Abstract
Leptospirillum ferriphilum strain UBK03 isolated from a mine in Jiangxi, China, is resistant to Ni2+ (30 to 40 mM). A four-gene nickel resistance cluster was identified and, when transformed into Escherichia coli, enabled growth in 6 mM nickel. Mutation experiments revealed that the genes ncrA, ncrB, and ncrC could confer nickel resistance in Escherichia coli, whereas the gene ncrY could have a negative effect on nickel resistance.
Bioleaching for the recovery of metals from ores recently received increased attention. This technology has the potential to overcome many problems faced by metal industries (14, 20, 36). The success of a bioleaching operation depends largely on the consortium of microorganisms present in the mining environment. As these mining bacteria can grow and thrive under ore conditions having high concentrations of heavy metals, they are naturally resistant to heavy metal toxicity (2, 9, 24). Although Rawlings et al. (6, 28, 35) have demonstrated that Leptospirillum ferriphilum is the most important microorganism in bioleaching to date, only one gene cluster, related to arsenic resistance (35), has been isolated from this organism so far. However, many metal resistance genes have been cloned from other acidophilic bacteria, such as the zinc and nickel resistance genes from Acidiphilium multivorum GS19h (13), the copper resistance genes from Acidithiobacillus ferrooxidans (8), and the cadmium resistance genes from Acidiphilium symbioticum (20).
Bacteria resistant to nickel have been isolated from ecosystems polluted by heavy metals, such as wastewater, mine refuse, industrial composts, and cooling waters of the metal processing industry (26). These bacteria are mainly Ralstonia eutropha CH34 (4, 5, 15, 19, 21), Alcaligenes denitrificans 4a-2 (33), Alcaligenes xylosoxydans 31A (32), Ralstonia eutropha KTO2 (32), Klebsiella oxytoca CCUG 15788 (34), Hafnia alvei 5-5 (25, 26), and Escherichia coli (29). The mechanism of nickel resistance in bacteria is due to the action of an operon-encoded, energy-dependent specific efflux system that pumps the cation from the cell, thereby lowering the intracellular concentration of the toxic metal (25).
The present study is aimed at characterizing the metal resistance of a new strain, L. ferriphilum UBK03, cloning its nickel resistance determinant, and studying the functions of these genes.
Characterization of L. ferriphilum UBK03.
L. ferriphilum UBK03 is a gram-negative, vibrio- or spiral-shaped bacterium that was isolated from a mine in Jiangxi, China (Table 1). It is an obligate chemolithotroph and grows optimally at 37°C in 9K inorganic medium within the pH range 1.3 to 2.0 (6). To identify this bacterium at the genetic level, a partial 16S rRNA gene of UBK03 was obtained by PCR using the primer pair 16sF/16sR (Table 1). The 16S rRNA gene sequence of UBK03 (GenBank accession no. DQ534052) was compared with other sequences in the NCBI nucleotide database and found to be identical to those of the L. ferriphilum strains Fairview (GenBank accession no. AF356830.1) and ATCC 49881 (GenBank accession no. AF356829.1).
TABLE 1.
Bacterial strains, plasmids, and primers used in this study
| Strain, plasmid, or primer | Relevant characteristics or sequencea | Source or reference(s) |
|---|---|---|
| Strains | ||
| L. ferrophilum | ||
| UBK03 | Wild type | This study |
| E. coli | ||
| JM109 | Host for plasmid amplification | Promega |
| Top10 | Host for plasmid amplification | Invitrogen |
| BL21 | F−dcm ompT hsdS (rB2 mB2) galDE3 | Novagen |
| CC118 | Δ(ara-leu)7697 ΔlacX74 ΔphoA20 galE galK thi rpsE rpoB argE(am) recA1 | 7, 10, 27 |
| Plasmids | ||
| pUC19 | lacZ′; ColE1 replicon, cloning vector, Ampr | Promega |
| pNR21 | 4.0-kb HindIII fragment from genomic DNA of strain UBK03, Ampr | This study |
| pHA-4 | Contains PhoA reporter gene | 7, 10, 27 |
| pWaldo-TEV-GFP | Contains GFP reporter gene | 7, 10, 27 |
| pNDA | pNR21 derivative deleting ncrA, Ampr | This study |
| pNDB | pNR21 derivative deleting ncrB, Ampr | This study |
| pNDC | pNR21 derivative deleting ncrC, Ampr | This study |
| pNDY | pNR21 derivative deleting ncrY, Ampr | This study |
| pLAFR1 | Contains tetracycline resistance cassette, Ampr, Tcr | 37 |
| pNTA | pNR21 containing insertion in ncrA, Ampr, Tcr | This study |
| pNTB | pNR21 containing insertion in ncrB, Ampr, Tcr | This study |
| pNTC | pNR21 containing insertion in ncrC, Ampr, Tcr | This study |
| pNTY | pNR21 containing insertion in ncrY, Ampr, Tcr | This study |
| Primers | ||
| 16sF | 5′-CATGGCCCATCAGCTAGTTG-3′ | This study |
| 16sR | 5′-GCGATTCCGACTTCATGAGG-3′ | This study |
| ncrAF | 5′-CTCGAGGATGCTCAACATTCTTTCTAA-3′ | This study |
| ncrAR | 5′-GGATCCCTTCTCTTTCTTCGGCCAGG-3′ | This study |
| ncrCF | 5′-CTCGAGATGACTGATTTTTCCACTCTTTT-3′ | This study |
| ncrCR | 5′-GGATCCGCTCGTTACGCCGATCCAGC-3′ | This study |
| BU_F | 5′-AAGCTTAGGAGAGGATAAGCCGATAA-3′ | This study |
| YD_R | 5′-GAGCTCTTAACGGTGAAATTAAGGTTCT-3′ | This study |
| AU_F | 5′-AAGCTTTCATTTTTTTCAGGTCCCTCT-3′ | This study |
| AU_R | 5′-GGATCCATCCTCTCCTGATACGTTGT-3′ | This study |
| CD_F | 5′-GGATCCTATCTTTAAGGCAAGCGAAACA-3′ | This study |
| BU_R | 5′-GGATCCAGAGTGGAAAAATCAGTCATG-3′ | This study |
| YD_F | 5′-GGATCCGTCGGCATTTACATGGGCTATC-3′ | This study |
| CU_R | 5′-GGATCCTCAGGCGTGAGATTCATACAG-3′ | This study |
Amp, ampicillin; Km, kanamycin; Tc, tetracycline. The restriction enzyme sites incorporated into the primers are shown in boldface in the primer sequences.
Metal resistance of L. ferriphilum UBK03.
Because L. ferriphilum has been proven by Rawlings et al. (6, 28, 35) to play the most important role in bioleaching, it might be resistant to metals. Thus, its resistance to some metals was examined by monitoring ferrous iron oxidation using a method to determine ferrous iron in the presence of ferric iron (17). The metal tolerance of strain UBK03 in 9K medium followed this order: Ni2+ (30 mM to 40 mM) > Zn2+ (20 mM to 30 mM) > Co2+ (5 mM to 10 mM) > Cu2+ (<5 mM) ≈ Cd2+ (<5 mM).
Cloning of nickel resistance genes from L. ferriphilum UBK03.
The genomic DNA of L. ferriphilum UBK03 was isolated as described previously (6). Partially digested 3- to 10-kb HindIII DNA fragments were used to construct a gene bank in the HindIII site of the vector pUC19 (Table 1). The recombinant vectors were introduced into E. coli JM109 with electroporation using Gene Pulser (Bio-Rad). When transformants were selected on LB plates (22, 31) containing 100 μg/ml of ampicillin and 3 mM NiCl2, three colonies appeared on the plates in 24 h, indicating that they expressed the genes for nickel resistance. The plasmids of these three positive colonies were isolated and analyzed with restriction enzymes. The results demonstrated that the recombinant plasmids, designated pNR21, pNR22, and pNR23, had 4.0-, 7.3-, and 5.6-kb DNA insertions in the vector pUC19, respectively. To determine whether the nickel resistance determinant was indeed located within these plasmids, the recombinant plasmids were extracted and transformed into the E. coli strain Top10. All three plasmids endowed E. coli Top10 with nickel resistance. Although the plasmids included DNA fragments of different lengths, all strains exhibited the same MIC of nickel, 6 mM. Together, these results suggested that the genes conferring nickel resistance were coded by the DNA fragment in each plasmid.
Sequence analysis of nickel resistance genes.
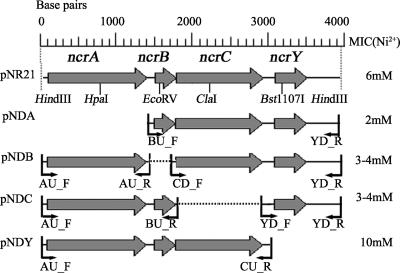
Sequencing of the three plasmids revealed a common 3,991-bp segment containing four open reading frames (NcrA, NcrB, NcrC, and NcrY), which localized to the 3,991-bp HindIII-HindIII fragment of pNR21 (GenBank accession no. DQ517331), as shown in Fig. 1. BLAST (1) and Pfam (3) searches were performed to identify putative gene functions. The similarities of these proteins with related sequences are shown in Table 2. As analyzed by TMHMM (18) and PSORT (23), the proteins NcrA, NcrC, and NcrY were predicted to be membrane proteins, whereas NcrB was predicted to be a globular protein expressed in the cytoplasm.
FIG. 1.
Physical map of the sequences of pNR21, pNDA, pNDB, pNDC, and pNDY. Gray arrows indicate putative open reading frames. Black little arrows indicate the primers (Table 1) used in constructing the plasmid. The dashed line represents the deleted DNA fragment. The values in the right column are the MICs of Ni2+ for Escherichia coli JM109 bearing the corresponding plasmids.
TABLE 2.
Comparison of proteins encoded by L. ferriphilum UBK03 with similar proteins
| Protein name | Sequence length (aaa) | Homologous protein (sequence length [aa], sequence identity [%], accession no.) |
|---|---|---|
| NcrA | 432 | NirA (356, 77.3, AAR82963.1) |
| NreB-like protein (382, 83, AAK38164) | ||
| NreB (440, 68, NP_436142.1) | ||
| NcrB | 89 | NirB (89, 98, AAR82964.1) |
| Protein of unknown function (88, 62, ZP_00427384.1) | ||
| Protein of unknown function (92, 58, ZP_00509556.1) | ||
| NcrC | 376 | NirC (307, 79, AAR82965.1) |
| Putative transmembrane protein (377, 71, NP_745112.1) | ||
| Putative transmembrane protein (373, 67, ZP_00509558.1) | ||
| NcrY | 136 | NirD (164, 75, AAR82966.1) |
| NcrY (63, 44, AF322866_4) | ||
| Possible exported protein (130, 35, NP_806781.1) |
aa, amino acids.
Southern hybridization analysis to confirm the source of the 3,991-bp fragment.
To determine whether the 3,991-bp fragment in pNR21 was derived from L. ferriphilum UBK03, Southern hybridization was performed. L. ferriphilum UBK03 total DNA was digested by HindIII, electrophoresed on a 1% agarose gel, transferred to a nylon membrane, and hybridized using sequences upstream of ncrA (1 to 747 bp) as the probe. The resulting Southern blot revealed a band around 4 kb, the same size as the cloned fragment (data not shown). This result indicated that the 3,991-bp nickel resistance gene cluster originated from L. ferriphilum UBK03 and is present as a single copy.
Topology analysis of proteins NcrA and NcrC.
Using C-terminal tagging with alkaline phosphatase (PhoA) and green fluorescent protein (GFP) (7, 10, 27), the locations of the C termini of NcrA and NcrC, which were exposed to the cytoplasm or the periplasm, were detected. Fragments of ncrA and ncrC were amplified by PCR using the ncrAF/ncrAR and ncrCF/ncrCR primer pairs (Table 1), and the resulting products were verified by sequencing. These two DNA fragments were inserted into the reporter vectors pHA-4 and pWaldo-TEV-GFP using the XhoI and BamHI restriction sites, respectively. Constructs of PhoA or GFP fusions were transformed into E. coli CC118 or E. coli BL21(DE3)/pLysS, respectively. The plasmids pHA-4 in E. coli CC118 and pWaldo-TEV-GFPe in E. coli BL21 were used as controls. The protein activities of PhoA and GFP were assayed as described previously (7, 10), and the results are shown in Table 3. GFP activity was detected only for the NcrA fusion, and PhoA activity was detected only for the NcrC fusion, indicating that the C terminus of NcrA was cytoplasmic and the C terminus of NcrC was periplasmic. Using this information, the topology models of these two proteins were derived (Fig. 2).
TABLE 3.
Protein activities of PhoA and GFP fusion proteinsa
| Fusion | PhoA activity | Raw GFP activity |
|---|---|---|
| NcrA | 0.6 ± 0.6 | 565.8 ± 17.6 |
| NcrC | 33.5 ± 1.5 | 66.2 ± 4.1 |
| CK | 1.9 ± 0.6 | 64.3 ± 3.3 |
The data shown represent means ± standard deviations. The CKs were E. coli CC118 containing pHA-4 and E. coli BL21 containing pWaldo-TEV-GFPe.
FIG. 2.
Predicted topology of proteins NcrA (A) and NcrC (B). The membrane-associated amino acid residues are boxed, the periplasmic residues are in bold, and the remaining residues are cytoplasmic. The regions are named using the letters M, P, and C for membrane, periplasm, and cytoplasm, respectively.
Identification of the nickel resistance of each cloned gene.
To evaluate the effect of each gene on nickel resistance, a series of independent deletion and TC-box insertion mutants were constructed using standard molecular genetic techniques (31). All constructs are listed in Table 1 and were obtained as described below.
We constructed the deletion plasmids pNDA, pNDB, pNDC, and pNDY (Table 1; Fig. 1) carrying ncrBCY, ncrACY, ncrABY, and ncrABC, respectively, by deleting ncrA (bp 1 to 1398), ncrB (bp 1409 to 1740), ncrC (bp 1782 to 2844), and ncrY (bp 2921 to 3337). Plasmids pNDA and pNDY (Fig. 1) were constructed by inserting the PCR products containing ncrBCY and ncrABC into pUC19 using HindIII-BamHI. Plasmids pNDB and pNDC (Fig. 1) were constructed by inserting DNA fragments ncrA and ncrAB into pUC19 using HindIII-BamHI and then ligating the PCR products containing ncrCY and ncrY into the generated plasmid using BamHI-SacI. The MICs of strains harboring the corresponding plasmids were measured, as shown in Fig. 1.
Insertion mutations were constructed by ligating the 1,188-bp tetracycline resistance cassette of plasmid pLAFR1 (TC box) (37) into unique restriction sites of plasmid pNR21. These sites, HpaI, EcoRV, ClaI, and Bst1107I, are located within ncrA, ncrB, ncrC, and ncrY, and were used to construct the insertion plasmids pNTA, pNTB, pNTC, and pNTY, respectively. The MICs of these strains containing plasmids pNTA, pNTB, pNTC, and pNTY were 2 mM, 4 to 5 mM, 4 to 5 mM, and 9 mM, respectively.
The MICs of strains harboring different mutant plasmids were analyzed by one-way analysis of variance, followed by Duncan's multiple-range test with an α value of 0.05. As a result, there were significant differences among mutations in the ncrA, ncrB, and ncrY strains. However, there were not significant differences between mutations in the ncrB and ncrC strains, as well as mutations in the ncrA strain and the control (pUC19).
Among the proteins encoded by pNR21, NcrA was the base of the nickel resistance system. When ncrA was mutated by either deletion (pNDA) or insertion (pNTA), the strains had the same MICs of nickel (2 mM) as the control (pUC19). NcrA was found to contain 10 transmembrane helices (Fig. 2), belongs to the major facilitator superfamily (Pfam accession no. PF07690) (30), and possesses a histidine-rich region in the C terminus, which might have high affinity to nickel (Fig. 2). This protein may form a transporter in the membrane, be the foundation of the nickel resistance complex (12), and require the presence of accessory proteins for maximal function.
NcrB is a cytoplasmic, histidine-rich, 89-amino-acid protein with unknown function (Pfam accession no. PF02583). When this gene was mutated by deletion (pNDB) or insertion (pNTB), the MIC of nickel was reduced from 6 mM (pNR21) to 3 to 4 mM. These results suggest that NcrB may assist in nickel efflux.
NcrC, similar to NcrA, is a membrane protein belonging to the high-affinity nickel transport protein family (Pfam accession no. PF03824). When this gene was mutated by deletion (pNDC) or insertion (pNTC), the MIC of nickel was reduced from 6 mM (pNR21) to 3 to 5 mM. These results suggest that NcrC also significantly contributes to nickel resistance. Topology analysis of NcrC (Fig. 2) revealed that at least half of the residues in the mid-region of the protein are cytoplasmic, with high frequencies of histidine (9%) and charged residues (41%), which are aspartic acid, glutamic acid, histidine, lysine, and arginine. These residues have high affinity for nickel and might act to chelate the nickel cation in the cytoplasm (11, 16, 29).
In contrast to NcrA, NcrB, and NcrC, which endow E. coli JM109 with nickel resistance, NcrY acts in the opposite manner. Mutations in the ncrY strain (pNDY and pNTY) increased the MIC of nickel from 6 mM (pNR21) to 9 to 10 mM. According to Pfam, NcrY is a predicted protein with unknown function (Pfam accession no. PF04076). In our system, NcrY could have a negative effect on nickel resistance through an unknown mechanism which will be studied in the future.
Acknowledgments
We thank Gunnar von Heijne for the vectors of PhoA-4, pWaldo-TEV-GFP, and host E. coli CC118.
This work was supported by the National Basic Research Program of China (973 Program; grant no. 2004CB719603).
Footnotes
Published ahead of print on 9 February 2007.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee, P. C. 2004. Genetics of metal resistance in acidophilic prokaryotes of acidic mine environments. Indian J. Exp. Biol. 42:9-25. [PubMed] [Google Scholar]
- 3.Bateman, A., L. Coin, R. Durbin, R. D. Finn, V. Hollich, S. Griffiths-Jones, A. Khanna, M. Marshall, S. Moxon, E. L. Sonnhammer, D. J. Studholme, C. Yeats, and S. R. Eddy. 2004. The Pfam protein families database. Nucleic Acids Res. 32:D138-D141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brim, H., M. Heyndrickx, P. de Vos, A. Wilmotte, D. Springael, H. G. Schlegel, and M. Mergeay. 1999. Amplified rDNA restriction analysis and further genotypic characterisation of metal-resistant soil bacteria and related facultative hydrogenotrophs. Syst. Appl. Microbiol. 22:258-268. [DOI] [PubMed] [Google Scholar]
- 5.Collard, J.-M., A. Provoost, S. Taghavi, and M. Mergeay. 1993. A new type of Alcaligenes eutrophus CH34 zinc resistance generated by mutations affecting regulation of the cnr cobalt-nickel resistance system. J. Bacteriol. 175:779-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coram, N. J., and D. E. Rawlings. 2002. Molecular relationship between two groups of the genus Leptospirillum and the finding that Leptospirillum ferriphilum sp. nov. dominates South African commercial biooxidation tanks that operate at 40°C. Appl. Environ. Microbiol. 68:838-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daley, D. O., M. Rapp, E. Granseth, K. Melen, D. Drew, and G. von Heijne. 2005. Global topology analysis of the Escherichia coli inner membrane proteome. Science 308:1321-1323. [DOI] [PubMed] [Google Scholar]
- 8.Das, A., J. M. Modak, and K. A. Natarajan. 1998. Surface chemical studies of Thiobacillus ferrooxidans with reference to copper tolerance. Antonie Leeuwenhoek 73:215-222. [DOI] [PubMed] [Google Scholar]
- 9.Dopson, M., C. Baker-Austin, P. R. Koppineedi, and P. L. Bond. 2003. Growth in sulfidic mineral environments: metal resistance mechanisms in acidophilic micro-organisms. Microbiology 149:1959-1970. [DOI] [PubMed] [Google Scholar]
- 10.Drew, D. E., G. von Heijne, P. Nordlund, and J. W. de Gier. 2001. Green fluorescent protein as an indicator to monitor membrane protein overexpression in Escherichia coli. FEBS Lett. 507:220-224. [DOI] [PubMed] [Google Scholar]
- 11.Eitinger, T., and B. Friedrich. 1994. A topological model for the high-affinity nickel transporter of Alcaligenes eutrophus. Mol. Microbiol. 12:1025-1032. [DOI] [PubMed] [Google Scholar]
- 12.García-Domínguez, M., L. Lopez-Maury, F. J. Florencio, and J. C. Reyes. 2000. A gene cluster involved in metal homeostasis in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 182:1507-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh, S., N. R. Mahapatra, and P. C. Banerjee. 1997. Metal resistance in Acidocella strains and plasmid-mediated transfer of this characteristic to Acidiphilium multivorum and Escherichia coli. Appl. Environ. Microbiol. 63:4523-4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosh, S., N. R. Mahapatra, S. Nandi, and P. C. Banerjee. 2005. Integration of metal-resistant determinants from the plasmid of an Acidocella strain into the chromosome of Escherichia coli DH5alpha. Curr. Microbiol. 50:28-32. [DOI] [PubMed] [Google Scholar]
- 15.Grass, G., C. Grosse, and D. H. Nies. 2000. Regulation of the cnr cobalt and nickel resistance determinant from Ralstonia sp. strain CH34. J. Bacteriol. 182:1390-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hebbeln, P., and T. Eitinger. 2004. Heterologous production and characterization of bacterial nickel/cobalt permeases. FEMS Microbiol. Lett. 230:129-135. [DOI] [PubMed] [Google Scholar]
- 17.Herrera, L., P. Ruiz, J. C. Aguillon, and A. Fehrmann. 1989. A new spectrophotometric method for the determination of ferrous iron in the presence of ferric iron. J. Chem. Technol. Biotechnol. 44:171-181. [Google Scholar]
- 18.Krogh, A., B. Larsson, G. von Heijne, and E. L. Sonnhammer. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305:567-580. [DOI] [PubMed] [Google Scholar]
- 19.Liesegang, H., K. Lemke, R. A. Siddiqui, and H. G. Schlegel. 1993. Characterization of the inducible nickel and cobalt resistance determinant cnr from pMOL28 of Alcaligenes eutrophus CH34. J. Bacteriol. 175:767-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahapatra, N. R., S. Ghosh, C. Deb, and P. C. Banerjee. 2002. Resistance to cadmium and zinc in Acidiphilium symbioticum KM2 is plasmid mediated. Curr. Microbiol. 45:180-186. [DOI] [PubMed] [Google Scholar]
- 21.Mergeay, M., D. Nies, H. G. Schlegel, J. Gerits, P. Charles, and F. Van Gijsegem. 1985. Alcaligenes eutrophus CH34 is a facultative chemolithotroph with plasmid-bound resistance to heavy metals. J. Bacteriol. 162:328-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 23.Nakai, K., and M. Kanehisa. 1991. Expert system for predicting protein localization sites in gram-negative bacteria. Proteins 11:95-110. [DOI] [PubMed] [Google Scholar]
- 24.Nies, D. H., and S. Silver. 1995. Ion efflux systems involved in bacterial metal resistances. J. Ind. Microbiol. 14:186-199. [DOI] [PubMed] [Google Scholar]
- 25.Park, J. E., H. G. Schlegel, H. G. Rhie, and H. S. Lee. 2004. Nucleotide sequence and expression of the ncr nickel and cobalt resistance in Hafnia alvei 5-5. Int. Microbiol. 7:27-34. [PubMed] [Google Scholar]
- 26.Park, J. E., K. E. Young, H. G. Schlegel, H. G. Rhie, and H. S. Lee. 2003. Conjugative plasmid mediated inducible nickel resistance in Hafnia alvei 5-5. Int. Microbiol. 6:57-64. [DOI] [PubMed] [Google Scholar]
- 27.Rapp, M., D. Drew, D. O. Daley, J. Nilsson, T. Carvalho, K. Melen, J. W. De Gier, and G. Von Heijne. 2004. Experimentally based topology models for E. coli inner membrane proteins. Protein Sci. 13:937-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rawlings, D. E., H. Tributsch, and G. S. Hansford. 1999. Reasons why ‘Leptospirillum’-like species rather than Thiobacillus ferrooxidans are the dominant iron-oxidizing bacteria in many commercial processes for the biooxidation of pyrite and related ores. Microbiology 145:5-13. [DOI] [PubMed] [Google Scholar]
- 29.Rodrigue, A., G. Effantin, and M.-A. Mandrand-Berthelot. 2005. Identification of rcnA (yohM), a nickel and cobalt resistance gene in Escherichia coli. J. Bacteriol. 187:2912-2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saier, M. H., Jr., J. T. Beatty, A. Goffeau, K. T. Harley, W. H. Heijne, S. C. Huang, D. L. Jack, P. S. Jahn, K. Lew, J. Liu, S. S. Pao, I. T. Paulsen, T. T. Tseng, and P. S. Virk. 1999. The major facilitator superfamily. J. Mol. Microbiol. Biotechnol. 1:257-279. [PubMed] [Google Scholar]
- 31.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 32.Schmidt, T., R.-D. Stoppel, and H. G. Schlegel. 1991. High-level nickel resistance in Alcaligenes xylosoxydans 31A and Alcaligenes eutrophus KTO2. Appl. Environ. Microbiol. 57:3301-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stoppel, R., and H. G. Schlegel. 1995. Nickel-resistant bacteria from anthropogenically nickel-polluted and naturally nickel-percolated ecosystems. Appl. Environ. Microbiol. 61:2276-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stoppel, R. D., M. Meyer, and H. G. Schlegel. 1995. The nickel resistance determinant cloned from the enterobacterium Klebsiella oxytoca: conjugational transfer, expression, regulation and DNA homologies to various nickel-resistant bacteria. Biometals 8:70-79. [DOI] [PubMed] [Google Scholar]
- 35.Tuffin, I. M., S. B. Hector, S. M. Deane, and D. E. Rawlings. 2006. Resistance determinants of a highly arsenic-resistant strain of Leptospirillum ferriphilum isolated from a commercial biooxidation tank. Appl. Environ. Microbiol. 72:2247-2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tyson, G. W., J. Chapman, P. Hugenholtz, E. E. Allen, R. J. Ram, P. M. Richardson, V. V. Solovyev, E. M. Rubin, D. S. Rokhsar, and J. F. Banfield. 2004. Community structure and metabolism through reconstruction of microbial genomes from the environment. Nature 428:37-43. [DOI] [PubMed] [Google Scholar]
- 37.Vanbleu, E., K. Marchal, and J. Vanderleyden. 2004. Genetic and physical map of the pLAFR1 vector. DNA Sequence 15:225-227. [DOI] [PubMed] [Google Scholar]