Intervention Trial (LIMIT-2) Lancet. 1992;339:1553–1558. doi: 10.1016/0140-6736(92)91828-v. [DOI] [PubMed] [Google Scholar]
- 95.Woods KL, Fletcher S. Long-term outcome after intravenous magnesium sulphate in suspected acute myocardial infarction: the second Leicester Intravenous Magnesium Intervention Trial (LIMIT-2) Lancet. 1994;343:816–819. doi: 10.1016/s0140-6736(94)92024-9. [DOI] [PubMed] [Google Scholar]
- 96.ISIS-4: a randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58,050 patients with suspected acute myocardial infarction. ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group. Lancet. 1995;345:669–685. [PubMed] [Google Scholar]
- 97.Early administration of intravenous magnesium to high-risk patients with acute myocardial infarction in the Magnesium in Coronaries (MAGIC) Trial: a randomised controlled trial. Lancet. 2002;360:1189–1196. doi: 10.1016/s0140-6736(02)11278-5. [DOI] [PubMed] [Google Scholar]
- 98.Kaye P, O'Sullivan I. The role of magnesium in the emergency department. Emerg Med J. 2002;19:288–291. doi: 10.1136/emj.19.4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Which anticonvulsant for women with eclampsia? Evidence from the Collaborative Eclampsia Trial. Lancet. 1995;345:1455–1463. [PubMed] [Google Scholar]
- 100.Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2000;359:1877–90. doi: 10.1016/s0140-6736(02)08778-0. [DOI] [PubMed] [Google Scholar]
- 101.Greene MF. Magnesium sulfate for preeclampsia. N Engl J Med. 2003;348:275–6. doi: 10.1056/NEJMp020166. [DOI] [PubMed] [Google Scholar]
- 102.Belfort MA, Anthony J, Saade GR, Allen JCJ. A comparison of magnesium sulfate and nimodipine for the prevention of eclampsia. N Engl J Med. 2003;348:304–11. doi: 10.1056/NEJMoa021180. [DOI] [PubMed] [Google Scholar]
- 103.Rodts-Palenik S, Morrison JC. Tocolysis: an update for the practitioner. Obstet Gynecol Surv. 2002;57:29–34. doi: 10.1097/00006254-200205001-00001. [DOI] [PubMed] [Google Scholar]
- 104.Huey CG, Chan KM, Wong ET, Nelson JM, Durand M. Los Angeles County-University of Southern California Medical Center clinical pathology case conference: extreme hypermagnesemia in a neonate. Clin Chem. 1995;41:615–618. [PubMed] [Google Scholar]
- 105.Oren S, Rapoport J, Zlotnik M, Brami JL, Heimer D, Chaimovitz C. Extreme hypermagnesemia due to ingestion of Dead Sea water. Nephron. 1987;47:199–201. doi: 10.1159/000184491. [DOI] [PubMed] [Google Scholar]
- 106.Jaing TH, Hung IJ, Chung HT, Lai CH, Liu WM, Chang KW. Acute hypermagnesemia: a rare complication of antacid administration after bone marrow transplantation. Clin Chim Acta. 2002;326:201–203. doi: 10.1016/s0009-8981(02)00308-x. [DOI] [PubMed] [Google Scholar]
- 107.Clark BA, Browns RS. Unsuspected morbid hypermagnesemia in elderly patients. Am J Nephrol. 1992;18:336–43. doi: 10.1159/000168469. [DOI] [PubMed] [Google Scholar]
- 108.Birrer RB, Shallash AJ, Totten V. Hypermagnesemia-induced fatality following Epsom salt gargles. J Emerge Med. 2002;822:185–188. doi: 10.1016/s0736-4679(01)00462-0. [DOI] [PubMed] [Google Scholar]
- 109.Hutchison AJ, Merchant M, Boulton HF, Hinchcliffe R, Gokal R. Calcium and magnesium mass transfer in peritoneal dialysis patients using 1.25 mmol/L calcium, 0.25 mmol/L magnesium dialysis fluid. Perit Dial Int. 1993;13:219–223. [PubMed] [Google Scholar]
- 110.Cholst IN, Steinberg SF, Tropper PJ, Fox HE, Segre GV, Bilezikian JP. The influence of hypermagnesemia on serum calcium and parathyroid hormone levels in human subjects. N Eng J Med. 1984;310:1221–1225. doi: 10.1056/NEJM198405103101904. [DOI] [PubMed] [Google Scholar]