Abstract
Weissella cibaria 110, isolated from the Thai fermented fish product plaa-som, was found to produce a bacteriocin active against some gram-positive bacteria. Bacteriocin activity was not eliminated by exposure to high temperatures or catalase but was destroyed by exposure to the proteolytic enzymes proteinase K and trypsin. The bacteriocin from W. cibaria 110 was purified, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis showed that the purified bacteriocin contained one protein band that was approximately 2.5 kDa in size. Mass spectrometry analysis showed the mass of the peptide to be approximately 3,487.8 Da. N-terminal amino acid sequence analysis was performed, and 27 amino acids were identified. Because it has no similarity to other known bacteriocins, this bacteriocin was defined as a new bacteriocin and termed weissellicin 110.
Lactic acid bacteria (LAB) have long played important roles in food technology. The LAB include a wide variety of cell types with various physiological and biochemical characteristics. The isolation of LAB from milk products, fermented foods, and plants has frequently been reported. The phylogeny of the bacteria classified currently in the genus Weissella was clarified in 1990 (16), and the taxonomy of Weissella species was further assessed in 1993 (5). Weissella species have been isolated from a variety of sources, and some of them play important roles in fermentation (1). Weissella cibaria was first described by Björkroth et al. (1) and later found in various kinds of fermented foods (6, 19).
Bacteriocins are peptides produced by bacteria that kill or inhibit the growth of closely related bacteria. Bacteriocins produced by LAB have attracted special interest as potential safe, alternative food preservatives (4, 8, 15). Many bacteriocins associated with Lactobacillus, Enterococcus, and Leuconostoc species have been described previously (4). However, bacteriocins from Weissella species remain rare, and to our knowledge, no bacteriocins from W. cibaria (1, 9) have been reported previously.
W. cibaria 110 (AB261010/DDBJ; DNA Data Bank of Japan [http://www.ddbj.nig.ac.jp/]) isolated from plaa-som (17), a fermented fish product from Thailand, was found to produce a bacteriocin active against some gram-positive bacteria. The present paper describes the purification and analysis of this bacteriocin and discusses its similarities to other known peptides. This is the first study to clarify the characteristics of a W. cibaria bacteriocin.
MATERIALS AND METHODS
W. cibaria 110.
Ten strains of W. cibaria were isolated from plaa-som samples collected from Bangkok, Thailand, and their activities against the indicator strain Lactobacillus sakei JCM 1157T were determined. Only strain 110 showed activity; this strain was identified using the API 50CHL kit, and the identification was confirmed using 16S rRNA sequence analysis. W. cibaria 110 was therefore used as the bacteriocin-producing strain in this study. Inhibitory activity was determined using the agar well diffusion assay described by Yanagida et al. (24).
Other bacterial strains and their culture conditions.
The culture conditions for strains used for the determination of the antibacterial spectrum are listed in Table 1.
TABLE 1.
Inhibition spectrum of the bacteriocin produced by W. cibaria 110
| Indicator strain | Medium | Incubation temp (°C) | Diam (mm) of zone of inhibitiona |
|---|---|---|---|
| L. sakei JCM 1157T | MRS | 30 | 13 |
| L. sanfranciscensis JCM 5668T | MRS | 30 | 10 |
| L. homohiochii JCM 1199T | MRS | 30 | 10 |
| L. coryniformis subsp. coryniformis JCM 1164T | MRS | 30 | 11 |
| L. brevis JCM 1059T | MRS | 30 | − |
| L. brevis JCM 1170 | MRS | 30 | − |
| L. acetotolerans JCM 3825T | MRS | 30 | 12 |
| L. paracasei subsp. paracasei JCM 1181 | MRS | 30 | − |
| L. plantarum subsp. plantarum JCM 1551 | MRS | 30 | − |
| L. jensenii JCM 1146T | MRS | 37 | − |
| L. delbrueckii subsp. bulgaricus JCM 1002T | MRS | 37 | − |
| L. gasseri JCM 1131T | MRS | 37 | − |
| L. vitulinus JCM 1143T | MRS | 37 | − |
| L. acidophilus JCM 1132T | MRS | 37 | − |
| Enterococcus faecalis JCM 5803T | MRS | 37 | − |
| Carnobacterium divergens JCM 5816T | MRS | 25 | − |
| Streptococcus salivarius JCM 5707T | MRS | 37 | − |
| W. kandleri JCM 5817T | MRS | 30 | 10 |
| W. confusa JCM 1093T | MRS | 30 | − |
| W. halotolerans JCM 1114T | MRS | 37 | 10 |
| W. minor JCM 1168T | MRS | 30 | − |
| W. paramesenteroides JCM 9890T | MRS | 30 | 13 |
| Leuconostoc mesenteroides subsp. mesenteroides JCM 6124T | MRS | 30 | − |
| Leuconostoc lactis JCM 6123T | MRS | 30 | 11 |
| Listeria monocytogenes NCIMB 13726 | PBNb | 30 | − |
| Escherichia coli JCM 1649T | PBN | 37 | − |
| Bacillus cereus JCM 2152T | PBN | 37 | − |
| Staphylococcus aureus JCM 2151 | PBN | 37 | − |
Wells (8 mm in diameter) were filled with 100 μl of supernatant from the W. cibaria culture. −, no inhibitory zone observed.
PBN broth (pH 7.3) included the following components: 0.5% peptone, 0.3% beef extract, and 0.8% NaCl.
Optimum temperature for growth and bacteriocin production.
To study the optimum temperature for growth and bacteriocin production, 100 μl of the overnight bacterial culture was inoculated into 5 ml of lactobacillus Man-Rogosa-Sharpe (MRS) broth (Difco, Sparks, MD) and then incubated at 15, 20, 25, 30, 37, or 45°C for 14 h. After incubation, the optical density at 660 nm was determined and bacteriocin activity was checked by determining the size of the zone of inhibition around the well (8 mm in diameter) in the agar well diffusion assay.
Effects of enzymes and heat on bacteriocin activity.
To evaluate heat stability, samples of neutralized supernatant fluid from the W. cibaria 110 culture were incubated at 80°C for 30 min, 90°C for 30 min, 100°C for 30 min, 110°C for 15 min, or 121°C for 15 min. To analyze sensitivity to various enzymes, neutralized supernatant fluid was treated with proteinase K (Merck, Darmstadt, Germany), trypsin (Wako, Osaka, Japan), or catalase (Wako, Osaka, Japan) at 30 IU mg−1 and 37°C for 5 h. After the treatments described above, bacteriocin activity was determined using the agar well diffusion assay.
Production of the bacteriocin by W. cibaria 110.
To study bacteriocin production, 5 ml of the overnight bacterial culture was inoculated into 1 liter of MRS medium. At specific time intervals, 1-ml samples were removed and the optical density at 660 nm of the culture and the arbitrary activity units (AU) ml−1 (reciprocal of the highest dilution at which activity was still obtained) of the bacteriocin were determined according to the method of Henderson et al. (13). L. sakei JCM 1157T was used as the indicator strain. The incubation temperature was set based on the results obtained from the optimum growth temperature analysis.
Purification of the bacteriocin.
Cell-free culture supernatant (2.5 liters) was prepared and then purified by ammonium sulfate precipitation (40%) and column chromatography with a hydrophobic column of phenyl-650M TOYOPEARL (Tosoh, Tokyo, Japan) and Sep-Pak C18 cartridges (Waters, Milford, MA) (24). Eluted bacteriocin fractions from Sep-Pak C18 cartridges were freeze-dried and then stored at 4°C.
Molecular size approximation.
The molecular size of the purified bacteriocin was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) by following the method described by Yanagida et al. (24). Bacteriocin size was estimated using rainbow-colored protein molecular mass markers (Amersham Biosciences, Piscataway, NJ).
Mass spectrometry.
The molecular mass of the purified bacteriocin was determined by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry (MS) using a mass spectrometer (Microflex; Bruker, Bremen, Germany) (14).
The molecular mass of the purified bacteriocin was also determined by liquid chromatography with an ion trap mass spectrometer (LC/MSD Trap XCT; Agilent, CA).
N-terminal amino acid sequence analyses.
The activity of the purified bacteriocin was confirmed on the SDS-PAGE gel, and the gel was then blotted onto polyvinylidene difluoride membranes and stained with CBB R-250 (Wako, Osaka, Japan). The objective bands were cut out and analyzed, and the N-terminal amino acid sequence was determined by Edman degradation on a protein sequencer (model 491; Applied Biosystems, Foster City, CA).
RESULTS
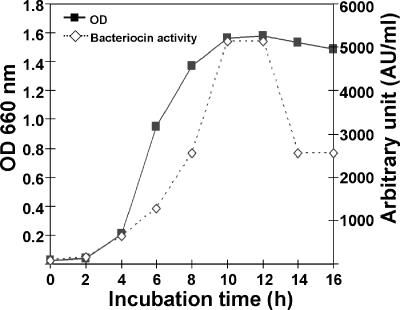
Maximum cell numbers and activity against the indicator strain L. sakei JCM 1157T were observed at 30°C (Table 2). The highest bacteriocin titers (5,120 AU ml−1) were obtained after 10 h of incubation at 30°C, and the highest cell density, based on the optical density at 660 nm, was observed after 10 to12 h of incubation (Fig. 1).
TABLE 2.
Effects of various factors on bacteriocin produced by W. cibaria 110
| Factor | OD660a of W. cibaria culture | Diam (mm) of zone of inhibitionb |
|---|---|---|
| Growth temp (°C) | ||
| 15 | 0.125 | − |
| 20 | 1.364 | − |
| 25 | 1.419 | 12 |
| 30 | 1.502 | 13 |
| 37 | 1.394 | 11 |
| 45 | 0.169 | − |
| Treatment with enzyme | ||
| Control | 13 | |
| Proteinase K | − | |
| Trypsin | − | |
| Catalase | 12 | |
| Heat | ||
| Control | 13 | |
| 30 min at 80°C | 13 | |
| 30 min at 90°C | 13 | |
| 30 min at 100°C | 13 | |
| 15 min at 110°C | 13 | |
| 15 min at 121°C | 13 |
OD660, optical density at 660 nm.
Wells (8 mm in diameter) were filled with 100 μl of supernatant from the W. cibaria 110 culture. L. sakei JCM 1157T was used as the indicator strain. −, no inhibitory zone observed.
FIG. 1.
Production of bacteriocin during the growth of W. cibaria 110. OD 660 nm, optical density at 660 nm.
The effects of enzymes and heat on the inhibitory agent from W. cibaria 110 are shown in Table 2. The bacteriocin was inactivated by proteinase K or trypsin but not affected by treatment with catalase. The bacteriocin was considered to be heat stable, as activity remained after heating at 121°C for 15 min.
The neutralized supernatant from W. cibaria 110 showed activity against some gram-positive bacteria, as listed in Table 1, but had no activity against Listeria monocytogenes.
The molecular mass of the purified bacteriocin was approximately 2.5 kDa, according to SDS-PAGE (Fig. 2).
FIG. 2.
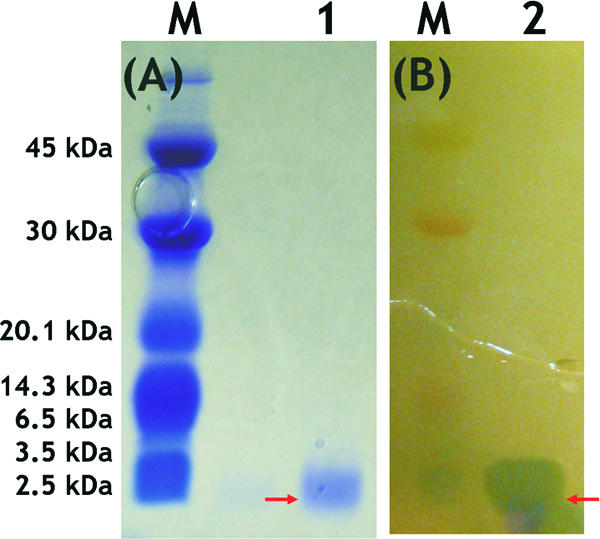
SDS-PAGE analysis of purified bacteriocin from W. cibaria 110. (A) CBB-stained gel. (B) Gel placed onto MRS agar surface overlaid with L. sakei JCM 1157T. Lanes M, low-molecular-mass standards; lanes 1 and 2, purified bacteriocin from W. cibaria 110.
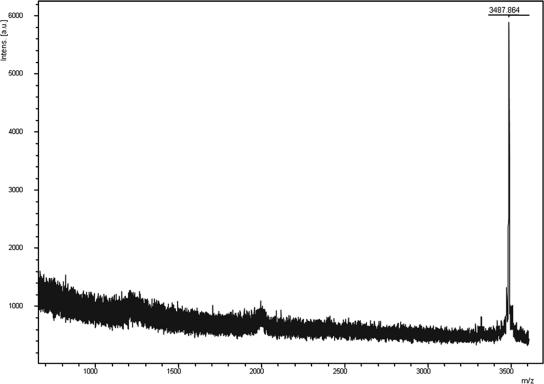
The purified bacteriocin was analyzed by MALDI-TOF MS, and the result revealed a major peak at 3,487.86 Da (Fig. 3). The same sample subjected to liquid chromatography electrospray ionization MS revealed one peak with an identified molecular mass of 3,490.8 Da; this result coincided with the expected molecular mass. N-terminal amino acid analysis of this band revealed the following partial sequence: NH2-SDKNNVFFQIGKRYVAPVLYXFGKXAE, where X represents unidentified amino acids. MALDI-TOF MS analysis of the protein after tryptic digestion confirmed the identities of amino acids 1 to 12 and 14 to 24 and revealed that the 21st amino acid was tryptophan (W) and the 25th amino acid was glycine (G) (data not shown). No corresponding protein sequence was found in the database (DNA Data Bank of Japan [http://www.ddbj.nig.ac.jp/]) or NCBI BLAST (http://www.ncbi.nlm.nih.gov/BLAST/). Based on the results described above, we strongly suggest that W. cibaria 110 produces a novel, natural bacteriocin and have termed this bacteriocin weissellicin 110.
FIG. 3.
MALDI MS-determined mass spectrum of weissellicin 110 (mass, 3,487.86 Da).
DISCUSSION
Bacteriocins produced by LAB such as Lactobacillus (12, 25), Enterococcus (2, 3, 11, 24, 25), Leuconostoc (7, 10), Streptococcus (22), and Carnobacterium (20, 23) species have been frequently reported in previous studies. However, studies of bacteriocins from Weissella sp. remain scarce (18). To our knowledge, this is the first study of a bacteriocin from W. cibaria.
The optimum growth temperature always has an influence on the production of bacteriocins (7, 21). The results of our investigations of growth temperature and bacteriocin production indicated that W. cibaria 110 had higher bacteriocin production when incubated at 30°C for 10 to 12 h than when incubated at other temperatures (Fig. 1). The sensitivity of the substance to proteinase K and trypsin is proof of its proteinaceous nature. In addition, no effect was observed after treatment with catalase; this finding provided evidence that the active agent did not originate from H2O2. The same treatments were later performed with the purified bacteriocin, and the results were confirmed (data not shown).
Weissellicin 110 showed a narrow spectrum of inhibition of other LAB. Unlike most class II bacteriocins produced by LAB, weissellicin 110 had no activity against Listeria monocytogenes. This characteristic may limit its potential application in fermented foods.
The molecular mass of the 27-amino-acid peptide was calculated using the tool Compute pl/Mw from the ExPASy proteomics server (http://ca.expasy.org), and a result of 3,205.71 Da was obtained. However, results from MALDI-TOF MS and liquid chromatography electrospray ionization MS indicated that the true molecular mass of the bacteriocin was approximately 3,488 Da (Fig. 3). We therefore believe that two or three of the amino acids remain unknown in this study. To clarify this result, an advanced analysis such as PCR DNA sequencing analysis will be conducted in the future.
In conclusion, the evidence presented in this report indicates that W. cibaria 110 isolated from plaa-som produces a novel bacteriocin, which we have named weissellicin 110. Weissellicin 110 is stable after high-temperature treatment but has a narrow spectrum of inhibition of other LAB and does not inhibit Listeria monocytogenes. Future work in our laboratory will focus on the clarification of the full amino acid sequence of weissellicin 110 and the possibility of applying weissellicin 110 as a biopreservative.
Acknowledgments
We thank Tsutomu Takayanagi for his technical assistance during the N-terminal amino acid sequence analyses. We also thank Yan Kwok of the Vaccine Research and Development Center at the National Health Research Institutes for his assistance and patience in helping us understand the analysis of mass spectrometry and for discussions about protein purification.
Footnotes
Published ahead of print on 9 February 2007.
REFERENCES
- 1.Björkroth, K. J., U. Schillinger, R. Geisen, N. Weiss, B. Hoste, W. H. Holzapfel, H. J. Korkeala, and P. Vandamme. 2002. Taxonomic study of Weissella confusa and description of Weissella cibaria sp. nov., detected in food and clinical samples. Int. J. Syst. Evol. Microbiol. 52:141-148. [DOI] [PubMed] [Google Scholar]
- 2.Casaus, P., T. Nilsen, L. M. Cintas, I. F. Nes, P. E. Hernández, and H. Holo. 1997. Enterocin B, a new bacteriocin from Enterococcus faecium T136 which can act synergistically with enterocin A. Microbiology 143:2287-2294. [DOI] [PubMed] [Google Scholar]
- 3.Cintas, L. M., P. Casaus, C. Herraz, L. S. Håvarstein, H. Helge, P. Hernández, and I. F. nes. 2000. Biochemical and genetic evidence that Enterococcus faecium L50 produces enterocins L50A and L50B, the sec-dependent enterocin P, and a novel bacteriocin secreted without an N-terminal extension termed enterocin Q. J. Bacteriol. 182:6806-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cleveland, J., T. J. Montville, I. F. Nes, and M. L. Chikindas. 2001. Bacteriocins: safe, natural antimicrobials for food preservation. A review. Int. J. Food Microbiol. 71:1-20. [DOI] [PubMed] [Google Scholar]
- 5.Collins, M. D., J. Samelis, J. Metaxopoulos, and S. Wallbanks. 1993. Taxonomic studies on some leuconostoc-like organisms from fermented sausages: description of a new genus Weissella for the Leuconostoc paramesenteroides group of species. J. Appl. Bacteriol. 75:595-603. [DOI] [PubMed] [Google Scholar]
- 6.De Vuyst, L., V. Schrijvers, S. Paramithiotis, B. Hoste, M. Vancanneyt, J. Swings, G. Kalantzopoulos, E. Tsakalidou, and W. Messens. 2002. The biodiversity of lactic acid bacteria in Greek traditional wheat sourdoughs is reflected in both composition and metabolite formation. Appl. Environ. Microbiol. 68:6059-6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drosinos, E. H., M. Mataragas, P. Nasis, M. Galiotou, and J. Metaxopoulos. 2005. Growth and bacteriocin production kinetics of Leuconostoc mesenteroides E131. J. Appl. Microbiol. 99:1314-1323. [DOI] [PubMed] [Google Scholar]
- 8.Ennahar, S., K. Sonomoto, and A. Ishizaki. 1999. Class IIa bacteriocins from lactic acid bacteria: antibacterial activity and food preservation. J. Biosci. Bioeng. 87:705-716. [DOI] [PubMed] [Google Scholar]
- 9.Ennahar, S., and Y. Cai. 2004. Genetic evidence that Weissella kimchii Choi et al. 2002 is a later heterotypic synonym of Weissella cibaria Björkroth et al. 2002. Int. J. Syst. Evol. Microbiol. 54:463-465. [DOI] [PubMed] [Google Scholar]
- 10.Fang, W., B. B. Budde, and H. Siegumfeldt. 2006. Leucocins 4010 from Leuconostoc carnosum cause a matrix related decrease in intracellular pH of Listeria monocytogenes. FEMS Microbiol. Lett. 258:208-213. [DOI] [PubMed] [Google Scholar]
- 11.Foulquié Moreno, M. R., R. Callewaert, B. Devreese, J. Van Beeumen, and L. De Vuyst. 2003. Isolation and biochemical characterization of enterocins produced by enterococci from different sources. J. Appl. Microbiol. 94:214-229. [DOI] [PubMed] [Google Scholar]
- 12.Garver, K. I., and P. M. Muriana. 1994. Purification and partial amino acid sequence of curvaticin FS47, a heat-stable bacteriocin produced by Lactobacillus curvatus FS47. Appl. Environ. Microbiol. 60:2191-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henderson, J. T., A. L. Chopko, and P. D. Van Wassenaar. 1992. Purification and primary structure of pediocin PA-1 produced by Pediococcus acidilactici PAC-1.0. Arch. Biochem. Biophys. 295:5-12. [DOI] [PubMed] [Google Scholar]
- 14.Hindré, T., S. Didelot, J. P. Le Pennec, D. Haras, A. Dufour, and K. Vallée-Réhel. 2003. Bacteriocin detection from whole bacteria by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl. Environ. Microbiol. 69:1051-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klaenhammer, T. R. 1988. Bacteriocins of lactic acid bacteria. Biochimie 70:337-349. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Murcia, A. J., and M. D. Collins. 1990. A phylogenetic analysis of the genus Leuconostoc based on reverse transcriptase sequencing of 16S rRNA. FEMS Microbiol. Lett. 58:73-83. [DOI] [PubMed] [Google Scholar]
- 17.Paludan-Müller, C., M. Madsen, P. Sophanodora, L. Gram, and P. L. Moller. 2002. Fermentation and microflora of plaa-som, a thai fermented fish product prepared with different salt concentrations. Int. J. Food Microbiol. 73:61-70. [DOI] [PubMed] [Google Scholar]
- 18.Papathanasopoulos, M. A., F. Krier, A. M. Revol-Junelles, G. Lefebvre, J. P. Le Caer, A. von Holy, and J. W. Hastings. 1997. Multiple bacteriocin production by Leuconostoc mesenteroides TA33a and other Leuconostoc/Weissella strains. Curr. Microbiol. 35:331-335. [DOI] [PubMed] [Google Scholar]
- 19.Santos, E. M., I. Jaime, J. Rovira, U. Lyhs, H. Korkeala, and J. Bjorkroth. 2005. Characterization and identification of lactic acid bacteria in “morcilla de Burgos.” Int. J. Food Microbiol. 97:285-296. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki, M., T. Yamamoto, Y. Kawai, N. Inoue, and K. Yamazaki. 2005. Mode of action of piscicocin CS526 produced by Carnobacterium piscicola CS526. J. Appl. Microbiol. 98:1146-1151. [DOI] [PubMed] [Google Scholar]
- 21.Van den Berghe, E., G. Skourtas, E. Tsakalidou, and L. De Vuyst. 2006. Streptococcus macedonicus ACA-DC 198 produces the lantibiotic, macedocin, at temperature and pH conditions that prevail during cheese manufacture. Int. J. Food Microbiol. 107:138-147. [DOI] [PubMed] [Google Scholar]
- 22.Xiao, H., X. Chen, M. Chen, S. Tang, X. Zhao, and L. Huan. 2004. Bovicin HJ50, a novel lantibiotic produced by Streptococcus bovis HJ50. Microbiology 150:103-108. [DOI] [PubMed] [Google Scholar]
- 23.Yamazaki, K., M. Suzuki, Y. Kawai, N. Inoue, and T. J. Montville. 2005. Purification and characterization of a novel class IIa bacteriocin, piscicocin CS526, from surimi-associated Carnobacterium piscicola CS526. Appl. Environ. Microbiol. 71:554-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yanagida, F., Y.-S. Chen, T. Onda, and T. Shinohara. 2005. Durancin L28-1A, a new bacteriocin from Enterococcus durans L28-1, isolated from soil. Lett. Appl. Microbiol. 40:430-435. [DOI] [PubMed] [Google Scholar]
- 25.Yanagida, F., Y.-S. Chen, and T. Shinohara. 2006. Searching for bacteriocin-producing lactic acid bacteria in soil. J. Gen. Appl. Microbiol. 52:21-28. [DOI] [PubMed] [Google Scholar]