Abstract
Species-specific primers and a multiplex PCR assay were developed for the simultaneous identification and differentiation of Pseudomonas fragi, P. lundensis, and P. putida based on the coamplification of different portions of the small subunit of the carbamoyl phosphate synthase gene (carA). The carA multiplex PCR was used to detect the presence of the three Pseudomonas species from beef, chicken, and pork samples and proved to be effective in showing their evolution during the storage of meat.
Several species of the genus Pseudomonas are very often recognized as the principal causative agents of the spoilage of fresh foods stored aerobically. Members of the Pseudomonas fluorescens group, along with the psychrotrophic P. fragi, P. lundensis, and P. putida, are usually involved in spoilage of milk, meat, and fish, even during storage at low temperatures. These bacteria are often isolated from spoiled meat (10, 12, 18).
The molecular detection and identification of microorganisms is widely used in food microbiology. However, only limited information is now available on the molecular detection of spoilage bacteria, and the development of appropriate strategies for their rapid identification and monitoring is needed. Some molecular approaches, such as ribotyping, PCR amplification of the 16S-23S rRNA gene spacer region, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis, have been exploited for the analysis of the diversity of Pseudomonas isolates from foods (4, 13, 19, 21).
The molecular identification of Pseudomonas is often difficult and controversial. The sequence analysis of the 16S rRNA gene is widely employed for the identification of bacteria; however, this region is not satisfactorily discriminating between the species of Pseudomonas. Phylogenetic studies have highlighted that inferred phylogenies based on the 16S rRNA gene lack resolution at the intrageneric level because of its low rate of evolution (1, 15, 20). In recent studies on the spoilage-related microbiota of beef, we also realized that it was difficult to achieve an unequivocal identification of Pseudomonas at the species level, even though variable regions of the 16S rRNA gene were analyzed (7, 17). Several authors have evaluated the use of alternative sequences for the identification and phylogenetic studies of Pseudomonas spp. For this purpose, the sequences of the carA, recA, gyrB, fliC, and rpoD genes of Pseudomonas species have been determined (2, 11, 20). The sequences of the carbamoyl phosphate synthase gene small subunit (carA) of several Pseudomonas spp. of environmental origin have been determined by Hilario et al. (11). However, the carA sequences for species of food interest, such as P. fragi and P. lundensis, were not considered.
In this study, the carA gene sequence was used as a target in order to design species-specific primers to selectively and simultaneously detect P. fragi, P. lundensis, and P. putida from meat.
carA gene sequencing and primer design.
The Pseudomonas strains used in this study are listed in Table 1. They were cultivated aerobically on nutrient agar (Oxoid, Milan, Italy) at 20°C and stored in nutrient broth with 20% glycerol at −20°C. DNA extraction was carried out from a loopful of grown culture on nutrient agar plates according to the method of Marmur (14).
TABLE 1.
Pseudomonas strains used in this study and identification by species-specific carA PCR assay
| Species | Strain | Sourcea | Origin | Reference |
carA PCR result for:
|
||
|---|---|---|---|---|---|---|---|
| P. putida | P. fragi | P. lundensis | |||||
| P. fragi | DSM3456T | DSMZ | − | + | − | ||
| P. lundensis | DSM6252T | DSMZ | − | − | + | ||
| P. putida | DSM291T | DSMZ | + | − | − | ||
| P. aeruginosa | DSM50071T | DSMZ | − | − | − | ||
| P. aeruginosa | DSM27853 | DSMZ | − | − | − | ||
| P. agarici | DSM11810T | DSMZ | − | − | − | ||
| P. chlororaphis | DSM50082 | DSMZ | − | − | − | ||
| P. cichorii | DSM50259T | DSMZ | − | − | − | ||
| P. costantinii | DSM16734T | DSMZ | − | − | − | ||
| P. flavescens | DSM12071T | DSMZ | − | − | − | ||
| P. fluorescens | DSM50091 | DSMZ | − | − | − | ||
| P. fluorescens | DSM50108 | DSMZ | − | − | − | ||
| P. fluorescens | DSM50415 | DSMZ | − | − | − | ||
| P. marginalis | DSM13124T | DSMZ | − | − | − | ||
| P. putida biotype A | DSM50208 | DSMZ | + | − | − | ||
| P. putida biotype B | DSM50222 | DSMZ | −c | − | − | ||
| P. stutzeri | DSM5190T | DSMZ | − | − | − | ||
| P. syringae | DSM1241 | DSMZ | − | − | − | ||
| P. syringae | DSM10604 | DSMZ | − | − | − | ||
| P. fragi | PMK37 | DTA | Cheese | 16 | − | + | − |
| P. lundensis | PMK52 | DTA | Cheese | 16 | − | − | + |
| P. putida | PMK32 | DTA | Cheese | 16 | − | − | − |
| Pseudomonas sp. | L1114 | DFST | Fish | 19 | − | + | − |
| Pseudomonas sp. | L414 | DFST | Fish | 19 | − | + | − |
| Pseudomonas sp. | L47 | DFST | Fish | 19 | − | + | − |
| Pseudomonas sp. | L514 | DFST | Fish | 19 | − | − | − |
| Pseudomonas sp. | L128 | DFST | Fish | 19 | − | − | − |
| Pseudomonas sp. | L1110 | DFST | Fish | 19 | − | − | − |
| Pseudomonas sp. | 25P | DSAb | Meat | − | − | − | |
| P. fragi | 24P | DSA | Meat | − | + | − | |
| P. fragi | 26P | DSA | Meat | − | + | − | |
| P. fragi | 27P | DSA | Meat | − | + | − | |
| Pseudomonas sp. | 28P | DSA | Meat | − | − | − | |
| Pseudomonas sp. | 29P | DSA | Meat | − | − | − | |
| P. fragi | 30P | DSA | Meat | − | + | − | |
| Pseudomonas sp. | 33M | DSA | Meat | − | − | − | |
| Pseudomonas sp. | 39M | DSA | Meat | − | − | − | |
| Pseudomonas sp. | 1P2 | DSA | Cheese | − | − | − | |
| Pseudomonas sp. | 6P2 | DSA | Cheese | − | − | + | |
| P. fluorescens | PSEflu4 | DISTAM | Milk | − | − | − | |
| P. fluorescens | PSEflu24 | DISTAM | Salad | − | − | − | |
| P. fluorescens | PSEflu13 | DISTAM | Salad | − | − | − | |
| P. fluorescens biovar C | PSEflu14 | DISTAM | Salad | − | − | − | |
| P. fluorescens biovar G | PSEflu20 | DISTAM | Salad | − | − | − | |
| P. fluorescens | PSEflu22 | DISTAM | Salad | − | − | − | |
| P. fragi | 1S 63 | VSA | Fish | 8 | − | + | − |
| P. fragi | 4S 72 | VSA | Fish | 8 | − | + | − |
| P. fragi | F188 | VSA | Meat | 9 | − | + | − |
| P. fragi | F271 | VSA | Fish | 9 | − | + | − |
| P. fragi | 101M5 | VSA | Meat | 9 | − | + | − |
| P. lundensis | F31 | VSA | Meat | 9 | − | − | + |
| P. putida biotype A | F385 | VSA | 9 | − | − | − | |
| P. putida biotype A | F164 | VSA | 9 | + | − | − | |
| P. putida biotype A | F196 | VSA | 9 | + | − | − | |
| P. putida biotype B | F292 | VSA | 9 | − | − | − | |
DSMZ, Deutsche Sammlung von Mikroorganismen und Zelkulturen, Braunschweig, Germany; DTA, Departamento de Tecnologia de Alimentos, INIA, Madrid, Spain; DFST, Department of Food Science and Technology, University of Athens, Athens, Greece; DSA, Dipartimento di Scienza degli Alimenti Università degli Studi di Napoli Federico II, Portici, Italy; DISTAM, Dipartimento di Scienze e Tecnologie Alimentari e Microbiologiche, Università di Milano, Milano, Italy; VSA, Dipartimento di Scienze e Tecnologie Veterinarie per la Sicurezza Alimentare, Università di Milano, Milano, Italy.
Strains previously identified by Microlog System 2 (Biolog, Hayward, CA) according to the manufacturer's instructions.
A spurious PCR product of less than 200 bp was detected after amplification of genomic DNA from this strain.
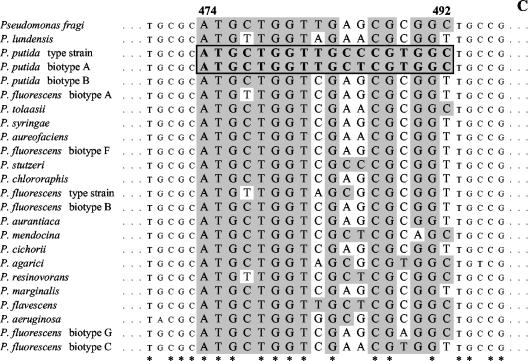
The carA gene of P. fragi DSM3456 and that of P. lundensis DSM6252 were amplified by using primers and conditions reported by Hilario et al. (11); the PCR products (700 bp) were sequenced by using a deoxy terminator cycle sequencing kit (Perkin-Elmer Applied Biosystems). carA sequence accession numbers were determined for P. fragi DSM3456T and P. lundensis DSM6252T (see below). Sequence alignment between the determined carA genes and the previously sequenced carA genes (11) was performed by MacDNasis Pro v3.0.7 (Hitachi Software Engineering Europe S.A., Olivet Cedex, France). The alignment is shown in Fig. 1, where the sequence heterogeneity used for a species-specific primer design is highlighted. Three different forward primers were designed for the specific amplification of fragments of the carA gene of P. fragi, P. lundensis, and P. putida. A specific identification of P. fluorescens would also be of interest in food microbiology. However, in our case it was impossible to design a species-specific probe targeting all the biotypes of P. fluorescens because they showed very high sequence variability within the sequence of the carA gene (Fig. 1).
FIG. 1.
Sequence alignment of variable portions of the carA gene sequences of selected Pseudomonas spp. (A) The accession numbers of the sequences used for the alignment are indicated. Variable sequences are highlighted in white, and consensus sequences are indicated by asterisks. The sequences used to design primers (Table 2) for the species-specific detection of P. lundensis (A), P. fragi (B), and P. putida (C) are boxed and reported in bold.
Multiplex PCR amplification of the carA gene.
Multiplex PCR amplifications were performed in a programmable heating incubator (MyCycler; Bio-Rad, Milan, Italy). Each mixture (final volume, 50 μl) contained 20 ng of each template DNA, each deoxynucleoside triphosphate at a concentration of 0.25 mM, 2.5 mM MgCl2, 5 μl of 10× PCR buffer (Invitrogen, Milan, Italy), and 2.5 U of Taq polymerase. The PCR conditions are reported in Table 2; the reverse primer (11) was used at concentration of 0.6 μM in each PCR. The PCR products were run in 2% agarose electrophoresis gels for 45 min at 150 V. A 16S rRNA gene amplification was also performed prior to multiplex PCR using the conditions previously described (3). This amplification was used in order to check that the DNA was suitable for PCR amplification and to avoid false negatives.
TABLE 2.
Specific oligonucleotide sequences of the carA gene used as forward primers in this study
| Name | Sequence | Expected sizea | Source | Concentration in multiplex PCR | PCR conditions (30×) |
|---|---|---|---|---|---|
| putF | 5′-ATG CTG GTT GCY CGT GGC-3′ | 230 bp | This study | 0.2 μM | 10 s at 94°C |
| fraF | 5′-CGT CAG CAC CGA AAA AGC C-3′ | 370 bp | This study | 0.2 μM | 10 s at 94°C |
| lunF | 5′-TGT GGC GAT TGC AGG CAT T-3′ | 530 bp | This study | 0.2 μM | 20 s at 59°C |
Size of the PCR product obtained using the reverse primer for the amplification of the carA gene (11) at concentration of 0.6 μM in each PCR.
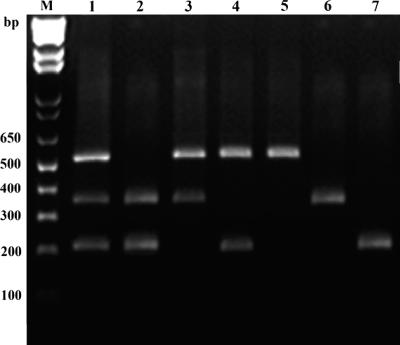
DNAs from the strains P. fragi DSM3456T, P. lundensis DSM6252T, and P. putida DSM291T were used for the optimization of the PCR conditions. The species-specific assay was shown to give the specific amplification products in uniplex, duplex, and multiplex PCR assays involving the three Pseudomonas species (Fig. 2), suggesting the potential for a simultaneous detection and identification of the three spoilage bacteria. The detection limits for each species were found to be 1 ng and 5 ng of DNA template per PCR for uniplex and multiplex PCR, respectively. The PCR products showed the expected sizes of 530, 370, and 230 bp for P. lundensis, P. fragi, and P. putida, respectively (Fig. 2).
FIG. 2.
Results of multiplex (lane 1), duplex (lanes 2 to 4), and uniplex (lanes 5 to 7) PCR obtained using mixtures of DNA templates from P. lundensis DSM6252T (lane 5), P. fragi DSM3456T (lane 6), and P. putida DSM291T (lane 7). M, 1-kb Ladder Plus (Invitrogen).
The multiplex PCR assay was validated by using DNA extracted from different Pseudomonas species and strains, and the results are reported in Table 1. Species of Pseudomonas different from the ones targeted by the multiplex PCR gave no PCR product, confirming the specificity of the assay. The expected results were not obtained in only a few cases. Our forward primer for the specific amplification of the P. putida carA gene was designed on the basis of the carA sequences of P. putida DSM291T and P. putida DSM50208 biotype A. The sequence of the primer differs from the sequence of biotype B in several nucleotides (Fig. 1). However, a spurious PCR product slightly shorter than 200 bp was obtained when the DNA from P. putida DSM50222 (biotype B) was used in our experiments. The carA gene sequence of P. putida DSM50222 was determined, and it did not show zones of possible annealing of the primers used (Fig. 1), indicating that the PCR product could be a genomic fragment outside the carA gene. Moreover, the DNAs of P. putida PMK32, F385, and F292, isolated in other studies and identified by biochemical tests, gave no PCR product, suggesting the possession of a different sequence in the primer alignment zone. Strains L414 and L47 were identified as P. fragi strains according to our assay (Table 1); however, they were previously isolated from fish and were reported as belonging to the same sodium dodecyl sulfate-polyacrylamide gel electrophoresis cluster as P. lundensis (19). In the above-described cases, the strains might have been misidentified in the previous characterization.
Detection of P. fragi, P. lundensis, and P. putida from meat.
Twelve beef steak, 10 pork steak, and 11 chicken breast samples were used. Each meat sample was analyzed soon after the purchase and after 8 days of aerobic storage at 5°C, when the meat presented objective signs of spoilage. Samples (25 g) were homogenized in quarter-strength Ringer's solution (Oxoid). Decimal dilutions were prepared and plated in triplicate on Pseudomonas agar with cetrimide-fucidin-cephaloridine selective supplement (Oxoid); the plates were incubated at 20°C for 48 h. After plate counts, all the colonies present on the surface of each countable plate were collected in bulk as previously described (5) by suspending them in a suitable volume of quarter-strength Ringer's solution. The bulk cells were harvested with a sterile pipette and stored by freezing at −20°C. For DNA extraction, 100 μl of bulk suspension (optical density at 600 nm [absorbance], 1) was centrifuged at 17,000 × g for 5 min, and the pellet was washed in 100 μl of TE buffer (10 mM Tris, 0.1 mM EDTA), centrifuged at 17,000 × g for 5 min, resuspended in 20 μl of sterile water, and boiled for 10 min. After boiling, the samples were centrifuged for 1 min (17,000 × g), and 1 μl of the supernatant was employed in the multiplex PCR assays as described above.
Most of the samples showed Pseudomonas loads higher than 104 CFU g−1 at time zero increasing to a range of 107 to 109 CFU g−1 after 8 days of aerobic storage at 5°C (Table 3). The multiplex PCR was performed on the bulk cells from the countable plates in order to investigate the occurrence of our target species as dominant bacteria in the spoilage process. The analysis of bulk cells from countable plates has been often used for a rapid identification of mixed microbial species from food without the need for isolation (5, 6, 7). In this case, the initial contamination of the beef samples as revealed by multiplex PCR of bulk colonies was imputable to P. fragi in all the cases except for sample N (Table 3). When none of the species was detected (samples G and N), the viable counts were probably given by a Pseudomonas species different from the ones targeted by our assay. Fifty percent of the chicken breast samples at time zero were contaminated by P. fragi. After spoilage, all the chicken samples were contaminated by P. fragi and P. putida, while P. lundensis was found in samples O, Q, R, and S (Table 3). Most of the pork samples were contaminated by both P. fragi and P. putida, while P. lundensis was found at time zero only in samples M2, M6, and M10. After 8 days, 60% of the pork samples were spoiled by P. fragi only (Table 3).
TABLE 3.
Viable counts of Pseudomonas spp. and results of carA multiplex PCR assays in meat samples at time zero and after aerobic storage at 5°C for 8 days
| Sample | CFU (g−1) ata:
|
carA multiplex PCR assay result (bulk cells from countable plates) atb:
|
||
|---|---|---|---|---|
| Time zero | 8 days | Time zero | 8 days | |
| A (beef) | 2.80 × 107 | 4.30 × 108 | P. fragi | P. fragi |
| B (beef) | 1.45 × 105 | 2.20 × 109 | P. fragi | P. fragi |
| C (beef) | 3.80 × 105 | 1.32 × 109 | P. fragi | P. fragi |
| D (beef) | 1.40 × 104 | 3.56 × 108 | P. fragi | P. fragi |
| E (beef) | 1.20 × 106 | 1.45 × 109 | P. fragi | P. fragi |
| F (beef) | 4.81 × 107 | 3.32 × 109 | P. fragi | P. fragi |
| G (beef) | 1.20 × 107 | 9.40 × 108 | P. fragi | − |
| H (beef) | 1.74 × 104 | 2.12 × 108 | P. fragi | P. fragi |
| I (beef) | 1.27 × 105 | 3.50 × 108 | P. fragi | P. fragi |
| L (beef) | 2.81 × 104 | 8.80 × 108 | P. fragi | P. fragi |
| M (beef) | 4.81 × 103 | 6.10 × 108 | P. fragi | P. fragi |
| N (beef) | 1.17 × 106 | 4.20 × 108 | − | − |
| O (chicken) | 1.12 × 106 | 3.80 × 108 | P. fragi | P. fragi, P. putida, P. lundensis |
| P (chicken) | 3.64 × 106 | 1.43 × 108 | P. fragi | P. fragi, P. putida |
| Q (chicken) | 1.65 × 106 | 6.50 × 108 | P. fragi | P. fragi, P. putida, P. lundensis |
| R (chicken) | 4.50 × 105 | 3.10 × 108 | P. fragi, P. putida | P. fragi, P. putida, P. lundensis |
| S (chicken) | 1.43 × 106 | 2.60 × 108 | − | P. fragi, P. putida, P. lundensis |
| T (chicken) | 1.04 × 106 | 2.54 × 107 | P. fragi | P. fragi, P. putida |
| U (chicken) | 8.30 × 104 | 3.60 × 108 | P. fragi, P. putida | P. fragi, P. putida |
| V (chicken) | 8.20 × 104 | 1.06 × 109 | P. fragi, P. putida | P. fragi, P. putida |
| W (chicken) | 2.64 × 104 | 6.50 × 107 | P. fragi, P. putida | P. fragi, P. putida |
| X (chicken) | 1.22 × 106 | 1.10 × 109 | P. fragi | P. fragi, P. putida |
| Y (chicken) | 2.20 × 104 | 1.34 × 109 | P. fragi, P. putida | P. fragi, P. putida |
| M1 (pork) | 3.45 × 106 | 7.80 × 109 | P. fragi, P. putida | P. fragi, P. putida |
| M2 (pork) | 1.52 × 104 | 3.90 × 109 | P. fragi, P. putida, P. lundensis | P. fragi, P. putida |
| M3 (pork) | 7.60 × 104 | 7.20 × 109 | P. fragi, P. putida | P. fragi, P. putida |
| M4 (pork) | 5.60 × 104 | 3.40 × 109 | P. fragi, P. putida | P. fragi |
| M5 (pork) | 2.11 × 106 | 6.00 × 109 | P. fragi, P. putida | P. fragi |
| M6 (pork) | 3.60 × 106 | 1.53 × 109 | P. fragi, P. putida, P. lundensis | P. fragi, P. putida |
| M7 (pork) | 5.50 × 104 | 1.19 × 109 | P. fragi, P. putida | P. fragi |
| M8 (pork) | 5.60 × 105 | 1.95 × 109 | P. fragi, P. putida | P. fragi |
| M9 (pork) | 6.50 × 104 | 7.90 × 108 | P. fragi, P. putida | P. fragi |
| M10 (pork) | 2.72 × 105 | 2.61 × 109 | P. fragi, P. putida, P. lundensis | P. fragi |
Values are expressed as the means based on triplicate plating. Standard deviations were always lower than 20% of the means.
A dash indicates the absence of the targeted species on the countable plates; it does not implicate their absence at a lower concentration.
The evolution of the Pseudomonas species during meat storage (Table 3) is the result of the competition between the targeted species and between them and other microorganisms developing during the spoilage. The interpretation of such results in further research could provide important insights into the microbial ecology associated with the storage of fresh foods. From the results of this study, it appears that P. fragi can play a significant role in the spoilage of the three different kinds of meat. This is in agreement with other reports (10, 18); even though this microorganism is often associated with the spoilage of several foods, it has been recognized that meat may be its ecological niche for several reasons, including its need for iron and its peculiar system for proteolytic enzyme release (12). As far as we know, this is the first molecular assay developed for the identification of P. fragi, P. lundensis, and P. putida. The carA multiplex PCR assay can give a rapid diagnosis of the possible spoilage-causing agents with a direct analysis of colonies from selective media. A direct identification could be very useful to recognize the Pseudomonas spp. occurring during meat storage in studies of shelf life determination and improvement. A rapid and reliable identification of Pseudomonas species can be fundamental for a better understanding of the microbial ecology associated with meat spoilage.
Nucleotide sequence accession numbers.
Nucleotide sequence accession numbers for the carA gene sequences are as follows: DQ647053 for P. fragi DSM3456T, DQ647052 for P. lundensis DSM6252T, and EF363547 for P. putida DSM50222.
Acknowledgments
This work was supported by a grant from MIPAF (Ministero delle Politiche Agricole e Forestali) projects SIQUALTECA and STANDBEEF.
We thank Maria G. De Falco for technical collaboration and the colleagues from the departments indicated in Table 1 for providing Pseudomonas strains.
Footnotes
Published ahead of print on 9 February 2007.
REFERENCES
- 1.Anzai, Y., H. Kim, J.-Y. Park, H. Wakabayashi, and H. Oyaizu. 2000. Phylogenetic affiliation of the pseudomonads based on 16S rRNA sequence. Int. J. Syst. Evol. Microbiol. 50:1563-1589. [DOI] [PubMed] [Google Scholar]
- 2.Bellingham, N. F., J. A. W. Morgan, J. R. Saunders, and C. Winstanley. 2001. Flagellin gene sequence variation in the genus Pseudomonas. Syst. Appl. Microbiol. 24:157-165. [DOI] [PubMed] [Google Scholar]
- 3.Blaiotta, G., C. Pennacchia, D. Ercolini, G. Moschetti, and F. Villani. 2003. Combining denaturing gradient gel electrophoresis of 16S rDNA V3 region and 16S-23S rDNA spacer region polymorphism analyses for the identification of staphylococci from Italian fermented sausages. Syst. Appl. Microbiol. 26:423-433. [DOI] [PubMed] [Google Scholar]
- 4.Dogan, B., and K. J. Boor. 2003. Genetic diversity and spoilage potential among Pseudomonas spp. isolated from fluid milk products and dairy processing plants. Appl. Environ. Microbiol. 69:130-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ercolini, D., G. Moschetti, G. Blaiotta, and S. Coppola. 2001. The potential of a polyphasic PCR-DGGE approach in evaluating microbial diversity of natural whey cultures for water-buffalo mozzarella cheese production: bias of “culture dependent” and “culture independent” approaches. Syst. Appl. Microbiol. 24:610-617. [DOI] [PubMed] [Google Scholar]
- 6.Ercolini, D. 2004. PCR-DGGE fingerprinting: novel strategies for detection of microbes in food. J. Microbiol. Methods 56:297-314. [DOI] [PubMed] [Google Scholar]
- 7.Ercolini, D., F. Russo, E. Torrieri, P. Masi, and F. Villani. 2006. Changes in the spoilage-related microbiota of beef during refrigerated storage under different packaging conditions. Appl. Environ. Microbiol. 72:4663-4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gennari, M., S. Tomaselli, and V. Cotrona. 1999. The microflora of fresh and spoiled sardines (Sardina pilchardus) caught in Adriatic (Mediterranean) sea and stored in ice. Food Microbiol. 16:15-28. [Google Scholar]
- 9.Gennari, M., and F. Dragotto. 1992. A study of the incidence of different fluorescent Pseudomonas species and biovars in the microflora of fresh and spoiled meat and fish, raw milk, cheese, soil and water. J. Appl. Microbiol. 72:281-288. [DOI] [PubMed] [Google Scholar]
- 10.Gill, C. O. 2003. Active packaging in practice: meat, p. 378-396. In H. Ahvenainem (ed.), Novel food packaging technology. Woodhead Publishing Limited and CRC Press LLC, Boca Raton, FL.
- 11.Hilario, E., T. R. Buckley, and J. M. Young. 2004. Improved resolution of the phylogenetic relationships among Pseudomonas by the combined analysis of atpD, carA, recA and 16S rDNA. Antonie Leeuwenhoek 86:51-64. [DOI] [PubMed] [Google Scholar]
- 12.Labadie, J. 1999. Consequences of packaging on bacterial growth. Meat is an ecological niche. Meat Sci. 52:299-305. [DOI] [PubMed] [Google Scholar]
- 13.Locatelli, L., S. Tarnawski, J. Hamelin, P. Rossi, M. Aragno, and N. Fromin. 2002. Specific PCR amplification for the genus Pseudomonas targeting the 3′ half of the 16S rDNA and the whole 16S-23S rDNA spacer. Syst. Appl. Microbiol. 25:220-227. [DOI] [PubMed] [Google Scholar]
- 14.Marmur, J. 1961. A procedure for the isolation of deoxyribonucleic acid from microorganisms. J. Mol. Biol. 3:208-218. [Google Scholar]
- 15.Moore, E. R. B., M. Mau, A. Arnscheidt, E. C. Böttger, R. A. Huston, M. D. Collins, Y. van de Peer, R. de Wachter, and K. N. Timmis. 1996. The determination and comparison of the 16S rRNA gene sequences of species of the genus Pseudomonas (sensu stricto) and estimation of the natural intrageneric relationships. Syst. Appl. Microbiol. 19:478-492. [Google Scholar]
- 16.Morales, P., E. Fernandez-Garcia, and M. Nuñez. 2005. Production of volatile compounds in cheese by Pseudomonas fragi strains of dairy origin. J. Food Prot. 68:1399-1407. [DOI] [PubMed] [Google Scholar]
- 17.Russo, F., D. Ercolini, G. Mauriello, and F. Villani. 2006. Behaviour of Brochothrix thermosphacta in presence of other meat spoilage microbial groups. Food Microbiol. 23:797-802. [DOI] [PubMed] [Google Scholar]
- 18.Stanbridge, L. H., and A. R. Davies. 1998. The microbiology of chill-stored meat, p. 175-177. In A. Davies and R. Board (ed.), Microbiology of meat and poultry. Blackie Academic & Professional, London, United Kingdom.
- 19.Tryfinopoulou, P., E. Tsakalidou, and G.-J. E. Nychas. 2002. Characterization of Pseudomonas spp. associated with spoilage of gilt-head sea bream stored under various conditions. Appl. Environ. Microbiol. 68:65-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamamoto, S., H. Kasai, D. L. Arnold, R. W. Jackson, A. Viavian, and S. Harayama. 2000. Phylogeny of the genus Pseudomonas: intrageneric structure reconstructed from the nucleotide sequences of gyrB and rpoD genes. Microbiology 146:2385-2394. [DOI] [PubMed] [Google Scholar]
- 21.Wiedmann, M., D. Weilmeier, S. S. Dineen, R. Ralyea, and K. J. Boor. 2000. Molecular and phenotypic characterization of Pseudomonas spp. isolated from milk. Appl. Environ. Microbiol. 66:2085-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]