Abstract
Sb(III) oxidation was documented in an Agrobacterium tumefaciens isolate that can also oxidize As(III). Equivalent Sb(III) oxidation rates were observed in the parental wild-type organism and in two well-characterized mutants that cannot oxidize As(III) for fundamentally different reasons. Therefore, despite the literature suggesting that Sb(III) and As(III) may be biochemical analogs, Sb(III) oxidation is catalyzed by a pathway different than that used for As(III). Sb(III) and As(III) oxidation was also observed for an eukaryotic acidothermophilic alga belonging to the order Cyanidiales, implying that the ability to oxidize metalloids may be phylogenetically widespread.
Antimony (Sb) occurs globally in fresh and marine waters and in soils, with the most common mineral form being stibnite (Sb2S3) (6). Sb is typically found with arsenic (As), another group V element having similar chemistry and toxicity, and is recognized by the U.S. Environmental Protection Agency as a priority pollutant (8, 14). Unlike microbe-As interactions, for which significant progress has been made in defining the genetics and physiology of microbial arsenite [As(III)] oxidation and arsenate [As(V)] reduction (reviewed in references 19 and 20), microbe-Sb interactions are poorly understood, and as a consequence, the geomicrobiology of Sb is as yet essentially undefined. Sb(III) biomethylation has been reported (5), but otherwise, reports of microbe-Sb redox interactions are restricted to studies of an organism referred to as Stibiobacter senarmontii and described as being capable of oxidizing the mineral senarmontite to form Sb2O5 (reviewed in reference 6). In the intervening 3 decades, no further characterization or confirmation of this organism has been reported, nor have there been reports of other microorganisms having the capacity to oxidize Sb(III).
Given the structural similarities between As and Sb, the presence of one could influence the biological interactions of the other (4). Indeed, both As(III) and Sb(III) will induce the microbial ars-based arsenic defense response (11), both are taken up via the same aquaglyceroporin channel, GlyF (16), and in bacteria they are both extruded by the same porter, ArsB (16). These observations suggest the possibility that the same enzymatic pathways used for As(III) oxidation may also be used for Sb(III). In recent studies regarding the genetics underlying As(III) oxidation in Agrobacterium tumefaciens, we discovered regulatory (aoxR) and Na+:H+ antiporter (mrpB) mutants which are defective in As(III) oxidation (12, 13). In subsequent investigations, we found the wild-type A. tumefaciens strain to also be capable of oxidizing Sb(III). This provided an exceptional opportunity to directly determine whether As(III) oxidation and Sb(III) oxidation share similar enzymes.
As and Sb speciation and analysis.
Borohydride reduction-based speciation was used for both As and Sb analysis. As(III) speciation protocols were as previously described (10). At near-neutral pH, Sb(III) and methyl-Sb species are converted to their corresponding volatile stibines, whereas unmethylated Sb(V) is not (3). For Sb speciation, cell-free culture samples (diluted to a volume of 5 ml) were buffered by the addition of Tris-HCl (1 ml, 1 M, pH 6), and then borohydride (1 ml, 1% NaBH4 in 0.1% NaOH) was added. Any potential stibines were driven from the sample by purging with N2 gas for 5 minutes. Following removal of Sb(III) as stibine, residual unmethylated Sb(V) was determined by inductively coupled plasma mass spectrometry (Agilent 7500ce) with operation in normal plasma mode by use of standard parameters and with Sb quantified at m/z 121 and 123. For all experiments, Sb(III) was provided as potassium antimonyl tartrate because of the very poor aqueous solubility of antimony trioxide. The tartrate complex does not interfere with cellular access to Sb(III) for uptake or extrusion (16) or with the capacity of Sb(III) to interact with regulatory proteins (11). Calibration curves were prepared with potassium antimonyl tartrate in 2% nitric acid with standards ranging in concentration from 1 to 100 ppb. As controls, potassium antimonyl tartrate and trimethyl-Sb(V) standards [Sb(CH3)3Cl2, 100 ppb in water; Sigma-Aldrich] were reduced with borohydride as described above to verify the complete conversion of these compounds to their corresponding stibines. After reduction of these standards, no Sb was detected in the solution by using inductively coupled plasma mass spectrometry analysis.
Sb(III) oxidation in A. tumefaciens.
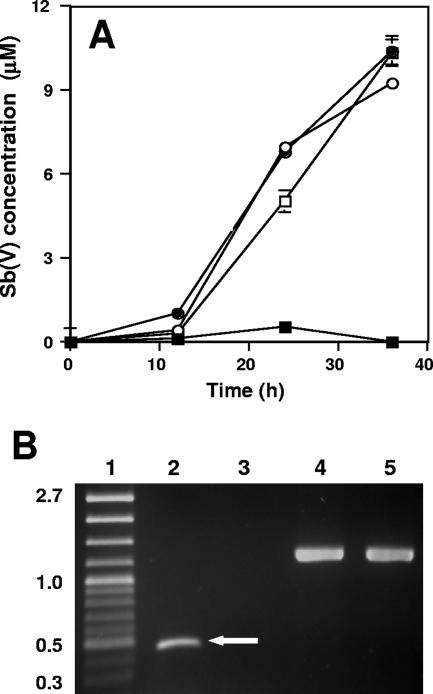
The wild-type A. tumefaciens strain 5A and the aoxR and mrpB mutants were all found to oxidize Sb(III) at the same rate (Fig. 1A). As expected for these culturing conditions and genotypes (12, 13), expression of the As(III) oxidase structural genes, aoxAB, was observed in the wild-type strain but not in the aoxR regulatory mutant (Fig. 1B). This provided evidence that Sb(III) oxidation occurred in the absence of detectable aoxAB expression and that Sb(III) does not induce aoxAB expression from an Sb(III)-sensitive promoter within the aox operon. Control reverse transcriptase PCRs (RT-PCRs) using primers specific for 16S rRNA demonstrated that MSUAt1 RNA was a suitable template for RT-PCR (Fig. 1B).
FIG. 1.
A. tumefaciens oxidation of Sb(III). (A) Sb(V) formation in the wild-type strain 5A (□), the aoxR mutant (•), the mrpB mutant (○), and uninoculated controls (▪). Data are averages from duplicate cultures, and error bars (where visible) represent the range of the two values. (B) RT-PCR amplicons documenting expression of aoxAB in strain 5A but not in the aoxR mutant strain MSUAt1. Protocols for RNA extraction from mid- to late-log-phase cells, RT-PCR conditions and primers, and absence of DNA contamination were all as previously described (12). Lanes: 1, molecular mass standards in kb; 2, wild-type strain 5A total RNA amplified with aoxAB-spanning primers (arrow denotes aoxAB RT-PCR amplicon); 3, aoxR mutant MSUAt1 total RNA amplified with aoxAB-spanning primers; 4, wild-type strain 5A total RNA amplified with 16S rRNA gene primers; 5, aoxR mutant total RNA amplified with 16S rRNA gene primers. All strains were cultured in a minimal mannitol medium as previously described (12). Cultures were amended with 50 μM Sb(III) (as potassium antimonyl tartrate). All experiments were repeated at least twice, and in each case experimental treatments (inoculated and uninoculated) were at least in duplicate.
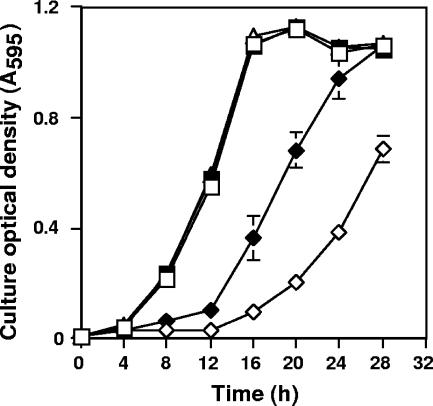
Growth responses of the wild-type strain to Sb(III) and As(III) were quite different. Cultures appeared to experience little toxicity from 50 μM As(III), because growth was the same as that in the absence of As(III) (Fig. 2). By contrast, the same concentration of Sb(III) appeared very toxic, as judged by the poorer growth (Fig. 2), although preculturing in 50 μM Sb(III) for 1 culture cycle resulted in better growth when the wild-type strain was subcultured into the same medium containing Sb(III). The lag phase was appreciably shorter, and the subsequent culture growth rates appeared to be similar to those for As(III)-treated or control cultures. Allowing for the 12-h lag phase, the final culture densities of Sb(III)-preexposed cells were the same as those for cultures grown in the presence or absence of 50 μM As(III) (Fig. 2).
FIG. 2.
Comparison of the toxicities of As(III) and Sb(III) to the wild-type A. tumefaciens strain 5A. Toxicity was estimated by growth response. Cells were first cultured in minimal mannitol ammonium broth in the presence (preexposed) or absence (naïve) of 50 μM As(III) or Sb(III) and then inoculated into the same medium amended with or without 50 μM As(III) or Sb(III). □, As-naïve strain 5A grown in the absence of 50 μM As(III); ▪, As-naïve strain 5A cultured with 50 μM As(III); ▵, As(III)-preexposed strain 5A cultured with 50 μM As(III); ⋄, Sb-naïve strain 5A cultured with 50 μM Sb(III); ⧫, Sb(III)-preexposed strain 5A cultured with 50 μM Sb(III). Symbols represent the means of at least duplicate cultures, and error bars (where visible) represent the range of the two values.
Eukaryotic microbial As(III) and Sb(III) oxidation.
Geothermal environments are known to be enriched with As and Sb (17), and so the above-described experiments were extended to examine the As/Sb oxidation capacity of an eukaryotic thermoacidophilic alga (belonging to the order Cyanidiales) isolated from Dragon Spring in Norris Geyser Basin, Yellowstone National Park (15). This organism was originally isolated as a single well-isolated colony that was subjected to three rounds of single-colony transfer on Allen's medium (1) solidified with 2% Gelrite. Based on visual inspection of the plates, the algal culture was pure and without satellite colonies near the dark green algal colonies.
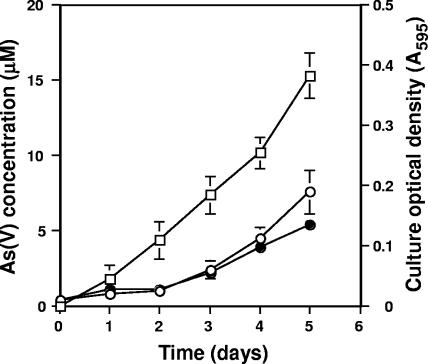
This alga, strain 5508, was found to be capable of oxidizing As(III) to As(V) (Fig. 3), the first verified pure-culture eukaryotic microorganism documented to do so. As(III) oxidation in the algal cultures could be detected within 24 h and appeared constitutive, as the As(III) oxidation profiles were the same for cells preexposed to As(III) and for As(III)-naïve cells (results not shown). Furthermore, growth appeared relatively unimpaired at the As(III) concentration used (Fig. 3). As(III) oxidation was not observed for uninoculated controls (results not shown).
FIG. 3.
Growth and As(V) formation by the Cyanidiales alga isolate CCMEE 5508. •, growth in the presence of 20 μM As(III); ○, growth in the absence of As(III); □, As(V) accumulated as a result of As(III) oxidation. No As(III) oxidation occurred in uninoculated controls or in killed controls (results not shown). Data represent the mean of duplicate cultures, and error bars (where visible) represent the range of the two values. The thermoacidophilic alga used in this work was grown at 42°C under constant illumination from cool-white fluorescent lamps (80 μE·m−2·s−2) in Allen medium (1) amended to contain 250 μM phosphate and 20 μM As(III) in closed serum bottles with headspace containing filter (0.2 μm)-sterilized CO2 (100% initial concentration). As controls, serum bottles containing uninoculated medium or autoclave-killed cells were included, with the headspace of each connected to that of the inoculated serum bottle via sterile Tygon tubing. This precaution was taken to verify that the Sb(III) oxidation observed in these experiments was not abiotic auto-oxidation due to the photosynthetically derived O2 produced by the algae.
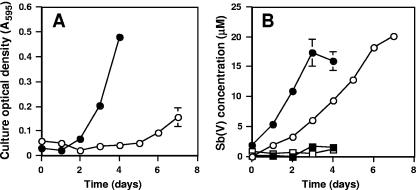
Equally significant and novel was our observation that this eukaryote was able to oxidize Sb(III) (Fig. 4). As with A. tumefaciens, Sb(III) appeared much more inhibitive to growth. Growth of Sb(III)-naïve cells was delayed for several days and was poor relative to that observed for Sb-preexposed cultures or for cultures incubated with As(III) (Fig. 3 and 4A). Sb(III) oxidation by Sb(III)-naïve cells was also slower than by Sb(III)-preexposed cells. In the former, near-complete Sb(III) oxidation required roughly 8 days, whereas in the latter case Sb(III) oxidation proceeded rapidly, reaching apparent completion within 4 days (Fig. 4B). We note with interest that algal cells preexposed to Sb(III) actually grew more robustly than cells grown with or without As(III) (Fig. 3 and 4A).
FIG. 4.
Growth response to Sb(III) and Sb(V) formation by the Cyanidiales alga isolate CCMEE 5508. (A) Growth of Sb(III)-naïve (○) and Sb(III)-preexposed (•) algal cultures. (B) Sb(V) formation in CCMEE 5508 cultures that were Sb(III) naïve (○) or Sb(III) preexposed (•). No Sb(III) oxidation occurred in uninoculated controls (□) or in killed controls (▪). Cultures were grown as described in the legend to Fig. 3 except that 20 μM Sb(III) was substituted for 20 μM As(III). Data in both panels represent the mean of four replicate cultures. Error bars (where visible) represent 1 standard deviation of the mean.
For the purposes of these experiments, the pure-culture status of this alga was established by several methods. First, extensive phase-contrast microscopy (×1,000 magnification) examination and monitoring of cultures was conducted throughout all experiments. In addition, PCR of culture nucleic acid extracts was employed as a molecularly based method for detecting organisms that may have been present at low levels and less easily observed with microscopy. For the latter, DNA was extracted from the algal cultures using methods we have described previously (18), followed by PCR amplification using the universal reverse primer Univ1392r coupled with either the Bacteria-biased forward primer Bac1070f or the Archaea-biased forward primer A931f, both as described by Ferris et al. (7). We also attempted to grow heterotrophs by inoculating 0.5% yeast extract Gelrite plates (pH 3.5) with 10- to 100-μl aliquots of stationary-phase algal cultures and incubating them at 42°C in the dark. All approaches failed to detect prokaryotes: microscopic examinations were consistently void of any cell morphology other than 8- to 10-μm-diameter spheres representing the algae, sequencing of PCR clones consistently yielded chloroplast and mitochondrial 16S rRNA gene signatures (as determined by BLAST analysis [2]), and inoculated yeast extract plates failed to demonstrate any microbial growth.
As(III) and Sb(III) are similar in that they are both quite toxic, and in bacteria thus far examined their properties appear interchangeable with respect to gene regulation and membrane trafficking (11, 16). Based on the results of our studies, however, it would appear that the biochemistries required for the oxidation of these chemically similar metalloids are different. Sb(III) oxidation was observed for two distinctly different and characterized mutants that we have previously shown to be incapable of As(III) oxidation (12, 13). In the case of the aoxR mutant, the As(III) oxidation defect derives from transposon insertional inactivation of aoxR, which encodes an apparent cognate regulatory protein member of a two-component signal transduction system involved in the regulation of As(III) oxidation in A. tumefaciens (12). In addition, however, aoxR is part of the aoxS-aoxR-aoxA-aoxB-cytC2 operon (aoxS being promoter proximal), and thus the same transposon that insertionally inactivates aoxR (12) also has polar effects on the expression of the As(III) oxidase structural genes aoxAB. Therefore, the complete lack of aoxAB expression in experiments with this mutant (Fig. 1B) was completely consistent with our previous report (12) and provides strong evidence that the As(III) oxidase enzyme is not involved in Sb(III) oxidation in this organism (Fig. 1A). Further evidence that Sb(III) oxidation occurs by a separate biochemical pathway comes from the results obtained with the mrpB mutant. MrpB is part of a multisubunit Na+:H+ antiporter (reviewed in reference 21) that was found to be essential for As(III) oxidation (13). While we have yet to determine the exact nature of how this antiporter is involved in As(III) oxidation, it nevertheless is not required for Sb(III) oxidation (Fig. 1A).
The experiments demonstrating As(III) oxidation in an acidothermophilic alga (Fig. 3) serve to offer firm documentation that eukaryotic microorganisms such as algae may potentially make significant contributions to arsenic cycling. A previous report of As(III) oxidation in a freshwater alga (9) was not accompanied by evidence that the culture did not contain prokaryotic organisms, which must be verified since prokaryote As(III) oxidation is well documented (see reviews in references 19 and 20). To our knowledge, Sb redox transformations have never been reported for an eukaryotic microorganism before, and thus the finding that this same alga will also oxidize Sb(III) considerably extends our appreciation of the extent to which eukaryotic microorganisms may participate in metalloid redox transformations. If marine algae can be shown to carry out similar redox transformations, then the implications of the observations reported herein take on significantly greater importance with respect to scale.
The response of this alga to Sb(III) was similar to that observed with A. tumefaciens in that both organisms exhibited growth responses that suggested Sb(III) is considerably more toxic than As(III) (Fig. 2 and Fig. 4A). Both organisms also appeared able to adjust to Sb(III) toxicity, as growth of both was considerably stronger after having been preexposed to Sb(III) for 1 culture cycle. The cellular responses of both organisms to Sb(III) and their apparent abilities to adapt suggest they contain an organized cellular response to Sb toxicity. When combined with the genetic evidence showing that Sb(III) oxidation occurs in the absence of enzymes and cellular functions that are essential for As(III) oxidation, we conclude that cellular mechanisms and enzymes for coping with and oxidizing Sb(III) are separate from those used for As(III). The genetic tractability of the A. tumefaciens strain 5A (12, 13) provides the opportunity to begin examining microbe-Sb interactions, which we are now exploring. Furthermore, the discovery of Sb(III) oxidation in an eukaryotic alga suggests this property may be phylogenetically widespread.
Acknowledgments
This study was supported by the NASA Exobiology Program (NAG5-8807), the USDA Soil Processes Program (2002-35107-12268), and the Montana Agricultural Experiment Station (911310), and minor support was provided by the National Science Foundation Microbial Observatory Program (MCB-0132022).
Footnotes
Published ahead of print on 16 February 2007.
REFERENCES
- 1.Allen, M. B. 1959. Studies with Cyanidium caldarium, an anomalously pigmented chlorophyte. Arch. Microbiol. 32:270-277. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. L. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreae, M. O., J.-F. Asmodé, P. Foster, and L. Van't Dack. 1981. Determination of antimony(III), antimony(V), and methylantimony species in natural waters by atomic absorption spectrometry with hydride generation. Anal. Chem. 53:1766-1771. [Google Scholar]
- 4.Andrews, P., W. R. Cullen, and E. Polishchuk. 2000. Arsenic and antimony biomethylation by Scopulariopsis brevicaulis: interaction of arsenic and antimony compounds. Environ. Sci. Technol. 34:2249-2253. [Google Scholar]
- 5.Bentley, R., and T. G. Chasteen. 2002. Microbial methylation of metalloids: arsenic, antimony, and bismuth. Microbiol. Mol. Biol. Rev. 66:250-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehrlich, H. L. 2002. Geomicrobiology, 4th ed. Marcel Dekker, New York, NY.
- 7.Ferris, M. J., G. Muyzer, and D. M. Ward. 1996. Denaturing gradient gel electrophoresis profiles of 16S rRNA-defined populations inhabiting a hot spring microbial mat community. Appl. Environ. Microbiol. 62:340-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filella, M., N. Belzile, and Y. W. Chen. 2002. Antimony in the environment: a review focused on natural waters. I. Occurrence. Earth Sci. Rev. 57:125-176. [Google Scholar]
- 9.Hasegawa, H., Y. Sohrin, K. Seki, M. Sato, K. Norisuye, K. Naito, and M. Matsui. 2001. Biosynthesis and release of methylarsenic compounds during the growth of freshwater algae. Chemosphere 43:265-272. [DOI] [PubMed] [Google Scholar]
- 10.Jackson, C. R., H. W. Langner, J. Donahoe-Christiansen, W. P. Inskeep, and T. R. McDermott. 2001. Molecular analysis of microbial community structure in an arsenite-oxidizing acidic thermal spring. Environ. Microbiol. 3:532-542. [DOI] [PubMed] [Google Scholar]
- 11.Ji, G., and S. Silver. 1992. Regulation and expression of the arsenic resistance operon from Staphylococcus aureus plasmid pI258. J. Bacteriol. 174:3684-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kashyap, D. R., L. M. Botero, W. L. Franck, D. J. Hassett, and T. R. McDermott. 2006. Complex regulation of arsenite oxidation in Agrobacterium tumefaciens. J. Bacteriol. 188:1081-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kashyap, D. R., L. M. Botero, C. Lehr, D. J. Hassett, and T. R. McDermott. 2006. A Na+:H+ antiporter and molybdate transporter are essential for arsenite oxidation in Agrobacterium tumefaciens. J. Bacteriol. 188:1577-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krachler, M., M. Burrow, and H. Emons. 1999. Optimized procedure for the determination of antimony in lipid-rich environmental matrices by flow injection hydride generation atomic absorption spectrometry. Analyst 124:923-926. [DOI] [PubMed] [Google Scholar]
- 15.Lehr, C. R., S. D. Frank, T. B. Norris, S. D'Imperio, A. V. Kalinin, J. A. Toplin, R. W. Castenholz, and T. R. McDermott. 2007. Cyanidia (Cyanidiales) population diversity and dynamics in an acid-sulfate chloride spring in Yellowstone National Park. J. Phycol. 43:3-14. [Google Scholar]
- 16.Meng, Y.-L., Z. Liu, and B. P. Rosen. 2004. As(III) and Sb(III) uptake by GlpF and efflux by ArsB in Escherichia coli. J. Biol. Chem. 279:18334-18341. [DOI] [PubMed] [Google Scholar]
- 17.Nordstrom, D. K., J. W. Bakk, and R. B. McCleskey. 2005. Ground water to surface water: chemistry of thermal outflows in Yellowstone National Park, p. 73-94. In W. P. Inskeep and T. R. McDermott (ed.) Geothermal biology and geochemistry in Yellowstone National Park. Thermal Biology Institute, Bozeman, MT.
- 18.Norris, T. B., J. Wraith, R. C. Castenholz, and T. R. McDermott. 2002. Soil microbial community diversity after a recent geothermal heating event. Appl. Environ. Microbiol. 68:6300-6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silver, S., and L. T. Phung. 2005. Genes and enzymes involved in bacterial oxidation and reduction of inorganic arsenic. Appl. Environ. Microbiol. 71:599-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stolz, J. F., P. Basu, J. M. Santini, and R. S. Oremland. 2006. Arsenic and selenium in microbial metabolism. Annu. Rev. Microbiol. 60:107-130. [DOI] [PubMed] [Google Scholar]
- 21.Swartz, T. H., S. Ikewada, O. Ishikawa, M. Ito, and T. A. Krulwich. 2005. The Mrp system: a giant among monovalent cation/proton antiporters? Extremophiles 9:345-354. [DOI] [PubMed] [Google Scholar]