Abstract
A bovine-specific cDNA microarray was used to characterize gene expression in the bovine rectoanal junction mucosa in response to Escherichia coli O157:H7 colonization, and results were confirmed using quantitative real-time PCR. The results showed involvement of cell processes including immune response, cell structure/dynamics, signal transduction, intercellular communication, and metabolism.
Enterohemorrhagic Escherichia coli O157:H7 is an important food-borne pathogen that causes hemorrhagic colitis with life-threatening sequelae in humans (16). Healthy ruminants, especially cattle, are the reservoir of this pathogen (2), and human infections are associated with direct or indirect contact with ruminant feces (13, 19). It is accepted that reduction of E. coli O157:H7 among ruminants will decrease the incidence of human infections.
The persistence of E. coli O157:H7 in individual cattle can vary greatly from several days to many weeks (5, 32), and both host and bacterial processes affect colonization. Multiple bacterial factors have been identified as contributing to the adherence of E. coli O157:H7 to host mucosa (7, 25, 27, 28); however, the molecular mechanisms that result in bacterial colonization have not been elucidated. Recent studies provide compelling evidence that the bovine lymphoid follicular-rich rectoanal junction (RAJ) mucosa is the primary site of E. coli O157:H7 colonization in cattle (14, 17). The reason(s) for this tropism and the host response(s) in this interaction are not understood.
To increase our knowledge about the bovine cell response to E. coli O157:H7, we undertook a cDNA microarray experiment to characterize bovine gene expression in RAJ tissue of cattle colonized with E. coli O157:H7. Four groups of 5- to 10-month-old Holstein steers (three per group) were used in this study. The experimental design included comparing challenges with E. coli O157:H7 strain ATCC 43895 (American Type Culture Collection, Manassas, VA) to challenges with Luria-Bertani broth (no bacteria, the negative control), a noncolonizing Shiga toxin-producing E. coli (STEC) (E. coli ONT:H25 SH3, non-O157 noncolonizing STEC control; referred to as SH3), and a colonizing non-O157 STEC (E. coli ONT:H25 SH2, non-O157-colonizing STEC control; referred to as SH2) (23). The negative control reduced the variability of gene expression caused by the procedure of challenge and tissue preparation. The STEC colonizing and noncolonizing controls allowed us to distinguish gene responses specific for STEC colonization or general to E. coli application.
All the challenges were performed by a rectal application of bacteria, as previously described (24), except that a single dose of 107 CFU was given to each animal. Unlike the oral dose, rectal application of bacteria allowed for very accurate analyses of early events after bacterial challenge. RAJ mucosa swab (RAMS) samples were collected and cultured as previously described (6, 23) at 6 h, day 1, day 3, and day 7 and then once a week for at least 1 month after bacterial challenge. Representative isolates from all animals on all culture-positive sampling days had restricted chromosomal DNA pulsed-field gel electrophoresis patterns identical to the original challenge strains (data not shown).
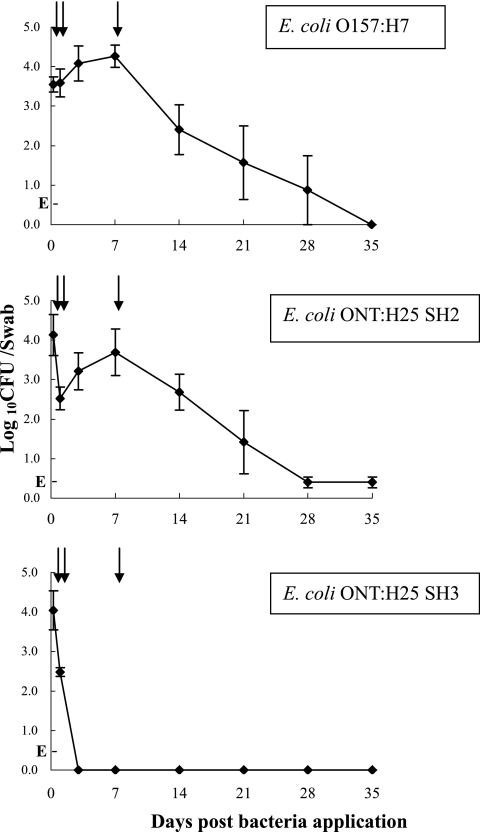
E. coli O157:H7 and SH2 persisted at the bovine RAJ mucosa for more than 2 weeks in all animals and reached the highest concentrations of bacteria at the 7th day postchallenge (Fig. 1), indicating that animals were colonized with these E. coli strains. In contrast, SH3 was cultured from the RAJ mucosa for only 1 day and was cleared from all three animals by day 3 postchallenge (Fig. 1), indicating that this strain did not colonize the cattle.
FIG. 1.
Bacterial persistence at the RAJ mucosa after challenge with E. coli O157:H7 or STEC strains. Three steers per group were given a single rectal application of 1 × 107 CFU of E. coli O157:H7 (ATCC 43895), E. coli ONT:H25 strain SH2, or E. coli ONT:H25 strain SH3. The average CFU (±standard errors) of E. coli cells/swab detected by RAMS culture are shown. E, RAMS sample culture positive for E. coli only after enrichment and indicating the cell number is ≤30 CFU/swab. Arrows indicate the times when RAJ mucosa tissue samples were taken: 6 h, day 1, and day 7 after bacterial challenges.
RAJ mucosal tissue samples were obtained at 6 h, day 1, and day 7 after bacterial challenges using a bone curette (Fisher Scientific Company LLC, Pittsburgh, PA). At each sampling time, three pieces of tissue (10 to 20 g/piece) were taken from the dorsal, right, and left sides of the RAJ mucosa 7 to 10 cm from the anus, immediately placed into 2 ml of RNAlater (QIAGEN Ltd., Valencia, CA) to stabilize the total RNA, and stored at −80°C until further processing.
Three pieces of tissue from each sampling time were combined for total-RNA extraction. Highly pure total RNA was extracted with an RNeasy minikit, and contaminating DNA was removed using an RNase-free DNase set according to the manufacturer's protocol (QIAGEN Ltd.). Aminoallyl-labeled cDNA was reverse transcribed from 5 μg of total RNA using a PowerScript fluorescent labeling kit (Clontech Laboratories Inc., Mountain View, CA) and reacted with Cy3 or Cy5 N-hydroxysuccinimide dye (Cy3 or Cy5) esters (Amersham Biosciences Ltd. [now GE Health], Piscataway, NJ) according to the manufacturer's protocol.
Microarray experiments used a standard reference design (3). Bovine-specific cDNA microarrays containing 13,824 total spots, consisting of 4,608 bovine expressed sequence tags, were generated from gastrointestinal, mammary, and pituitary tissues (rich in immune cells) (26). BLAST analysis of all the sequences identified 1,676 unique genes. The cDNA clone names, sequences, and/or gene information are available on a World Wide Web-accessible database (http://amadeus.biosci.arizona.edu/bovine/).
Microarray hybridizations were performed by standard procedures (4), the images were analyzed using GenePix software (version 6.0; Axon Instruments, Union City, CA), and data were imported into SAS 8.0 (SAS Institute Inc., Cary, NC) for further statistical analysis (20). The change (n-fold) of gene expression and the P value for each gene were calculated from the ratio of the average microarray signals from challenged cattle (E. coli O157:H7, SH2, or SH3) to signals from the negative control using Student's t test (n = 3). Although many analyses use a 2-fold change as the cutoff to identify differential gene expression, we used more-stringent criteria that coupled a ≥1.5-fold change with a P value of ≤0.05. Genes were categorized based on the function of their product most likely related to this study (e.g., the NADPH oxidase gene was categorized in immune systems because of its relation to the neutrophil respiratory burst).
Comparisons of the microarray results from all challenges at all sampling times showed 49 genes differentially expressed (up or down) only in the E. coli O157:H7 group and not in either of the other challenge groups (SH2 or SH3), indicating that they were unique to E. coli O157:H7 carriage (Table 1). However, 32 genes were associated with both E. coli O157:H7 and SH2 colonization, indicating they may be a generalized response to STEC colonization. Most of these genes were differentially expressed at days 1 and 7 after bacterial challenge (Table 1). Very little differential gene expression could be attributed to the procedure of applying bacterial cells to the RAJ mucosa, as only six genes had differential expression among all the groups (Table 1).
TABLE 1.
Bovine gene expression unique to E. coli O157:H7 or STEC colonization
| Sampling time | Total no. of genes differentially expresseda | No. of genes unique to E. coli O157:H7b | No. of genes responding to STEC:
|
|
|---|---|---|---|---|
| Colonizationc | Applicationd | |||
| 6 h | 14 (+) | 5 | 1 | 3 |
| 6 (−) | 4 | 0 | 0 | |
| Day 1 | 35 (+) | 15 | 4 | 0 |
| 9 (−) | 3 | 0 | 0 | |
| Day 7 | 22 (+) | 10 | 3 | 0 |
| 49 (−) | 12 | 24 | 3 | |
Genes differentially expressed following E. coli O157:H7 challenge. +, up-regulated; −, down-regulated. Criteria for differential expression were a P value <0.05 and a change of >1.5-fold.
Gene expression changed only among cattle colonized with E. coli O157:H7 and not with other challenges.
Gene expression changed among cattle colonized with E. coli O157:H7 or E. coli ONT:H25 SH2 but not among cattle challenged with noncolonizing E. coli ONT:H25 SH3.
Gene expression changed among cattle challenged with any of the three bacterial strains.
At 6 h after E. coli O157:H7 challenge, 20 genes exhibited significantly different expression compared to the negative control, most of which (70%) were up-regulated. Table 2 shows select genes with differential expression, and the complete data from this experiment are shown in Table S1 of supplemental material. Of these differentially expressed genes, five up-regulated genes (PVRL4, BTNL1, MAPRE1, SUMF2, and TNPO) and four down-regulated genes (TCAP, ARHGEF6, GCLC, and GS1-539F22) were unique to E. coli O157:H7 carriage. These genes included those encoding proteins involved in multiple cell processes such as immune response, cell growth and differentiation, cell structure dynamics, signal transduction, metabolism, and protein biosynthesis and modification (Table 2).
TABLE 2.
Description and differential expression of select genes
| Category and description of gene product | Sampling time | Gene symbola | GenBank accession no. |
E. coli O157:H7
|
E. coli ONT:H25 SH2d
|
E. coli ONT:H25 SH3d
|
|||
|---|---|---|---|---|---|---|---|---|---|
| Fold changeb | Pc | Fold changeb | Pc | Fold changeb | Pc | ||||
| Immune response | |||||||||
| Bos taurus poliovirus receptor-related 4 | 6 h | PVRL4 | BT020770 | 1.75 | <0.01 | 1.74 | 0.20 | 2.27 | 0.09 |
| Bos taurus polymeric immunoglobulin receptor | 6 h | PIGR | L04797 | 1.93 | 0.02 | 3.51 | 0.05 | 2.03 | 0.09 |
| Bos taurus protein similar to butyrophilin-like 9 | 6 h | BTNL1 | XM_591843 | 1.95 | 0.01 | 1.05 | 0.46 | 1.85 | 0.07 |
| Cell growth and differentiation | |||||||||
| Bos taurus titin-cap | 6 h | TCAP | XM_590918 | −1.72 | 0.01 | −1.50 | 0.24 | −1.13 | 0.90 |
| Bos taurus protein similar to keratinocyte growth factor precursor (fibroblast growth factor 7) | Day 1 | KGF | XM_869016 | 1.60 | 0.02 | 1.90 | 0.04 | 1.58 | 0.24 |
| Cell structure dynamics | |||||||||
| Homo sapiens microtubule-associated protein, RP/EB family, member 1 | 6 h | MAPRE1 | NM_012325 | 1.57 | 0.01 | 3.29 | 0.06 | 3.47 | 0.12 |
| Homo sapiens coatomer protein complex, subunit beta | Day 7 | COPB | NM_016451 | −1.82 | 0.04 | −2.09 | 0.02 | −1.28 | 0.24 |
| Sus scrofa desmin | Day 7 | DES | AF136188 | −2.09 | 0.01 | −2.72 | 0.01 | −1.52 | 0.18 |
| Bos taurus protein similar to cortactin isoform a | Day 7 | CTT | XM_583464 | −2.01 | 0.03 | −2.90 | 0.04 | 1.31 | 0.27 |
| Signal transduction | |||||||||
| Bos taurus protein similar to Rho guanine nucleotide exchange factor 6 | 6 h | ARHGEF6 | XM_613352 | −1.72 | 0.02 | −1.16 | 0.34 | −2.10 | 0.13 |
| Bos taurus protein similar to catenin (cadherin-associated protein), beta 1 | Day 1 | CTNNB1 | XM_877733 | 1.55 | 0.04 | 1.80 | 0.06 | 1.26 | 0.31 |
| Homo sapiens RAB43, member RAS oncogene family | Day 1 | RAB43 | BC082988 | 1.88 | 0.03 | 1.84 | 0.07 | 1.89 | 0.25 |
| Bos taurus caveolin 1 | Day 1 | CAV1 | AY823915 | 1.50 | 0.01 | 2.42 | 0.05 | 1.20 | 0.48 |
| Homo sapiens G protein-coupled receptor kinase interactor 2 | Day 1 | GIT2 | BC039880 | 1.69 | 0.04 | 3.10 | 0.10 | 1.82 | 0.19 |
| Bos taurus protein similar to putative GTP-binding protein RAY-like (Rab-like protein 4) | Day 1 | RAB4 | XM_589411 | −1.52 | 0.01 | −1.22 | 0.16 | −1.29 | 0.20 |
| Bos taurus protein similar to progesterone membrane binding protein | Day 1 | PMBP | XM_613630 | −1.53 | 0.01 | −1.24 | 0.11 | −1.55 | 0.12 |
| Intercellular communication | |||||||||
| Homo sapiens calcium channel, voltage dependent, L type, alpha 1C subunit | Day 7 | CACNA1C | NM_000719 | −1.66 | 0.03 | −2.83 | 0.05 | 1.14 | 0.70 |
| Metabolism | |||||||||
| Bos taurus sulfatase modifying factor 2 | 6 h | SUMF2 | XM_586469 | 2.08 | 0.04 | −2.27 | 0.06 | 1.42 | 0.76 |
| Bos taurus glutamate-cysteine ligase catalytic subunit | 6 h | GCLC | AY957499 | −1.92 | 0.01 | −7.80 | 0.13 | −1.16 | 0.39 |
| Protein biosynthesis and modification | |||||||||
| Bos taurus transportin mRNA | 6 h | TNPO | AY298813 | 2.08 | <0.01 | −1.23 | 0.30 | 2.32 | 0.06 |
| Bos taurus protein similar to signal peptidase complex subunit 1 (microsomal signal peptidase 12-kDa subunit) | Day 1 | SPCS1 | BC102144 | 1.75 | 0.01 | 2.50 | 0.02 | 1.40 | 0.18 |
| Others | |||||||||
| Homo sapiens BAC clone GS1-539F22 from chromosome 7 | 6 h | GS1-539F22 | AC005028 | −1.52 | 0.01 | −2.13 | 0.47 | −2.54 | 0.07 |
| Bos taurus hypothetical protein FLJ13096 | Day 1 | FLJ13096 | XM_865552 | 1.85 | 0.01 | 1.94 | 0.04 | 1.81 | 0.19 |
The gene symbol is an abbreviation of the gene description.
Changes for genes that are up-regulated (positive numbers) and down-regulated (negative numbers) in animals challenged with E. coli O157:H7 or ONT:H25 SH2 or SH3 relative to animals challenged with Luria-Bertani broth (nonbacterial, negative control). The array contained many genes that after sequence annotation were found to be analogous; therefore the average of these was used as the representative value for the annotated gene. Each value represents the mean of gene expression from three animals.
P was calculated by Student's t test from three biological replicates.
E. coli ONT:H25 SH2 is the non-O157 colonizing STEC control; E. coli ONT:H25 SH3 is the noncolonizing STEC control.
The largest numbers of differentially expressed annotated genes at 6 h after E. coli O157:H7 challenge belonged to the categories of immune response or metabolism. Among immune response-related genes was PIGR, encoding the polymeric immunoglobulin receptor, which can transport immunoglobulin A from the basal surface to the apical surface of intestinal epithelial cells (31). The up-regulation of expression of this gene may indicate that there was a specific local mucosal immune response to E. coli O157:H7 in the lymphoid follicular-rich RAJ mucosal tissue. This gene was also up-regulated among the cattle challenged with SH2, indicating that its activity may be induced by STEC at the bovine RAJ site. However, this local response was not strong enough to clear colonizing E. coli O157:H7 or SH2.
One day after E. coli O157:H7 challenge, 44 genes showed a significant expression change, with 35 genes (80%) up-regulated and 9 genes down-regulated. Table 2 shows select genes with differential expression, and the complete data from this experiment are shown in Table S2 in the supplemental material. Compared to the results at 6 h, more genes were unique to E. coli O157:H7 (15 up-regulated genes and 3 down-regulated genes; Table 1).
The differentially expressed genes at day 1 after bacterial challenge were in similar categories as the genes identified at 6 h postchallenge, but the largest number (six) were related to signal transduction (see Table S2 in the supplemental material). This category included five genes (CTNNB1, RAB43, GIT2, RAB4, and PMBP) unique to E. coli O157:H7 and one gene (CAV1) associated with STEC colonization (both E. coli O157:H7 and SH2). Interestingly, one gene (CTNNB1) encodes beta-catenin (cadherin-associated protein), a protein which can bind the cytoplasmic side of E-cadherin, mediating cell-cell adhesion and also binding to alpha-catenin, participating in the regulation of actin-containing cytoskeletal filaments (9, 33). Up-regulation of this gene may be related to the cell skeleton changes that contribute to E. coli O157:H7 adherence and interaction with bovine RAJ cells (18, 30).
Besides CAV1, three other genes (KGF, SPCS1, and FLJ13096) were also associated with STEC colonization (both E. coli O157:H7 and SH2) at day 1 after bacterial challenge. The product of KGF is keratinocyte growth factor precursor, a protein specific for epithelial cell growth (21). How increasing KGF gene expression is associated with increased colonization is not clear, but it may provide increased nutrients or surface area for E. coli persistence.
Seven days after E. coli O157:H7 challenge, 71 genes were differentially expressed. Among these genes, 22 (31%) exhibited significantly up-regulated expression and most were cell growth- and differentiation-related genes (see Table S3 in the supplemental material). In contrast, 49 genes (69%) were significantly down-regulated and related to multiple cellular processes (see Table S4 in the supplemental material). The majority of the down-regulated genes were related to protein biosynthesis, and most encoded ribosomal proteins. Most of the up-regulated genes (10 of 13 annotated genes) and 12 down-regulated genes were unique to E. coli O157:H7 carriage.
A significant finding at day 7 was that a large portion (38%) of the differentially expressed genes (3 up-regulated genes and 24 down-regulated genes) were associated with STEC colonization (both E. coli O157:H7 and SH2). Among these genes, three were related to cell structure dynamics (COPB, DES, and CTT) and were all down-regulated (Table 2). The product of gene CTT is cortactin, a protein that serves as an important regulator for actin polymerization in eukaryotic cells (22, 29). Cortactin is recruited to enterohemorrhagic E. coli adherence sites in HeLa cells and may contribute to the formation of pedestal-like structures in the characteristic attaching and effacing (A/E) lesions (1, 10). A/E lesion formation also occurs at the bovine terminal rectum with E. coli O157:H7 colonization (18). Therefore, down-regulation of the cortactin gene at day 7 may be a host response that contributes to clearance of the colonizing bacteria. As shown in Fig. 1, the total bacterial CFU/swab of both E. coli O157:H7 and SH2 decreased after day 7. However, it should be noted that the gene expression changes in this category indicated that host cell cytoskeleton changes in response to E. coli O157:H7 are complex and may involve other structural reorganization processes.
Another notable gene (CACNA1C) associated with STEC colonization (both E. coli O157:H7 and SH2) encodes a protein similar to the human L-type voltage-dependent calcium channel, alpha 1C subunit (Table 2). The product of this gene is a protein that transports extracellular calcium into cells (15) and that plays an important role in calcium-dependent signal transduction (11). One study shows that the adhesion of E. coli O157:H7 to human epithelial cells results in elevation of the second messengers, molecular free calcium, and inositol 1,4,5-triphosphate (8). We speculate that calcium-dependent signal transduction may play an important role in bovine cell interactions with E. coli O157:H7.
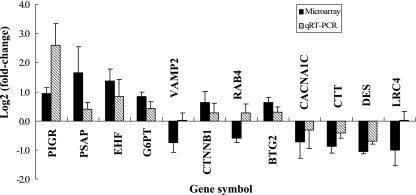
To confirm microarray reliability, expression levels of 12 representative genes were checked using quantitative real-time PCR (see Table S5 in the supplemental material). The genes were selected for their specific annotation information, for the availability of specific primer sets, for inclusion of multiple cell processes, and for representation of all the sampling times. Experiments were performed in triplicate for each gene, and the change (n-fold) was determined after normalization of each PCR product to the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene using the comparative-threshold method (12). Nine genes had up- or down-regulation similar to that measured by microarray (Fig. 2). Another three genes (VAMP2, RAB4, and LRC4) had no change or an opposite change to that measured by microarray (Fig. 2). However, gene expression at the mRNA level may not always equal the levels of protein expression. Further experiments are needed to confirm protein level changes and other cellular events key to cellular function.
FIG. 2.
Quantitative real-time PCR (qRT-PCR) validation of cDNA microarray data. Genes were differentially expressed at various times after bacterial challenge among cattle colonized with E. coli O157:H7. The bar shows the log2 value (ratio of E. coli O157:H7 to negative control). Total RNAs isolated from bovine RAJ cells from E. coli O157:H7-colonized cattle and non-bacterially challenged control cattle at 6 h, day 1, and day 7 after bacterial challenge were used as templates for SYBR green-based qRT-PCR processed on an ABI 7000 instrument. Quantification of gene expression was standardized relative to the internal control GAPDH gene.
This is the first time bovine gene expression in response to E. coli O157:H7 has been identified. It is also the first time that a microarray was used with in vivo tissue from cattle colonized by this human pathogen. Our results revealed that multiple cell processes were involved in the bovine RAJ mucosa response to E. coli O157:H7 and suggested that calcium-dependent signal transduction and cell structure dynamic changes may be important for E. coli O157:H7 bovine colonization. Our conclusions should be taken with some caution, however, due to the limitations of the microarray technique, gene annotations, and animal variation. Nevertheless, these results provide insight into the molecular mechanism of bovine cell response to E. coli O157:H7 colonization and identify several candidate genes for further study.
Supplementary Material
Acknowledgments
This work was supported, in part, by the Idaho Agriculture Experiment Station; the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service, grant number 04-04562; Public Health Service grants U54-AI-57141, P20-RR16454, and P20-RR15587 from the National Institutes of Health; and grants from the United Dairymen of Idaho and the Idaho Beef Council.
We thank Haiqing Sheng, Xianglu Li, Julie Kelsey, and Mark McGuire for assistance. We appreciate Robert Collier for providing the bovine microarrays and Chad Stiening for information about microarray data analysis. We also thank Lonie Austin and Duane Bull for animal handling.
Footnotes
Published ahead of print on 9 February 2007.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Cantarelli, V. V., A. Takahashi, Y. Akeda, K. Nagayama, and T. Honda. 2000. Interaction of enteropathogenic or enterohemorrhagic Escherichia coli with HeLa cells results in translocation of cortactin to the bacterial adherence site. Infect. Immun. 68:382-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapman, P. A., C. A. Siddons, A. T. Gerdan Malo, and M. A. Harkin. 1997. A 1-year study of Escherichia coli O157 in cattle, sheep, pigs and poultry. Epidemiol. Infect. 119:245-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Churchill, G. A. 2002. Fundamentals of experimental design for cDNA microarrays. Nat. Genet. 32(Suppl.):490-495. [DOI] [PubMed] [Google Scholar]
- 4.Coussens, P. M., C. J. Colvin, K. Wiersma, A. Abouzied, and S. Sipkovsky. 2002. Gene expression profiling of peripheral blood mononuclear cells from cattle infected with Mycobacterium paratuberculosis. Infect. Immun. 70:5494-5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cray, W. C., Jr., and H. W. Moon. 1995. Experimental infection of calves and adult cattle with Escherichia coli O157:H7. Appl. Environ. Microbiol. 61:1586-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis, M. A., D. D. Hancock, D. H. Rice, D. R. Call, R. DiGiacomo, M. Samadpour, and T. E. Besser. 2003. Feedstuffs as a vehicle of cattle exposure to Escherichia coli O157:H7 and Salmonella enterica. Vet. Microbiol. 95:199-210. [DOI] [PubMed] [Google Scholar]
- 7.Dytoc, M., R. Soni, F. Cockerill III, A. J. De, M. Louie, J. Brunton, and P. Sherman. 1993. Multiple determinants of verotoxin-producing Escherichia coli O157:H7 attachment-effacement. Infect. Immun. 61:3382-3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ismaili, A., D. J. Philpott, M. T. Dytoc, and P. M. Sherman. 1995. Signal transduction responses following adhesion of verocytotoxin-producing Escherichia coli. Infect. Immun. 63:3316-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobielak, A., and E. Fuchs. 2004. Alpha-catenin: at the junction of intercellular adhesion and actin dynamics. Nat. Rev. Mol. Cell. Biol. 5:614-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kodama, T. 2002. Mechanism of A/E lesion formation produced by enterohemorrhagic Escherichia coli O157—role of EspB, Tir and cortactin. Nippon Rinsho 60:1101-1107. [PubMed] [Google Scholar]
- 11.Lipscombe, D., T. D. Helton, and W. Xu. 2004. L-type calcium channels: the low down. J. Neurophysiol. 92:2633-2641. [DOI] [PubMed] [Google Scholar]
- 12.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 13.Locking, M. E., S. J. O'Brien, W. J. Reilly, E. M. Wright, D. M. Campbell, J. E. Coia, L. M. Browning, and C. N. Ramsay. 2001. Risk factors for sporadic cases of Escherichia coli O157 infection: the importance of contact with animal excreta. Epidemiol. Infect. 127:215-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Low, J. C., I. J. McKendrick, C. McKechnie, D. Fenlon, S. W. Naylor, C. Currie, D. G. Smith, L. Allison, and D. L. Gally. 2005. Rectal carriage of enterohemorrhagic Escherichia coli O157 in slaughtered cattle. Appl. Environ. Microbiol. 71:93-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moosmang, S., P. Lenhardt, N. Haider, F. Hofmann, and J. W. Wegener. 2005. Mouse models to study L-type calcium channel function. Pharmacol. Ther. 106:347-355. [DOI] [PubMed] [Google Scholar]
- 16.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naylor, S. W., J. C. Low, T. E. Besser, A. Mahajan, G. J. Gunn, M. C. Pearce, I. J. McKendrick, D. G. Smith, and D. L. Gally. 2003. Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonization of enterohemorrhagic Escherichia coli O157:H7 in the bovine host. Infect. Immun. 71:1505-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naylor, S. W., A. J. Roe, P. Nart, K. Spears, D. G. Smith, J. C. Low, and D. L. Gally. 2005. Escherichia coli O157:H7 forms attaching and effacing lesions at the terminal rectum of cattle and colonization requires the LEE4 operon. Microbiology 151:2773-2781. [DOI] [PubMed] [Google Scholar]
- 19.O'Brien, S. J., G. K. Adak, and C. Gilham. 2001. Contact with farming environment as a major risk factor for Shiga toxin (Vero cytotoxin)-producing Escherichia coli O157 infection in humans. Emerg. Infect. Dis. 7:1049-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quackenbush, J. 2002. Microarray data normalization and transformation. Nat. Genet. 32(Suppl.):496-501. [DOI] [PubMed] [Google Scholar]
- 21.Rubin, J. S., H. Osada, P. W. Finch, W. G. Taylor, S. Rudikoff, and S. A. Aaronson. 1989. Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc. Natl. Acad. Sci. USA 86:802-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selbach, M., and S. Backert. 2005. Cortactin: an Achilles' heel of the actin cytoskeleton targeted by pathogens. Trends Microbiol. 13:181-189. [DOI] [PubMed] [Google Scholar]
- 23.Sheng, H., M. A. Davis, H. J. Knecht, D. D. Hancock, D. J. Van, and C. J. Hovde. 2005. Characterization of a Shiga toxin-, intimin-, and enterotoxin hemolysin-producing Escherichia coli ONT:H25 strain commonly isolated from healthy cattle. J. Clin. Microbiol. 43:3213-3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheng, H., M. A. Davis, H. J. Knecht, and C. J. Hovde. 2004. Rectal administration of Escherichia coli O157:H7: novel model for colonization of ruminants. Appl. Environ. Microbiol. 70:4588-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sherman, P., F. Cockerill III, R. Soni, and J. Brunton. 1991. Outer membranes are competitive inhibitors of Escherichia coli O157:H7 adherence to epithelial cells. Infect. Immun. 59:890-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stiening, C. M., J. Hoying, A. Hoying, D. Henderson, P. Gentry, Y. Kobayashi, and R. Collier. 2005. Characterization of a 4,600 gene bovine microarray. J. Anim. Sci. 81(Suppl. 1):300. [Google Scholar]
- 27.Tatsuno, I., H. Kimura, A. Okutani, K. Kanamaru, H. Abe, S. Nagai, K. Makino, H. Shinagawa, M. Yoshida, K. Sato, J. Nakamoto, T. Tobe, and C. Sasakawa. 2000. Isolation and characterization of mini-Tn5Km2 insertion mutants of enterohemorrhagic Escherichia coli O157:H7 deficient in adherence to Caco-2 cells. Infect. Immun. 68:5943-5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torres, A. G., and J. B. Kaper. 2003. Multiple elements controlling adherence of enterohemorrhagic Escherichia coli O157:H7 to HeLa cells. Infect. Immun. 71:4985-4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uruno, T., J. Liu, P. Zhang, Y. Fan, C. Egile, R. Li, S. C. Mueller, and X. Zhan. 2001. Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat. Cell Biol. 3:259-266. [DOI] [PubMed] [Google Scholar]
- 30.Vlisidou, I., F. Dziva, R. M. La Ragione, A. Best, J. Garmendia, P. Hawes, P. Monaghan, S. A. Cawthraw, G. Frankel, M. J. Woodward, and M. P. Stevens. 2006. Role of intimin-tir interactions and the tir-cytoskeleton coupling protein in the colonization of calves and lambs by Escherichia coli O157:H7. Infect. Immun. 74:758-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woof, J. M., and M. A. Kerr. 2006. The function of immunoglobulin A in immunity. J. Pathol. 208:270-282. [DOI] [PubMed] [Google Scholar]
- 32.Wray, C., I. M. McLaren, L. P. Randall, and G. R. Pearson. 2000. Natural and experimental infection of normal cattle with Escherichia coli O157. Vet. Rec. 147:65-68. [DOI] [PubMed] [Google Scholar]
- 33.Yap, A. S., W. M. Brieher, and B. M. Gumbiner. 1997. Molecular and functional analysis of cadherin-based adherens junctions. Annu. Rev. Cell Dev. Biol. 13:119-146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.