Abstract
Commercial turkey flocks in North Carolina have been found to be colonized frequently with Campylobacter coli strains that are resistant to several antimicrobials (tetracycline, streptomycin, erythromycin, kanamycin, and ciprofloxacin/nalidixic acid). Such strains have been designated multidrug resistant (MDR). However, the population structure of MDR C. coli from turkeys remains poorly characterized. In this study, an analysis of multilocus sequence typing (MLST)-based sequence types (STs) of 59 MDR strains from turkeys revealed that the majority of these strains corresponded to one of 14 different STs, with three STs accounting for 41 (69%) of the strains. The major STs were turkey specific, and most (87%) of the strains with these STs were resistant to the entire panel of antibiotics mentioned above. Some (13%) of the strains with these STs were susceptible to just one or two of the antibiotics in this panel. Further subtyping using fla typing and pulsed-field gel electrophoresis with SmaI and KpnI revealed that the major MDR STs corresponded to strains of related but distinct subtypes, providing evidence for genomic diversification within these STs. These findings suggest that MDR strains of C. coli from turkeys have a clonal population structure characterized by the presence of a relatively small number of clonal groups that appear to be disseminated in the turkey production system. In addition, the observed correlation between STs and the MDR profiles of the microbes indicates that MLST-based typing holds potential for source-tracking applications specific to the animal source (turkeys) and the antimicrobial resistance profile (MDR status) of C. coli.
Campylobacter spp. are the leading bacterial cause of gastroenteritis in the industrialized world and are responsible for almost 500 million cases worldwide each year (11). Occasionally, severe complications may involve reactive arthritis and the immune system-mediated polyneuropathies Guillain-Barré syndrome and Miller-Fisher syndrome (18). Most human infections (85 to 95%) involve Campylobacter jejuni, with C. coli accounting for the majority of the remainder (11). Campylobacter spp. are found naturally in the intestines of many meat animals such as poultry (turkeys and chickens), cattle, and swine. Transmission to humans is thought to involve mainly contaminated food (especially poultry), untreated drinking water, or unpasteurized milk (2, 11). Although the disease is usually self-limiting, antibiotics may be used for treatment in severe cases (21). When treatment of human campylobacteriosis is indicated, fluoroquinolones and erythromycin or other macrolides are typically used (21).
As these organisms are transferred from animals to humans, the emergence of antimicrobial resistance, possibly due to the common use of antibiotics in animal husbandry (3, 9, 14), is a cause for concern. The efficiency of fluoroquinolones for the treatment of human campylobacteriosis has been compromised by the emergence of a relatively high incidence of resistance to these antibiotics (9, 21). Multidrug-resistant strains of Campylobacter are especially of concern in regard to the treatment of human illness. C. coli isolates from poultry and other animals have been found to be more prone than C. jejuni isolates to harbor resistance to multiple antibiotics, including macrolides and fluoroquinolones (1, 3-5, 7, 12, 16, 19). Further information about the ecology, population structure, and reservoirs of multidrug-resistant isolates of Campylobacter would be beneficial in developing strategies aimed at reducing the prevalence of such microbes.
In the course of our laboratory's investigation of Campylobacter colonization of commercial turkeys, we found that turkeys in eastern North Carolina were frequently colonized by C. coli (15, 22). In addition, we observed extensive colonization of successive turkey flocks by the same (or closely related) strains of multidrug-resistant C. coli, with the bacteria harboring resistance to tetracycline, ampicillin, streptomycin, kanamycin, erythromycin, and ciprofloxacin/nalidixic acid (15). However, the reservoir for such multidrug-resistant C. coli strains remains unidentified and the population structure of these organisms is poorly understood.
Recent advances in DNA sequence-based subtyping involving multilocus sequence typing (MLST) (8, 17) along with other subtyping tools such as fla typing and pulsed-field gel electrophoresis (PFGE) (6, 23) can facilitate studies of such issues. Recently, MLST data from 488 C. coli strains from diverse meat animals in the United States suggested the feasibility of using MLST for the detection of host-associated alleles. At each of the seven MLST loci, alleles were identified that were either specific to or associated with a particular food animal (e.g., aspA32 was found only [25/25] in swine isolates and gltA65 was found predominantly [14/15] in chicken isolates). In addition, sequence types (STs) were strongly associated with the host, with many of these host-associated STs corresponding to multiple host-associated alleles (17).
In this study, our objective was to characterize the population structure of multidrug-resistant C. coli strains which were isolated from turkeys and which harbored resistance to tetracycline, streptomycin, erythromycin, kanamycin, and ciprofloxacin/nalidixic acid. The study provided evidence for a largely clonal population structure for these organisms and suggested associations between genotypes, hosts, and antibiotic resistance profiles that may be of relevance for source-tracking purposes.
MATERIALS AND METHODS
Campylobacter strains and growth conditions.
C. coli strains characterized in this study are listed in Table 1. These strains were chosen based on their antimicrobial susceptibility profiles and are part of our laboratory's Campylobacter strain collection. Of the 68 strains, 59 were multidrug resistant (MDR), harboring resistance to tetracycline, streptomycin, erythromycin, kanamycin, and ciprofloxacin/nalidixic acid, and 9 lacked some of these resistance attributes but had STs characteristic of the MDR strains. C. coli strains were isolated from turkeys in North Carolina (67 strains) and Virginia (1 strain) between 2001 and 2004. Turkeys were from 51 commercial farms operated by five different companies (integrators). Typically, different integrators have their own breeder hens for sources of the poults. The bacteria were isolated as described previously by using direct plating of fecal droppings and cecal contents from birds (22). Bacteria were grown on sheep blood agar (Remel, Lenexa, KS) at 42°C under microaerobic conditions and preserved at −80°C in 2-ml tubes containing 0.5 ml of brain heart infusion (Difco, Sparks, MD) with 20% glycerol.
TABLE 1.
Turkey-derived C. coli strains characterized in this study
| ST | Straina | Date of isolation | Age category of hostb | Company | Farm | fla type | PFGE (SmaI) pattern type | Antimicrobial resistance profilec |
|---|---|---|---|---|---|---|---|---|
| 889 | 700A (NCSU119) | Nov. 2001 | 4 | B | 34 | 21 | w | C/N,K,S,T,E |
| 4292c (NCSU051) | Jan. 2003 | 2 | B | 34 | 21 | w | C/N,K,S,T,E | |
| 6282 | Mar. 2004 | 3 | B | 37 | 19 | w | C/N,K,S,T,E | |
| 6698 | May 2004 | 4 | B | 37 | 21 | w | C/N,K,S,T,E | |
| 7156 (NCSU084) | June 2004 | 2 | B | 34 | 21 | x | C/N,K,S,T,E | |
| 8077 (NCSU105) | Aug. 2004 | 4 | B | 35 | 21 | x | C/N,K,S,T,E | |
| 8137 (NCSU109) | Aug. 2004 | 4 | B | 36 | 21 | x | C/N,K,S,T,E | |
| 7726 (NCSU093) | Aug. 2004 | 4 | B | 37 | 21 | x | C/N,K,S,T,E | |
| 1092 | 1004A (NCSU117) | Jan. 2002 | 1 | C | 48 | 12 | aa | C/N,K,S,T,E |
| 1101 | 119 (NCSU045) | June 2001 | 2 | B | 17 | 21 | h | C/N,K,S,T,E |
| *328A (NCSU118) | July 2001 | 2 | B | 33 | 17 | u | K,S,T,E | |
| 563 (NCSU047) | Sept. 2001 | 4 | B | 17 | 20 | i | C/N,K,S,T,E | |
| 598 (NCSU048) | Sept. 2001 | 4 | B | 17 | 21 | k | C/N,K,S,T,E | |
| SC 182 (NCSU123) | Apr. 2002 | 2 | D | 18 | 21 | m | C/N,K,S,T,E | |
| 1788 (NCSU050) | May 2002 | 4 | A | 17 | 21 | l | C/N,K,S,T,E | |
| SC 280 (NCSU125) | July 2003 | 2 | B | 19 | 17 | a | C/N,K,S,T,E | |
| 5988 (NCSU055) | Nov. 2003 | 3 | B | 20 | 21 | n | C/N,K,S,T,E | |
| 8035sp (NCSU120) | May 2004 | 4 | A | 22 | 21 | b | C/N,K,S,T,E | |
| *6636 | May 2004 | 4 | B | 34 | 12 | ll | K,T,E | |
| 6685 (NCSU068) | June 2004 | 3 | A | 23 | 21 | b | C/N,K,S,T,E | |
| 6811 (NCSU071) | June 2004 | 3 | A | 24 | 21 | b | C/N,K,S,T,E | |
| 7135 (NCSU082) | June 2004 | 4 | A | 25 | 21 | b | C/N,K,S,T,E | |
| 7144 (NCSU083) | June 2004 | 4 | A | 26 | 21 | b | C/N,K,S,T,E | |
| 6818 (NCSU072) | June 2004 | 2 | B | 28 | 21 | q | C/N,K,S,T,E | |
| 6851 (NCSU074) | June 2004 | 4 | A | 28 | 21 | b | C/N,K,S,T,E | |
| *6788 (NCSU070) | June 2004 | 2 | B | 29 | 14 | z | K,S,T,E | |
| 7432 (NCSU088) | July 2004 | 3 | A | 28 | 21 | b | C/N,K,S,T,E | |
| 7221 (NCSU085) | July 2004 | 2 | B | 27 | 20 | p | C/N,K,S,T,E | |
| *7390 (NCSU087) | July 2004 | 2 | B | 30 | 20 | s | C/N,K,T,E | |
| *8016 (NCSU101) | Aug. 2004 | 4 | B | 32 | 15 | t | K,S,T,E | |
| 7107 (NCSU081) | Aug. 2004 | 2 | B | 21 | 16 | o | C/N,K,S,T,E | |
| *8266 (NCSU113) | Nov. 2004 | 4 | B | 31 | 20 | v | K,T,E | |
| 1110 | 1511 (NCSU049) | Mar. 2002 | 3 | A | 47 | 13 | z | C/N,K,S,T,E |
| 1126 | 301 (NCSU046) | July 2001 | 2 | B | 1 | 20 | a | C/N,K,S,T,E |
| 1271 | Mar. 2002 | 3 | B | 14 | 2 | a | C/N,K,S,T,E | |
| SC 318 (NCSU126) | July 2003 | 3 | E | 2 | 21 | c | C/N,K,S,T,E | |
| SC 355 (NCSU128) | July 2003 | 2 | D | 3 | 21 | j | C/N,K,S,T,E | |
| 5963 (NCSU053) | Dec. 2003 | 3 | B | 4 | 20 | a | C/N,K,S,T,E | |
| 6076 (NCSU062) | Dec. 2003 | 4 | B | 5 | 19 | d | C/N,K,S,T,E | |
| 6780 (NCSU069) | June 2004 | 2 | B | 9 | 21 | j | C/N,K,S,T,E | |
| 6865 (NCSU075) | June 2004 | 2 | B | 10 | 6 | a | C/N,K,S,T,E | |
| 6840 (NCSU073) | June 2004 | 2 | B | 11 | 20 | f | C/N,K,S,T,E | |
| 8002 (NCSU100) | Aug. 2004 | 4 | A | 6 | 18 | e | C/N,K,S,T,E | |
| 8125 (NCSU107) | Aug. 2004 | 4 | B | 7 | 21 | a | C/N,K,S,T,E | |
| 8136 (NCSU108) | Aug. 2004 | 4 | B | 8 | 21 | jj | C/N,K,S,T,E | |
| 7877 (NCSU098) | Aug. 2004 | 4 | B | 12 | 20 | a | C/N,K,S,T,E | |
| 8286 (NCSU114) | Sept. 2004 | 4 | A | 13 | 21 | g | C/N,K,S,T,E | |
| 8387 | Sept. 2004 | 3 | B | 15 | 21 | r | C/N,K,S,T,E | |
| 8891 | Oct. 2004 | 4 | B | 16 | 21 | r | C/N,K,S,T,E | |
| 1149 | SC 347DE (NCSU127) | July 2003 | 1 | D | 40 | 21 | y | C/N,K,S,T,E |
| 5961 (NCSU052) | Dec. 2003 | 3 | B | 41 | 21 | o | C/N,K,S,T,E | |
| *6449 | Apr. 2004 | 4 | B | 44 | 20 | pp | C/N,K,T,E | |
| *6480 | Apr. 2004 | 3 | B | 44 | 21 | kk | C/N,K,T,E | |
| *6638 | Apr. 2004 | 4 | B | 44 | 21 | pp | C/N,K,T,E | |
| 6603 | May 2004 | 4 | B | 43 | 21 | ii | C/N,K,S,T,E | |
| 8325 (NCSU116) | Sept. 2004 | 4 | B | 42 | 21 | ff | C/N,K,S,T,E | |
| 1154 | SC 141 (NCSU121) | Apr. 2002 | 2 | E | 37 | 21 | a | C/N,K,S,T,E |
| SC 144 (NCSU122) | Apr. 2002 | 1 | E | 38 | 21 | a | C/N,K,S,T,E | |
| 5991 (NCSU056) | Nov. 2003 | 3 | B | 39 | 14 | a | C/N,K,S,T,E | |
| 1160 | 6072 (NCSU061) | Dec. 2003 | 3 | B | 49 | 21 | ee | C/N,K,S,T,E |
| 1163 | 6108 (NCSU066) | Dec. 2003 | 4 | B | 50 | 21 | gg | C/N,K,S,T,E |
| 1170 | SC 242 (NCSU124) | July 2003 | 1 | B | 48 | 21 | bb | C/N,K,S,T,E |
| 1171 | 6958 (NCSU078) | June 2004 | 2 | B | 48 | 8 | cc | C/N,K,S,T,E |
| 1184 | 7553 (NCSU090) | July 2004 | 2 | B | 51 | 10 | hh | C/N,K,S,T,E |
| 1193 | 1203 | Feb. 2002 | 3 | B | 46 | 14 | a | C/N,K,S,T,E |
| 1383 | Mar. 2002 | 3 | B | 47 | 21 | a | C/N,K,S,T,E | |
| 8030 (NCSU103) | Aug. 2004 | 4 | B | 45 | 20 | a | C/N,K,S,T,E | |
| 1198 | 8260 (NCSU112) | Sept. 2004 | 4 | B | 48 | 11 | dd | C/N,K,S,T,E |
Strains indicated by asterisks have susceptibilities to selected antimicrobials, as indicated under “Antimicrobial resistance profile.” NCSU designations are those used in a previous study (17).
Age categories 1, 2, 3, and 4 correspond to 1 to 2 weeks, 3 to 4 weeks, 5 to 7 weeks, and 8 to 21 weeks, respectively.
Strains were resistant to the indicated antimicrobials. C/N, ciprofloxacin/nalidixic acid; K, kanamycin; S, streptomycin; T, tetracycline; E, erythromycin.
DNA extractions and bacterial subtyping by fla typing, MLST, and PFGE.
Genomic DNA was extracted from bacteria grown on blood agar plates by using a DNeasy tissue kit (QIAGEN, Valencia, CA) as described previously (22). For fla typing, the entire flaA gene was amplified using PCR and the product was then digested with the enzyme DdeI (New England Biolabs, Waverly, MA) as described previously (22). The MLST-based STs of most of the strains listed in Table 1 were determined previously (17), and the same procedures were employed to determine the STs of the remaining strains. BioNumerics (version 4.01; Applied Maths, Saint-Martens-Latem, Belgium) was used to display putative phylogenetic relationships among the STs in the form of a minimum spanning tree as described previously (17). PFGE was performed using SmaI and KpnI (New England Biolabs) by following the protocol described by Ribot et al. (20) with a few minor modifications; specifically, the prerestriction step was eliminated and the gels were electrophoresed for 22 h. TIFF images of banding patterns resulting from PFGE and fla typing were analyzed by BioNumerics (version 4.6; Applied Maths). Grouping was performed with the band position tolerance of the Dice coefficient at 1.0%, and cluster analysis for genetic similarity was performed by using the unweighted-pair group method with arithmetic averages.
Antibiotic susceptibility determinations.
Susceptibilities to tetracycline, erythromycin, and ciprofloxacin were determined as described previously (15) with the agar dilution method according to the Clinical and Laboratory Standards Institute (CLSI) guidelines. C. jejuni ATCC 33560 (purchased from the American Type Culture Collection) was used as the quality control strain. In addition, we determined susceptibilities to streptomycin, kanamycin, and nalidixic acid by the agar dilution method. Quality control ranges have not yet been established by the CLSI for these antibiotics, and resistance was determined as described previously (15) by the growth of the bacteria following incubation at 42°C for 48 h with the following antibiotic concentrations: streptomycin, 15 μg/ml; kanamycin, 25 μg/ml; and nalidixic acid, 20 μg/ml. No growth of C. jejuni ATCC 33560 with any of these antimicrobials at the indicated concentrations was observed.
Results
A total of 108 C. coli strains from turkeys from North Carolina (n = 107) and Virginia (n = 1) have been characterized by MLST. Antibiotic susceptibility determinations for these strains identified 59 that were resistant to all six antibiotics routinely used in our laboratory for the characterization of Campylobacter isolates from meat animals (tetracycline, streptomycin, erythromycin, kanamycin, ciprofloxacin, and nalidixic acid). These 59 strains have been designated MDR. A variety of other antibiotic resistance profiles among the other strains were identified (data not shown). The 59 MDR strains were isolated at various time points between 2001 and 2004, as well as from multiple farms operated by five different companies (integrators). In addition, they were isolated from birds of diverse ages (1 to 21 weeks) (Table 1).
A small number of STs corresponding to the majority of the MDR isolates were identified.
A total of 14 different STs were identified among the 59 MDR C. coli strains (Table 1). However, three STs (ST-1101, ST-1126, and ST-889) accounted for the majority of the strains (29, 27, and 14%, respectively). ST-1149 was identified in four MDR strains, and two other STs (ST-1193 and ST-1154) were identified in three strains each. The remaining eight STs were identified in only one strain each.
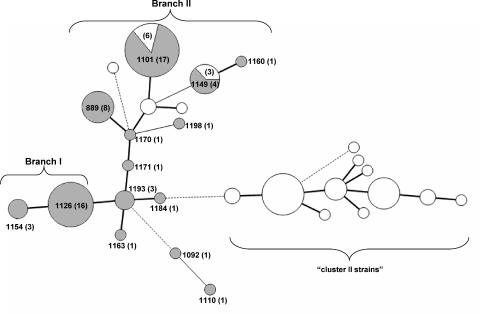
Nine of the 14 STs identified among MDR C. coli strains were found on two major branches of the minimum spanning tree incorporating all available STs of the 108 turkey-derived C. coli strains. STs on one branch (group I; 19 strains, all MDR) included ST-1154 and ST-1126, and those on the other branch (group II; 42 strains, 33 MDR) included ST-1171, ST-1170, ST-1198, ST-889, ST-1149, ST-1160, and ST-1101 (Fig. 1). No MDR isolates were identified among the distinct cluster (cluster II) that had been identified earlier (17) and that consisted of putatively chimeric C. coli isolates, mostly from turkeys, harboring the C. jejuni aspA103 allele (Fig. 1).
FIG. 1.
Placement of MDR strains in the minimum spanning tree of STs identified among C. coli strains from turkeys. STs of the 108 strains were analyzed using BioNumerics as described in Materials and Methods. STs are indicated by circles with the sizes proportional to the numbers of strains. Thick, short lines represent single-locus variants, while thin, longer lines represent double-locus variants. Dashed lines represent three or more allele differences. STs corresponding to MDR strains are labeled, and the total number of strains with each ST is indicated in parentheses. Shading represents MDR strains. Branch I and branch II correspond to branches of the minimum spanning tree harboring group I and group II STs, respectively. “Cluster II” strains were designated as described previously (17).
All strains with ST-1126 and ST-889 had the MDR phenotype. In contrast, strains that were susceptible to one or two of the antibiotics were identified among those corresponding to ST-1101 and ST-1149. Of the 23 ST-1101 strains, six were susceptible to one or two of the antibiotics (two to streptomycin, three to nalidixic acid/ciprofloxacin, and two to both streptomycin and nalidixic acid/ciprofloxacin), and of the seven ST-1149 isolates, three were sensitive to streptomycin (Table 1).
Most of the STs of the MDR strains were turkey specific, not having been encountered among C. coli isolates from other sources (17). The sole exception was ST-1170, which was identified in a single MDR C. coli strain and which had also been identified earlier in two chicken isolates (17). These two ST-1170 chicken isolates were not from our laboratory's strain collection, and their antibiotic resistance profiles are not known. Comparison of these STs with those detected in C. coli isolates from various meat animals (17) suggested that a significant fraction (31/59) of the MDR strains from turkeys were closely related (one- or two-allele differences) to C. coli strains from chickens, with the majority of the remaining MDR strains having STs closely related to those found among strains from swine (data not shown).
Distribution of the MDR C. coli strains.
The majority of the strains (101/108) characterized by MLST were obtained from turkeys grown under the control of two different companies, with 20 and 81 of the 108 strains being obtained from birds of companies A and B, respectively (Table 1). Samples from turkeys of both companies yielded ST-1101 and ST-1126 MDR strains; only ST-889 (eight strains) was limited to birds from one company (Table 1).
Precise determination of possible temporal trends in the prevalence of the major STs was hampered by the fact that the majority (67%) of the strains subtyped by MLST were obtained in 2004, with the remaining strains obtained in the previous three years (Table 1). Nonetheless, the major STs of MDR strains were identified both among strains from 2004 and among those obtained in the previous period. Furthermore, the major STs were detected in strains obtained from birds of different ages, without strong evidence for age-specific trends in prevalence. For instance, of the 23 strains of ST-1101, 13 (57%) were obtained from brooders (1 to 7 weeks of age) and 10 (44%) were obtained from grow-out birds (8 to 21 weeks); of the 16 ST-1126 strains, 9 (56%) and 7 (44%) were from brooders and from grow-out birds, respectively.
PFGE and fla typing revealed genomic diversification among MDR strains of the same STs.
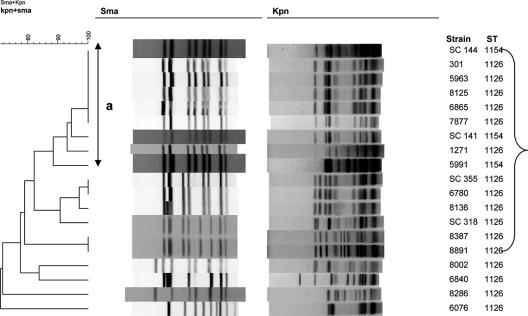
Analysis of the MDR strains with PFGE and fla typing revealed that the observed major STs and ST clusters could be partitioned further by these subtyping schemes. In group I (n = 19), a large PFGE-based cluster contained strains with ST-1126 and ST-1154 (Fig. 2). All 19 strains in this group were of the MDR phenotype. Nine distinct PFGE profiles based on SmaI and KpnI digests were detected among the 16 strains with ST-1126 (Fig. 2). However, one PFGE pattern based on SmaI, designated type a, was encountered among several isolates obtained between 2001 and 2004, and several other ST-1126 strains harbored closely related profiles, with a similarity to the type a profile of greater than 82% (Fig. 2). Two strains (6076 and 8286) with ST-1126 were unusual in having PFGE profiles highly different (72% similarity) from those of other strains of group I, including other ST-1126 strains (Fig. 2).
FIG. 2.
PFGE profiles of group I strains. Nineteen MDR strains were characterized by PFGE using SmaI and KpnI, and cluster analysis of the patterns was performed by BioNumerics as described in Materials and Methods. The line labeled “a” indicates strains with the same SmaI PFGE pattern (type a). The bracket indicates strains with closely related PFGE profiles.
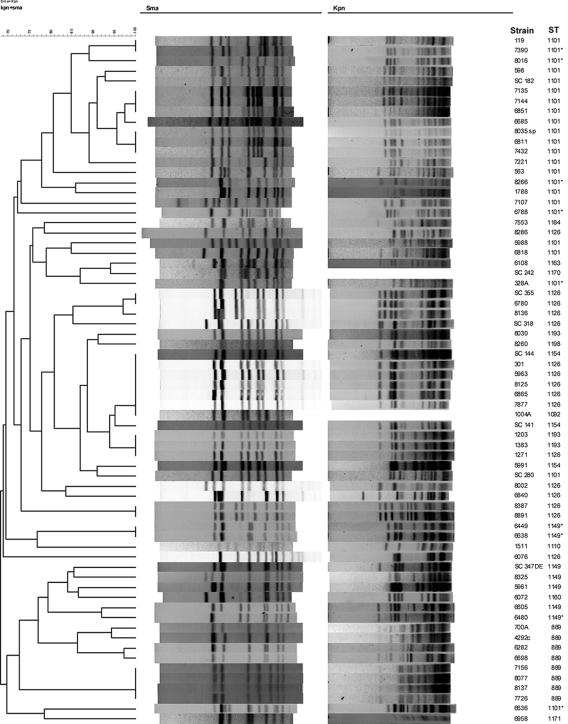
BioNumerics-based analysis of the PFGE profiles of group II strains resulted in two clusters (clusters A and B) (Fig. 3). Cluster A consisted of 26 strains which included all of the 23 ST-1101 strains. Of the 26 strains in this cluster, 20 had the MDR phenotype. Of the 17 MDR isolates with ST-1101, seven (8035sp, 6811, 7135, 6685, 7144, 6851, and 7432) were found to have the same or similar PFGE profiles by SmaI and KpnI (type b); the remaining strains had SmaI and KpnI patterns that differed by several bands from those of type b, with two strains (6788 and 7107) having markedly different PFGE profiles (Fig. 3). PFGE pattern similarity among ST-1101 strains was 67 to 100%. As mentioned above, of the 23 ST-1101 strains, 17 were MDR but 6 were found to be susceptible to streptomycin and/or the fluoroquinolones (nalidixic acid and ciprofloxacin) (Table 1). Two of these strains (7390 and 8016) had PFGE profiles that were indistinguishable from or closely related to those of MDR ST-1101 strains. The PFGE profiles of the other four strains were clearly diverse (Fig. 3).
FIG. 3.
PFGE profiles of group II strains. Forty-two strains were characterized by PFGE using SmaI and KpnI, and cluster analysis of the patterns was performed by BioNumerics as described in Materials and Methods. Strains which were not resistant to all six antibiotics are indicated by asterisks. The line labeled “b” indicates strains with the same or similar PFGE profiles (type b). Brackets indicate strains with closely related PFGE profiles.
The other cluster (cluster B) consisted of 16 strains (eight of ST-889 and seven of ST-1149). ST-889 strains were found to constitute two smaller clusters (four strains each) with 76% similarity to each other (Fig. 3). Among the ST-1149 strains, the four MDR strains (SC 347DE, 6603, 5961, and 8325) had closely related PFGE patterns with ca. 94% similarity (Fig. 3). Of the three ST-1149 strains that were susceptible to streptomycin but resistant to the other five antibiotics (6449, 6480, and 6638), two (6449 and 6638, obtained from the same flock in 2004) had identical PFGE profiles and the PFGE profile of the third strain (6480) had only a one-band difference from that of the MDR isolate 6603, which also had ST-1149 (Fig. 3).
Analysis of the fla profiles of the 68 strains identified 15 different fla types that varied in similarity from 55 to 91% (data not shown). Two fla types (types 20 and 21) were predominant, being detected in 10 and 40 strains, respectively (Table 1). Several different fla types were detected among strains of the major STs (six, seven, and two fla types among ST-1126, ST-1101, and ST-889 strains, respectively) (Table 1). Several strains that shared the same PFGE profiles also had the same fla types; for example, the seven strains with the type b PFGE profile all shared the type 21 fla profile. On the other hand, six different fla types were detected among 13 strains with PFGE pattern type a (Table 1).
A conserved SmaI PFGE pattern (type a) was disseminated among MDR strains of different STs.
PFGE characterization of MDR C. coli strains identified 13 strains of various STs as having a conserved SmaI pattern (type a) (Fig. 4). Of these 13 strains, 12 had identical or closely related patterns (one- to two-band differences) with KpnI but one strain, SC 280, differed by several (four to five) KpnI bands (Fig. 4). As mentioned earlier, six fla types, including the predominant types 20 and 21, were identified among these 13 strains (Table 2). In addition to ST-1126, found in six of these strains, three other STs were identified: all three isolates of ST-1154 (5991, SC 141, and SC 144) as well as the three isolates of ST-1193 (8030, 1383, and 1203) had this conserved PFGE pattern, along with one of the 17 isolates of ST-1101 (SC 280) (Table 2). With the exception of that of SC 280 (ST-1101), the STs of these strains were assembled into one group (group I) in the minimum spanning tree (Fig. 1).
FIG. 4.
PFGE profiles of 13 strains with various STs but a conserved PFGE profile (type a). Cluster analysis of the SmaI and KpnI patterns was performed by BioNumerics as described in Materials and Methods.
TABLE 2.
C. coli MDR strains with SmaI PFGE pattern type a
| Strain | Farm | Company | Year of isolation | fla type | ST |
|---|---|---|---|---|---|
| 301 | 1 | B | 2001 | 20 | 1126 |
| 5963 | 4 | B | 2003 | 20 | 1126 |
| 8125 | 7 | B | 2004 | 21 | 1126 |
| 7877 | 12 | B | 2004 | 20 | 1126 |
| 6865 | 10 | B | 2004 | 6 | 1126 |
| 1271 | 14 | B | 2002 | 2 | 1126 |
| SC 280 | 19 | B | 2003 | 17 | 1101 |
| 5991 | 39 | B | 2003 | 14 | 1154 |
| SC 141 | 37 | E | 2002 | 21 | 1154 |
| SC 144 | 38 | E | 2002 | 21 | 1154 |
| 8030 | 45 | B | 2004 | 20 | 1193 |
| 1203 | 46 | B | 2002 | 14 | 1193 |
| 1383 | 47 | B | 2002 | 21 | 1193 |
Comparative analysis of PFGE-based versus MLST-based clusters of the C. coli strains.
A BioNumerics-based cluster analysis of the SmaI and KpnI patterns of the 68 turkey isolates showed that in several cases the clustering of the strains was similar to that obtained with the minimum spanning tree based on MLST data (Fig. 5). For instance, in the middle of the BioNumerics cluster diagram, 12 strains with ST-1126 clustered with the closely related strains of ST-1154 and ST-1193 (Fig. 5). On the basis of MLST data, there was only a one-allele difference between ST-1126 and ST-1154 or ST-1193. Similarly, the single ST-1160 strain clustered together with strains of the related ST-1149 and ST-889 (Fig. 5). However, the single strain of ST-1198 was separated by PFGE-based clustering from other strains with group II STs and in fact clustered with group I isolates (Fig. 5).
FIG. 5.
PFGE-based clustering of C. coli strains investigated in this study. All 68 strains were analyzed by PFGE with SmaI and KpnI, and cluster analysis of the patterns was performed by BioNumerics as described in Materials and Methods. Two strains (1004 A and SC 242) could not be restricted with KpnI. Strains which were not resistant to all six antibiotics are indicated by asterisks.
Discussion
C. coli is implicated less frequently in human illness than C. jejuni but is still associated with many cases of acute gastroenteritis and constitutes a significant public health concern (13). This concern can be exacerbated by the high level of antibiotic resistance in C. coli, including resistance to antibiotics used for the treatment of human infections, such as ciprofloxacin and erythromycin. For reasons that remain unclear, C. coli has been repeatedly shown to be more likely than C. jejuni to harbor resistance to these antimicrobial agents (1, 3-5, 7, 12, 16, 19).
Among poultry-derived Campylobacter strains, strains from turkeys seem especially prone to be resistant to erythromycin and certain other antibiotics. Turkey strains are more likely to harbor resistance to both erythromycin and ciprofloxacin than strains from broilers (16), and strains from turkey meat at retail sites are more likely to be resistant to multiple antibiotics, including erythromycin, than their counterparts from chicken (12). A recent study with commercial turkeys in North Carolina showed that the birds were colonized frequently with C. coli strains that harbored resistance to multiple antibiotics, with genetically similar strains colonizing successive flocks in one farm (15).
In this study, the MDR phenotype (defined here as resistance to tetracycline, streptomycin, erythromycin, kanamycin, and nalidixic acid/ciprofloxacin) was encountered exclusively among turkey-derived C. coli isolates. None of the C. coli isolates obtained from other animals (e.g., broilers, swine, and cattle) in the same geographical region and investigated by MLST harbored this phenotype, even though several different antibiotic resistance profiles were identified. In our continuing studies with C. coli from turkeys and swine, strains with the MDR phenotype have been derived invariably from turkeys (S. Wright, R. Siletzky, and S. Kathariou, unpublished results).
The identification of certain major STs and ST clusters among the MDR strains suggested that a relatively small number of clonal groups was responsible for the majority of these strains. All of the major STs were specific to turkeys and were in fact not encountered among C. coli strains from swine from the same geographic region. These findings suggest that the MDR phenotype has evolved primarily among certain clonal groups of C. coli that colonize turkeys. Two of these clonal groups (ST-1101, with 17 MDR strains, and ST-1126, with 16 strains) were encountered repeatedly among organisms from different integrators and farms at several different time points between 2001 and 2004. A third, smaller clonal group, ST-889, was encountered among eight strains, obtained from four farms of one integrator. These findings suggest that these clonal groups have become disseminated and established in the turkey industry. In a previous study, we demonstrated that successive turkey flocks on a specific farm were colonized with MDR C. coli strains that had the same PFGE type and that fluctuations in the fla types in successive flocks could be detected (15).
One can speculate that MDR strains of the major ST lineages may have enhanced abilities to colonize turkeys or to persist in yet unidentified reservoirs, from which they become introduced repeatedly into the flocks. Antibiotic treatment regimens or other production practices in the turkey industry may have originally provided selection pressure for the acquisition of the MDR phenotype and the establishment and dissemination of the bacteria harboring it. Further studies are needed to determine the extent to which the MDR phenotype may be associated with the colonization of the host (turkeys) and with other aspects of the physiology of the organisms.
Even though it is also possible that the MDR phenotype emerges de novo in different flocks and farms, in our opinion this scenario is less likely, at least for the major STs 1101, 1126, and 889, since we would then expect to encounter these STs randomly among isolates with various antibiotic susceptibility profiles. Instead, all 16 strains with ST-1126, 17 of the 23 ST-1101 strains, and all eight ST-889 strains were MDR, even though they were derived from various locations and isolated at different times. Such data suggest that these lineages acquired the MDR phenotype and became disseminated subsequently. Preliminary data from our laboratory suggest that this phenotype is remarkably stable, persisting following numerous passages of the organisms in the absence of selection for antibiotic resistance (C. B. D'lima, unpublished results). In field strains with STs found among MDR organisms, susceptibilities to some of the antibiotics were encountered only among ST-1101 and ST-1149 strains. In all cases, susceptible strains lacked resistance to just one or two of the antibiotics (with nalidixic acid and ciprofloxacin considered to represent one antibiotic class). It is possible that such organisms have not yet acquired the corresponding resistance attributes. Alternatively, these strains may have evolved from MDR organisms by loss of certain resistance attributes, possibly through transformation with DNA from other strains that lacked such resistance.
The observed variation in genetic fingerprints of strains within the major MDR STs also supports the notion of disseminated lineages, with subsequent genomic diversification that can become evident with additional subtyping tools, such as PFGE and fla typing. On certain occasions, strains of the same ST harbored markedly different PFGE profiles, suggesting extensive diversification, possibly arising through genomic rearrangements, insertion sequences, and the excision or integration of other mobile elements that may be harbored by these strains. Some of the differences in PFGE patterns may also be due to the presence of megaplasmids, such as the plasmid identified in C. coli RM2228 (10). The genomic content of the MDR strains has not been characterized yet, and the possibly unique genetic attributes of these lineages (e.g., ST-1101, ST-1126, and ST-889) remain unidentified. Our findings suggest the usefulness of accompanying MLST with PFGE to enhance the resolution potential of strain typing.
In conclusion, we have provided evidence for the existence of certain clonal groups of C. coli that are disseminated among C. coli strains from turkeys in North Carolina, having been detected in organisms isolated at different times from diverse farms and companies and from birds of different age groups. The presently observed correlation between STs and the MDR profiles of the organisms suggests that MLST-based typing holds potential for source-tracking applications specific to the animal source (turkeys) as well as the antimicrobial resistance profile (MDR status) of C. coli. Further studies are needed to identify the possible reservoirs for these clones, to characterize the ecology and adaptations of the organisms, and to determine whether these clonal groups of MDR C. coli are encountered in isolates from other major turkey-growing regions in the United States or other countries.
Acknowledgments
Funding for this research was provided by U.S. Department of Agriculture grants NRI 2001-2099 and NRI 2003-0299.
We thank Steven Clark and the turkey company veterinarians and farm personnel for facilitating the collection of samples from which the strains were obtained. We appreciate the encouragement and support of all members of our laboratory.
Footnotes
Published ahead of print on 9 February 2007.
REFERENCES
- 1.Aarestrup, F. M., E. M. Nielsen, M. Madsen, and J. Engberg. 1997. Antimicrobial susceptibility patterns of thermophilic Campylobacter spp. from humans, pigs, cattle, and broilers in Denmark. Antimicrob. Agents Chemother. 41:2244-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adak, G. K., J. M. Cowden, S. Nicholas, and H. S. Evans. 1995. The Public Health Laboratory Service national case-control study of primary indigenous sporadic cases of campylobacter infection. Epidemiol. Infect. 115:15-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avrain, L., F. Humbert, R. L'Hospitalier, P. Sanders, C. Vernozy-Rozand, and I. Kempf. 2003. Antimicrobial resistance in Campylobacter from broilers: association with production type and antimicrobial use. Vet. Microbiol. 96:267-276. [DOI] [PubMed] [Google Scholar]
- 4.Bae, W., K. N. Kaya, D. D. Hancock, D. R. Call, Y. H. Park, and T. E. Besser. 2005. Prevalence and antimicrobial resistance of thermophilic Campylobacter spp. from cattle farms in Washington State. Appl. Environ. Microbiol. 71:169-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bywater, R., H. Deluyker, E. Deroover, A. de Jong, H. Marion, M. McConville, T. Rowan, T. Shryock, D. Shuster, V. Thomas, M. Valle, and J. Walters. 2004. A European survey of antimicrobial susceptibility among zoonotic and commensal bacteria isolated from food-producing animals. J. Antimicrob. Chemother. 54:744-754. [DOI] [PubMed] [Google Scholar]
- 6.de Boer, P., B. Duim, A. Rigter, J. van Der Plas, W. F. Jacobs-Reitsma, and J. A. Wagenaar. 2000. Computer-assisted analysis and epidemiological value of genotyping methods for Campylobacter jejuni and Campylobacter coli. J. Clin. Microbiol. 38:1940-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desmonts, M. H., F. Dufour-Gesbert, L. Avrain, and I. Kempf. 2004. Antimicrobial resistance in Campylobacter strains isolated from French broilers before and after antimicrobial growth promoter bans. J. Antimicrob. Chemother. 54:1025-1030. [DOI] [PubMed] [Google Scholar]
- 8.Dingle, K. E., F. M. Colles, D. Falush, and M. C. Maiden. 2005. Sequence typing and comparison of population biology of Campylobacter coli and Campylobacter jejuni. J. Clin. Microbiol. 43:340-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engberg, J., F. M. Aarestrup, D. E. Taylor, P. Gerner-Smidt, and I. Nachamkin. 2001. Quinolone and macrolide resistance in Campylobacter jejuni and C. coli: resistance mechanisms and trends in human isolates. Emerg. Infect. Dis. 7:24-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fouts, D. E., E. F. Mongodin, R. E. Mandrell, W. G. Miller, D. A. Rasko, J. Ravel, L. M. Brinkac, R. T. DeBoy, C. T. Parker, S. C. Daugherty, R. J. Dodson, A. S. Durkin, R. Madupu, S. A. Sullivan, J. U. Shetty, M. A. Ayodeji, A. Shvartsbeyn, M. C. Schatz, J. H. Badger, C. M. Fraser, and K. E. Nelson. 2005. Major structural differences and novel potential virulence mechanisms from the genomes of multiple Campylobacter species. PLoS Biol. 3:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman, C. R., J. Neimann, H. C. Wegener, and R. V. Tauxe. 2000. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations, p. 121-138. In I. Nachamkin and M. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, DC.
- 12.Ge, B., D. G. White, P. F. McDermott, W. Girard, S. Zhao, S. Hubert, and J. Meng. 2003. Antimicrobial-resistant Campylobacter species from retail raw meats. Appl. Environ. Microbiol. 69:3005-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillespie, I. A., S. J. O'Brien, J. A. Frost, G. K. Adak, P. Horby, A. V. Swan, M. J. Painter, and K. R. Neal. 2002. A case-case comparison of Campylobacter coli and Campylobacter jejuni infection: a tool for generating hypotheses. Emerg. Infect. Dis. 8:937-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen, L. B., and F. M. Aarestrup. 2001. Macrolide resistance in Campylobacter coli of animal origin in Denmark. Antimicrob. Agents Chemother. 45:371-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee, B. C., N. Reimers, H. J. Barnes, C. D'Lima, D. Carver, and S. Kathariou. 2005. Strain persistence and fluctuation of multiple-antibiotic resistant Campylobacter coli colonizing turkeys over successive production cycles. Foodborne Pathog. Dis. 2:103-110. [DOI] [PubMed] [Google Scholar]
- 16.Luangtongkum, T., T. Y. Morishita, A. J. Ison, S. Huang, P. F. McDermott, and Q. Zhang. 2006. Effect of conventional and organic production practices on the prevalence and antimicrobial resistance of Campylobacter spp. in poultry. Appl. Environ. Microbiol. 72:3600-3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller, W. G., M. D. Englen, S. Kathariou, I. V. Wesley, G. Wang, L. Pittenger-Alley, R. M. Siletz, W. Muraoka, P. J. Fedorka-Cray, and R. E. Mandrell. 2006. Identification of host-associated alleles by multilocus sequence typing of Campylobacter coli strains from food animals. Microbiology 152:245-255. [DOI] [PubMed] [Google Scholar]
- 18.Nachamkin, I., B. M. Allos, and T. Ho. 1998. Campylobacter species and Guillain-Barre syndrome. Clin. Microbiol. Rev. 11:555-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Payot, S., S. Dridi, M. Laroche, M. Federighi, and C. Magras. 2004. Prevalence and antimicrobial resistance of Campylobacter coli isolated from fattening pigs in France. Vet. Microbiol. 101:91-99. [DOI] [PubMed] [Google Scholar]
- 20.Ribot, E. M., C. Fitzgerald, K. Kubota, B. Swaminathan, and T. J. Barrett. 2001. Rapid pulsed-field gel electrophoresis protocol for subtyping of Campylobacter jejuni. J. Clin. Microbiol. 39:1889-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skirrow, M. B., and M. J. Blaser. 2000. Clinical aspects of Campylobacter infection, p. 69-88. In I. Nachamkin and M. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, DC.
- 22.Smith, K., N. Reimers, H. J. Barnes, B. C. Lee, R. Siletzky, and S. Kathariou. 2004. Campylobacter colonization of sibling turkey flocks reared under different management conditions. J. Food Prot. 67:1463-1468. [DOI] [PubMed] [Google Scholar]
- 23.Wassenaar, T. M., and D. G. Newell. 2000. Genotyping of Campylobacter spp. Appl. Environ. Microbiol. 66:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]