Abstract
Simvastatin is a semisynthetic derivative of the fungal polyketide lovastatin and is an important drug for lowering cholesterol levels in adults. We have developed a one-step, whole-cell biocatalytic process for the synthesis of simvastatin from monacolin J. By using an Escherichia coli strain overexpressing the previously discovered acyltransferase LovD (X. Xie, K. Watanabe, W. A. Wojcicki, C. C. Wang, and Y. Tang, Chem. Biol. 13:1161-1169, 2006), we were able to achieve >99% conversion of monacolin J to simvastatin without the use of any chemical protection steps. The key finding was a membrane-permeable substrate, α-dimethylbutyryl-S-methyl-mercaptopropionate, that was efficiently utilized by LovD as the acyl donor. The process was scaled up for gram-scale synthesis of simvastatin. We also demonstrated that simvastatin synthesized via this method can be readily purified from the fermentation broth with >90% recovery and >98% purity as determined by high-performance liquid chromatography. Bioconversion using high-cell-density, fed-batch fermentation was also examined. The whole-cell biocatalysis can therefore be an attractive alternative to currently used multistep semisynthetic transformations.
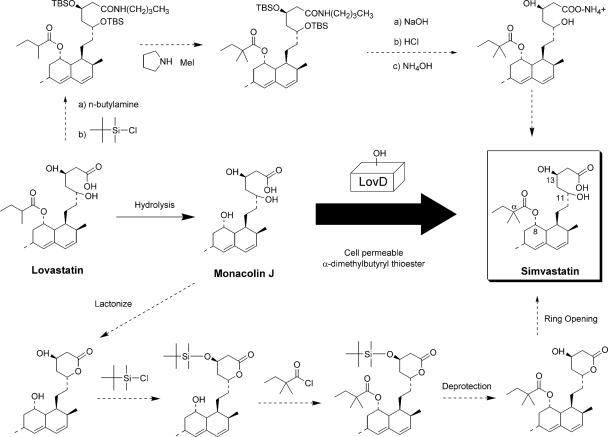
Simvastatin (Fig. 1) is a cholesterol-lowering compound that significantly reduces the risk of heart diseases associated with hypercholesterolemia (17). Simvastatin is marketed by Merck as Zocor and is the second-best-selling drug in the United States, with annual sales exceeding $5 billion. Simvastatin is a semisynthetic compound derived from the natural product lovastatin, which is a secondary metabolite produced by the filamentous fungus Aspergillus terreus (1, 3, 4, 8, 10). The synthesis of simvastatin from lovastatin is a multistep process and has been of intense interest because of its importance in the pharmaceutical industry (2, 5, 6, 16) (J. Lee, T. Ha, C. Park, H. Lee, G. Lee, and Y. Chang, U.S. patent application 20050080275; B. Morgan, M. Burk, M. Levin, Z. Zhu, J. Chaplin, K. Kustedjo, Z. Huang, and W. Greenberg, 6 May 2005, patent application PCT WO2005040107; B. P. Reddy, 21 July 2005, patent application PCT WO2005066150).
FIG. 1.
Chemical structures of lovastatin acid, monacolin J acid, and simvastatin acid. The biocatalytic reaction studied is the enzymatic conversion of monacolin J to simvastatin (thick arrow). LovD is able to regioselectively acylate the C8 hydroxyl group. Two commonly used semisynthetic processes are shown with dashed arrows.
Currently, two semisynthetic processes (2, 5) are widely used to synthesize simvastatin starting from lovastatin (Fig. 1). One commonly adapted process (5) starts with the hydrolysis of lovastatin to yield the key intermediate monacolin J, followed by the lactonization of the acid to protect the C11 hydroxyl group and trimethylsilylation protection of the C13 hydroxyl. The protected monacolin J is then subjected to acylation by α-dimethylbutyryl chloride to yield the protected form of simvastatin, which is subsequently deprotected to yield simvastatin. Both multistep processes shown in Fig. 1 are laborious, thus contributing to simvastatin being nearly five times more expensive than lovastatin. Therefore, a new semisynthetic scheme that can decrease the number of chemical transformations and increase the overall efficiency of the conversion can be of significant utility.
We have previously described the cloning and characterization of a dedicated acyltransferase from the lovastatin biosynthetic gene cluster that regioselectively transfers the α-methylbutyryl group from the lovastatin diketide synthase to the C8 hydroxyl group of monacolin J to yield lovastatin (18). We demonstrated that LovD has broad substrate specificity towards the acyl carrier, the acyl group, and the decalin core. Most notably, LovD was able to catalyze the direct acylation of monacolin J by α-dimethylbutyryl-S-N-acetylcysteamine (DMB-S-NAC) to afford simvastatin. The reaction is highly regiospecific towards the C8 alcohol only and is therefore a potential one-step process to produce simvastatin from monacolin J without employing protective chemistry.
In this report, we describe the development of a whole-cell biocatalytic process that is able to convert monacolin J to simvastatin in a highly efficient manner. By using a novel thioester as the acyl donor, we were able to achieve >99% conversion of simvastatin from monacolin J in a single step. The fermentation process can be easily scaled up to produce an industrial-scale yield of simvastatin.
MATERIALS AND METHODS
Synthesis of DMB-S-MMP.
Dimethylbutyryl chloride (1.76 ml, 12 mmol) was added slowly to a 50-ml solution of methyl-3-mercaptopropionate (1.30 ml, 12 mmol) and triethylamine (3.340 ml, 24 mmol) in diethyl ether at 0°C, and the solution was stirred for 2 h. The reaction was quenched with aqueous NH4Cl and extracted with 100 ml ethyl acetate twice. The organic layer was combined, dried, and evaporated to give a colorless liquid (2.6 g). The residue was purified with silica gel chromatography (ethyl acetate-hexane, 20/80) to yield pure α-dimethylbutyryl-S-methyl 3-mercaptopropionate (DMB-S-MMP) (2.35 g, 90% yield). 1H NMR: δ 3.87 (s, 3H), 3.09 (t, 2H, 7.1 Hz), 2.58 (t, 2H, 7.0 Hz), 1.59 (q, 2H, 7.5 Hz), 1.18 (s, 6H), 0.83 (t, 3H, 7.4 Hz); 13C NMR: δ 206.16, 172.26, 51.81, 50.04, 34.38, 33.73, 24.71, 23.60, 8.92.
Kinetic assay of LovD.
Expression and purification of LovD has been previously described in detail (18). The assays were performed at room temperature (RT) in 50 mM HEPES (pH 7.9). The initial velocities (ki) of the acyl substrates were determined with 10 μM LovD, 1 mM monacolin J, and 4 mM of the thioester. The reaction mixture at different time points was quenched by trifluoroacetic acid, extracted with ethyl acetate, evaporated to dryness, and redissolved in acetonitrile. The percent conversion of monacolin J to simvastatin was determined by high-performance liquid chromatography (HPLC) analysis (C18). The linear range of the turnover rate is reported as the ki in Table 1. To determine the Km of DMB-S-MMP, the concentration of monacolin J was fixed at 2 mM, while the concentration of DMB-S-MMP was varied from 0.2 mM to 10 mM. Dimethyl sulfoxide was added to a final concentration of 5% to facilitate the solubilization of DMB-S-MMP. To obtain Km values for monacolin J, the DMB-S-MMP concentration was fixed at 2 mM, while the concentration of monacolin J was varied from 0.2 mM to 5 mM.
TABLE 1.
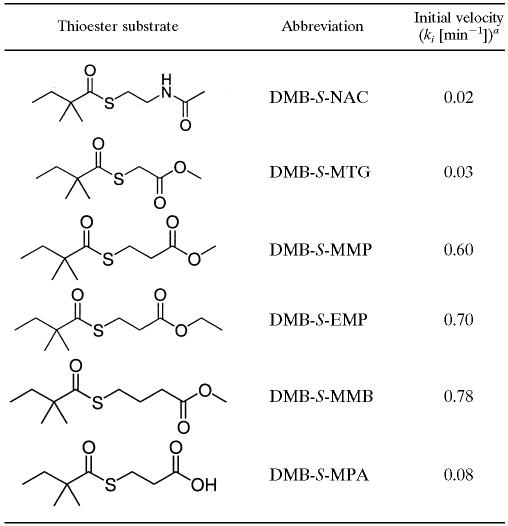
Comparison of initial velocities of thioester substrates
Reaction mixtures contained 1 mM monacolin J, 4 mM thioester substrate, 10 μM pure LovD, and 50 mM HEPES (pH 7.9). Initial velocity is defined as the rate of initial turnover in the linear range.
Whole-cell lysate activity assay.
A whole-cell lysate assay was used to determine the level of LovD activity under different fermentation conditions. For example, Escherichia coli strain BL21(DE3) transformed with pAW31 was grown in LB medium at 37°C to an optical density at 600 nm (OD600) of 0.5, at which time 100 μM IPTG (isopropyl-β-d-thiogalactopyranoside) was added to the culture, and expression was performed at RT for 16 h. A 7-ml aliquot of the culture was then harvested by centrifugation (4,000 × g for 20 min). The supernatant was removed, and the cell pellet was resuspended in 7 ml lysis buffer (20 mM Tris-HCl [pH 7.9], 500 mM NaCl). Cells were lysed by sonication on ice, and cell debris was removed by centrifugation (13,000 rpm for 10 min at 4°C). The lysate was then directly added to the in vitro assay mixture. The final assay mixture contained 50 mM HEPES (pH 7.9), 1 mM monacolin J, 4 mM DMB-S-MMP, and 5 μl cell lysate in a 25-μl final volume. The effective concentration of LovD in the fermentation broth was then calculated from the initial velocity using 0.6 min−1 as the turnover rate.
Low-density fermentation.
E. coli BL21(DE3)/pAW31 was grown in LB medium at 37°C to an OD600 of 0.5, at which time 100 μM IPTG was added to the culture, and expression was performed at RT for 16 h. A 10-ml culture was transferred to a 15-ml centrifuge tube. The cells were collected by centrifugation (4,000 × g for 10 min). The cell pellet was resuspended in 957 μl of the supernatant. The pH was adjusted to pH 7.9 with 1.0 N NaOH, followed by the addition of 33 μl 450 mM monacolin J (final concentration, 15 mM) and 6 μl pure DMB-S-MMP (final concentration, ∼25 mM). The culture was then shaken at 300 rpm at RT. At each time point, a 4-μl aliquot was removed from the reaction mixture and quenched in 300 μl ethyl acetate containing 1% trifluoroacetic acid. The organic phase was removed, evaporated, and redissolved in acetonitrile for HPLC analysis.
For larger-scale synthesis of simvastatin, the above-described procedure was scaled up starting from two 1-liter shake flask cultures of E. coli BL21(DE3)/pAW31 concentrated 10 times. After 16 h of expression at RT, the culture volume was concentrated to 200 ml to a final OD600 of 22. Monacolin J (sodium salt form) and DMB-S-MMP were added to final concentrations of 15 mM and 25 mM, respectively. The cells were shaken at RT, and HPLC was used to monitor the reaction progress. To purify the simvastatin from fermentation after the reaction was completed (>99% conversion as judged by HPLC), the following procedure was used. Cells were removed by centrifugation. The intracellular simvastatin was extracted by stirring the cell pellet in acetone. The acetone was then removed under reduced pressure, and the residue was dissolved in distilled water (dH2O) and filtered. The filtrate and the clear fermentation broth were combined, washed with equal volumes of n-hexane twice, and acidified to pH 2.0 with 6 N HCl. The white precipitate was recovered by filtration and was washed excessively with ice-cold dH2O to remove the coprecipitated α-dimethylbutyryl-S-mercaptopropionic acid (DMB-S-MPA). After drying of the filter cake under a vacuum, the solids were stirred in 200 ml of acetonitrile for 1 h, followed by filtration to remove insolubles. The filtrate was evaporated to dryness under reduced pressure to yield the acid form of simvastatin.
High-density F1 fed-batch fermentation.
Methods for F1 fed-batch fermentation and medium composition were adopted from methods described previously by Pfeifer et al. (14). We excluded the vitamin solution from both the fermentation medium and the feed medium. A starter culture was grown overnight in 5 ml of LB medium (with 35 mg/liter kanamycin) at 37°C and 250 rpm, and 1 ml was used to inoculate a 100-ml shake flask seed with F1 medium (with 35 mg/liter kanamycin). Ten milliliters of the seed F1 culture was used to inoculate a 2-liter Applikon Biobundle vessel containing 1 liter of F1 medium. Fermentation was conducted at 37°C, and the pH was maintained at 7.1 throughout the experiment with 1 M H2SO4 and half-concentrated NH4OH. Aeration was controlled at 0.2 to 0.4 liters/min, and agitation was maintained at 900 rpm. When the OD600 reading reached between 5 and 10, the temperature of the fermentation was reduced to RT, followed by the addition of 200 μM IPTG to induce protein expression. At the same time, a peristaltic pump delivered 0.08 ml/min of the feed solution to the fermentor.
Effective LovD activity and concentration at different stages of the fermentation were measured as described above. To prepare resting cells for bioconversion studies, the cells were centrifuged at 5,000 × g for 10 min, followed by gentle resuspension in the same volume of phosphate-buffered saline buffer (pH 7.4). Monacolin J and DMB-S-MMP were then added to the cell aliquots to initiate the synthesis of simvastatin.
RESULTS
Identification of a kinetically superior acyl donor.
We previously showed that LovD can utilize membrane-permeable thioesters as acyl donors in the transesterification reaction (18). Both DMB-S-NAC and α-dimethylbutyryl-S-methylthioglycolate (DMB-S-MTG) (Table 1) were shown to be substrates of LovD. The two substrates, however, both supported poor turnover in the synthesis of simvastatin, with apparent ki values of ∼0.02 per minute. In addition, when either substrate was utilized, the reaction suffered severe substrate inhibition at increasing concentrations of monacolin J. Therefore, the first priority in developing LovD into an industrially useful catalyst for the chembiosynthesis of simvastatin is to identify better substrates that can overcome these two limitations.
We synthesized several additional variants of DMB-S-MTG and assayed the catalytic properties in vitro. DMB-S-MMP, α-dimethylbutyryl-S-ethyl mercaptopropionate (DMB-S-EMP), and DMB-S-methyl mercaptobutyrate (DMB-S-MMB) were each synthesized by reacting α-dimethylbutyryl chloride with the corresponding free thiols. The initial turnover rates are compared in Table 1. Surprisingly, increasing the length of the thioester carrier by one carbon (from C2 in S-MTG to C3 in S-methyl mercaptopropionate and S-ethyl mercaptopropionate) significantly increased the turnover rate of the reaction. Inserting an additional carbon in S-methyl mercaptobutyrate (C4) resulted in an additional increase in the rate of transesterification. Under standard reaction conditions (1 mM monacolin J, 4 mM acyl substrate, 10 μM LovD, 50 mM HEPES [pH 7.9]), the initial turnover rates of esterification were 0.6, 0.7, and 0.78 min−1 for DMB-S-MMP, DMB-S-EMP, and DMB-S-MMB, respectively. The >30-fold increases in rates reflect that the relative positions of the carbonyl groups to the thioester linkage are critical for binding to LovD. In contrast, when DMB-S-MPA was used as the acyl donor, the initial turnover rate decreased significantly, to 0.08 per min, indicating that the free acid (likely a sodium salt) binds poorly to the LovD active site.
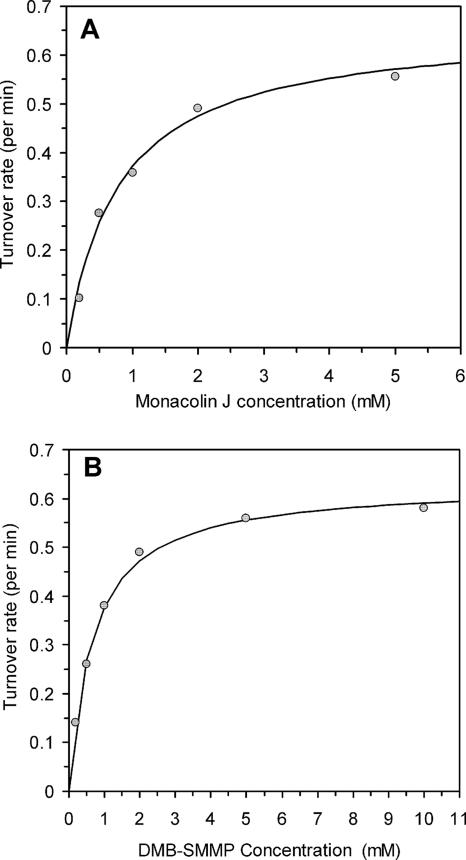
We chose DMB-S-MMP as the most suitable acyl thioester for biocatalysis because the precursor thiol (methyl mercaptopropionate) is significantly cheaper than the other starting materials. The kinetic parameters of the acylation reaction when DMB-S-MMP is used as the acyl donor were determined and are shown in Fig. 2. To obtain the Km values of either substrate in the transacylation reaction, we fixed the concentration of one substrate while varying the concentration of the other substrate to obtain a Michaelis-Menten kinetic curve. Figure 2A shows the reaction turnover rate (V/Eo) as a function of the monacolin J concentration. In contrast to previously assayed substrates, no substrate inhibition by monacolin J was observed, and the Km was determined to be 0.78 ± 0.12 mM. The turnover rate of LovD at various amounts of DMB-S-MMP was similarly determined by fixing the monacolin J concentration at 2 mM (Fig. 2B). The Km of DMB-S-MMP was shown to be 0.67 ± 0.04 mM. In both titrations, the kcat of the reaction was determined to be 0.66 ± 0.03 min−1. Our kinetic analysis clearly demonstrates that DMB-S-MMP is a kinetically superior substrate compared to previously reported thioesters. The level of substrate inhibition in a Ping Pong Bi Bi reaction depends upon the relative Km of the two substrates. When DMB-S-MMP is used as the acyl substrate, its relatively lower Km allows it to bind to LovD readily and is therefore not inhibited by increasing monacolin J concentrations. This important property therefore allows the development of a batch biocatalytic process in contrast to a fed-batch process in which the monacolin J has to be continuously supplied to keep its concentration low and minimize substrate inhibition.
FIG. 2.
Kinetic analysis of LovD-catalyzed acylation of monacolin J to yield simvastatin using DMB-S-MMP as the acyl thioester. The y axis is expressed as the catalytic turnover (V/Eo). (A) Michaelis-Menten kinetics of LovD as a function of monacolin J concentration at a fixed DMB-SMMP concentration of 2 mM. No substrate inhibition was observed. The Km (monacolin J) was 0.78 ± 0.12 mM. (B) Michaelis-Menten kinetics of LovD as a function of DMB-S-MMP concentration at a fixed monacolin J concentration of 2 mM. The Km (DMB-S-MMP) was 0.67 ± 0.04 mM. In both assays, the kcat was estimated to be 0.66 ± 0.03 min−1.
We previously observed that acyl-S-MTG was rapidly hydrolyzed when added to an E. coli fermentation broth (18). To test the stability of the newly identified acyl donor under in vivo conditions, neat DMB-S-MMP was added to LB medium inoculated with E. coli BL21(DE3) cells, and the culture was grown at 37°C overnight (16 h). We observed ∼20% degradation of the substrate to a predominantly more polar compound. Compared to known compounds, using HPLC, we identified the major degradation product to be DMB-S-MPA, which may arise through the action of endogenous E. coli lipases. We concluded that when DMB-S-MMP is supplied in excess in the batch reaction, the low extent of degradation will not limit the bioconversion of monacolin J to simvastatin.
Whole-cell biocatalysis.
Equipped with the significantly more efficient DMB-S-MMP thioester, we studied the conversion of monacolin J to simvastatin using E. coli as a whole-cell biocatalyst. A BL21(DE3) strain transformed with the pET28a-derived expression plasmid pAW31 was used as the microbial host. Expression of LovD was performed for 16 h at RT, and the level of expression was visualized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. To quantify the active amount of LovD expressed under different growth conditions and in different media, we employed an activity assay in which the whole-cell lysate was directly added to a reaction assay mixture containing 1 mM monacolin J and 4 mM DMB-S-MMP in 50 mM HEPES (pH 7.9). The conversion of monacolin J to simvastatin was quantified by HPLC, and the apparent concentration of LovD was estimated by using a ki value of 0.6 min−1 (Table 1). Under low-cell-density conditions, LovD is expressed at rates that are almost three times higher from LB medium (96 mg/liter) than from F1 minimal medium (34 mg/liter).
We then tested the chembiosynthesis of simvastatin in LB medium. After expression of LovD overnight, a 10-ml aliquot of the cells was concentrated 10-fold to 1 ml in LB. The concentration step was performed to achieve a high-cell-density environment mimicking fermentation conditions and to obtain a higher effective concentration of LovD (final concentration, approximately 20 μM). The sodium salt form of monacolin J was added to a final concentration of 15 mM (from a stock of 450 mM in water), and neat DMB-S-MMP was added to a final concentration of 25 mM. The reaction mixture was then shaken rigorously at RT. Samples were taken periodically to check the conversion of monacolin J to simvastatin (Fig. 3A).
FIG. 3.
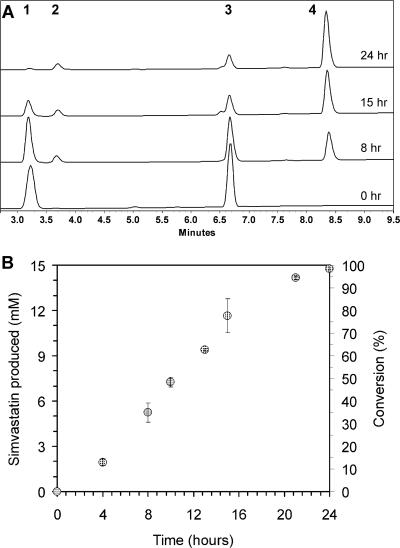
Low-density fermentation and biocatalysis. E. coli strain BL21(DE3)/pAW31 expressing LovD overnight was concentrated 10 times to a final OD600 of 22. The substrates monacolin J and DMB-S-MMP were added to final concentrations of 15 and 25 mM, respectively. The conversion was monitored as a function of time by HPLC analysis. (A) HPLC traces of the time course study. The labeled peaks are monacolin J (lactonized form) (1), DMB-S-MPA as a result of DMB-S-MMP hydrolysis (2), DMB-S-MMP (3), and simvastatin (lactonized form) (4). (B) Conversion of monacolin J to simvastatin as a function of time. The final conversion at 24 h was 99%. The data points are averaged values of two runs.
As shown in Fig. 3B, the simple, low-density culture of BL21(DE3)/pAW31 was a robust whole-cell biocatalyst for the synthesis of simvastatin. An initial lag phase of 2 h was observed at the onset of adding the two substrates, followed by rapid conversion with a linear reaction velocity of ∼0.73 mM per h. The reaction rate slowed at higher conversion rates (>95%) as the result of monacolin J depletion. Within 24 h, 99% of monacolin J was converted to simvastatin. Based on HPLC analysis, no degradation of either monacolin J or simvastatin by cellular metabolism was observed. We observed a ∼20% decrease in the OD600 readings of the culture after 24 h, suggesting that the whole-cell process may be maintained for prolonged catalysis.
We reported previously that LovD is able to hydrolyze lovastatin into monacolin J in vitro with a kcat of 0.21 min−1 (18). To examine whether we can couple the hydrolytic step together with the acylation step in a single fermentation run, we assayed the rate of lovastatin hydrolysis by BL21(DE3)/pAW31. Under the same in vivo conditions described above, we observed a slow rate of monacolin J formation. Starting with 0.5 mM lovastatin (sodium salt form), we achieved 91% hydrolysis in 48 h, with a conversion rate of ∼0.01 mM per h, nearly 75-fold slower than the rate of the acylation reaction. This is in sharp contrast to the in vitro kinetic results in which the hydrolysis rate is only three times slower than the rate of the acylation reaction. The significant attenuation of the lovastatin hydrolysis rate in vivo is likely due to the permeation barrier of the cell membranes towards the more hydrophobic lovastatin. We attempted to alleviate the membrane permeability barrier by using an alternative strain, BL21(DE3)/pXXK2, in which LovD is cloned with an N-terminal pelB signal sequence for localization into the periplasm space. No improvement in the hydrolytic activity was observed for this strain, indicating that the impermeability of the outer plasma membrane is the main transport obstacle.
Recovery and purification of simvastatin.
To access the recovery yield of simvastatin from the whole-cell biocatalytic process, we increased the scale of the bioconversion to a final volume of 200 ml. This was achieved by concentrating 2 liters of the expression strain 10 times in LB medium after overnight expression of LovD. The sodium salt forms of monacolin J and DMB-S-MMP were added to a final concentration of 15 mM and 25 mM, respectively, and the progress of the reaction was monitored by HPLC. We observed an identical conversion kinetic in the larger-scale process, and >99% conversion was obtained 24 h after the addition of substrates. Simvastatin obtained from the chemobiosynthetic route can be readily purified from the fermentation broth without using chromatography steps. A centrifugation step was used to separate the cells and the fermentation broth. Intracellular simvastatin was recovered by stirring the cell pellet in acetone, followed by evaporation and redissolving in dH2O. The aqueous solution containing intracellular simvastatin was combined with the fermentation broth and was washed with n-hexane to remove DMB-S-MMP. The aqueous solution was then acidified with 6 N HCl to pH 2.0, which resulted in the precipitation of the free-acid forms of simvastatin and DMB-S-MPA. The latter contaminant can be removed by filtration and washing the filter cake with excessive dH2O. The acidified filtrate contained <1% of the total amounts of simvastatin recovered. Nearly pure simvastatin can be recovered by washing the filter cake with acetonitrile, followed by evaporation of the filtrate (final recovery of 1.13 g [90%]; final purity [as determined by HPLC], 98%).
High-cell-density fermentation.
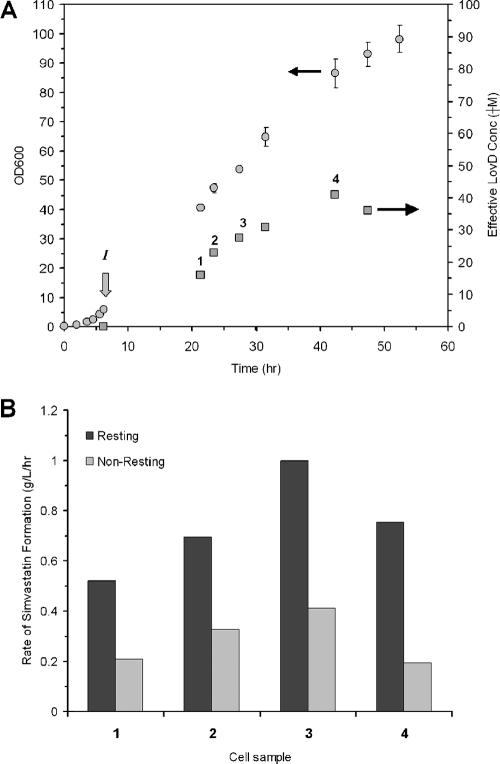
We explored the activity of the whole-cell biocatalyst by using a high-cell-density fermentor. The effective LovD concentration measured from a batch fermentor using terrific broth as the medium and that from a fed-batch run using F1 minimal medium were 980 and 1,500 mg/liter, respectively (the reported yields represent the highest observed yield under the respective conditions; the conversion observed was used to estimate the LovD concentration with a turnover rate of 0.6 min−1). Using the fed-batch process adopted from that reported previously by Pfeifer et al. (14), we were able to obtain cultures with very high cell densities and LovD activities (Fig. 4A).
FIG. 4.
High-density fermentation and biocatalysis. (A) Fed-batch fermentation (500 ml) with F1 minimal medium. At an OD600 (circles) of 5.93 (I), the temperature of the fermentation was decreased to and maintained at RT. IPTG was added to a final concentration of 200 μM, and the feeding was initiated and maintained at 0.08 ml/min. The effective concentrations of LovD (μM) at different points of the fermentation were measured (squares). (B) Rate of conversion of monacolin J to simvastatin by cells at four different points during the fermentation (indicated by 1, 2, 3, and 4) in A. The cells are either made “resting” by shifting to 50 mM HEPES (pH 7.9) or “nonresting” without medium change.
Due to the high cost of the starting materials, we were not able to perform a bioconversion using the entire fermentation broth. Instead, we sampled aliquots of the culture at various points during the fermentation run (Fig. 4A). The activities LovD at each time point were determined using the whole-cell lysate assay described in Materials and Methods. We then performed bioconversion using 1 ml of the culture directly and measured the rate of simvastatin formation (nonresting cells) (Fig. 4B). Alternatively, we also examined the properties of the whole-cell biocatalyst after resuspension of the cell pellets in phosphate-buffered saline (pH 7.4) (resting cells). As can be seen in Fig. 4B, at each time point, high conversion rates were obtained when the cells were placed in a resting environment. In addition, the total activity of LovD in the culture is not linearly correlated to the rate of conversion. For example, resting cells from time point 3 exhibited the highest rate of simvastatin conversion at nearly 1 g/liter/h, despite having less total LovD activity than cells obtained from time point 4. The exceptional robustness of these cells allowed them to be able to complete the conversion of 15 mM monacolin J to simvastatin in less than 8 h.
DISCUSSION
The key enabling finding in this study was the identification of significantly more efficient membrane-permeable acyl donors for the acylation reaction. We previously identified DMB-S-NAC as being a substrate of LovD (18). However, the acylation reaction proceeded with poor turnover, and as a result of weak substrate binding to LovD, the second substrate, monacolin J, became a competitive inhibitor of DMB-S-NAC. The latter property is especially detrimental since it forces the concentration of monacolin J to be kept at a minimum during the bioconversion. Both DMB-S-MMP and DMB-S-MMB are superior to DMB-S-NAC as acyl donors. The kcat value of DMB-S-MMP is approximately 30-fold higher through a subtle structural alteration of the acyl carrier, while the Km value is now comparable to that of monacolin J, hence eliminating substrate inhibition of monacolin J. Furthermore, the S-methyl 3-mercaptopropionate thiol precursor is significantly less expensive than S-N-acetylcysteamine. Considering that acyl-S-N-acetylcysteamine thioesters are used prevalently in the precursor-directed biosynthesis of natural products (7), the thioester acyl carriers described in this work may also find important utility in other engineered biosynthesis applications.
We attempted to develop an in vitro biocatalytic process for converting monacolin J to simvastatin. We reasoned that a crudely purified LovD may be useful to overcome any transport and purification difficulties associated with whole-cell fermentation. LovD can be readily fractioned from a majority of other cellular proteins by a single 30% ammonium sulfate precipitation step. More than 98% of the LovD activity measured from whole-cell lysates can be reconstituted by resolubilizing the precipitated pellet in 50 mM HEPES (pH 7.9). However, LovD precipitates readily (hours) at high protein concentrations (∼100 μM) and slowly (days) at lower concentrations (∼10 μM) under assay conditions at RT. Interestingly, the precipitated LovD protein can be resolubilized upon dilution into the same buffer and regains nearly all activity.
When moderate amounts of monacolin J (>5 mM) were used in a batch in vitro process, we were not able to achieve >60% equilibrium conversion of monacolin J into simvastatin, even after prolonged incubation. This is likely due to a competing reaction in which simvastatin is hydrolyzed back to monacolin J by LovD. When the simvastatin concentration reaches the millimolar range, the velocity of the hydrolysis reaction reaches the maximum value (kcat = 0.3 min−1) and therefore significantly impedes the overall net rate of acylation. Taking the stability of LovD and the reverse reaction into account, we concluded that the in vitro reaction will not be a useful process for the large-scale production of simvastatin.
Interestingly, the reverse reaction was not a limiting factor under in vivo conditions when the statin concentration was between 10 and 15 mM (4 to 6 g/liter), as evident in the high rate of conversion (>99%) of monacolin J into simvastatin achieved in this study. We reason that after monacolin J has been converted into simvastatin, multidrug exporters such as the AcrAB-TolC tripartite complex are able to extrude simvastatin from the inner membrane or the periplasm to the medium (13). The impermeability of the E. coli outer membrane prevents the reentry of the hydrophobic simvastatin. The more polar monacolin J is able to continuously diffuse through the membrane and serve as a substrate of LovD. Together, the E. coli outer membrane and its efflux pumps effectively decrease the intracellular concentration of simvastatin available for hydrolysis, hence attenuating the rate of the undesirable reverse reaction and maximizing the conversion of the desired reaction. However, the efflux pumps normally expressed by E. coli may be overloaded when the substrate concentrations are increased to >20 mM. Under elevated concentrations of monacolin J (and hence simvastatin) and DMB-S-MMP, we were able to achieve a maximum conversion of approximately 85 to 90%. Examination of the distribution of simvastatin in the culture revealed that a significant amount (>20%) is localized inside the cells, hence likely leading to increased levels of product hydrolysis. It is unknown whether simvastatin was partitioned in the inner membrane, the cytoplasm, or the periplasm. We are currently examining methods to increase the rate of simvastatin export through the overexpression of selected multidrug efflux pumps (11).
In this report, we have developed a highly robust whole-cell biocatalytic process for the synthesis of simvastatin from monacolin J. Using E. coli as the host, a laboratory-scale process capable of gram-scale synthesis has been implemented. Additionally, a straightforward downstream purification scheme has been devised for the facile recovery and purification of the product. We are currently using rational and directed-evolution approaches to improve the catalytic turnover rates of LovD. With an optimization of LovD properties, as well as metabolic engineering of the host to improve the throughput of the conversion, the chemobiosynthetic route of affording simvastatin can be a competitive and attractive alternative to the synthetic routes shown in Fig. 1.
Acknowledgments
This work was supported by the American Heart Association (grant 0535069N to Y.T.) and a UCLA engineering school faculty start-up grant.
We thank James C. Liao and Rachel Chen for helpful discussions. X.X. thanks Michael Connor and Todd Ambo for assistance with fermentation experiments.
Footnotes
Published ahead of print on 2 February 2007.
REFERENCES
- 1.Alberts, A. W., J. Chen, G. Kuron, V. Hunt, J. Huff, C. Hoffman, J. Rothrock, M. Lopez, H. Joshua, E. Harris, A. Patchett, R. Monaghan, S. Currie, E. Stapley, G. Albers-Schonberg, O. Hensens, J. Hirshfield, K. Hoogsteen, J. Liesch, and J. Springer. 1980. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc. Natl. Acad. Sci. USA 77:3957-3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Askin, D., T. R. Verhoeven, T. M.-H. Liu, and I. Shinkai. 1991. Synthesis of synvinolin: extremely high conversion alkylation of an ester enolate. J. Org. Chem. 56:4929-4932. [Google Scholar]
- 3.Endo, A. 1980. Monacolin K, a new hypocholesterolemic agent that specifically inhibits 3-hydroxy-3-methylglutaryl coenzyme A reductase. J Antibiot. (Tokyo) 33:334-336. [DOI] [PubMed] [Google Scholar]
- 4.Hendrickson, L., C. R. Davis, C. Roach, D. K. Nguyen, T. Aldrich, P. C. McAda, and C. D. Reeves. 1999. Lovastatin biosynthesis in Aspergillus terreus: characterization of blocked mutants, enzyme activities and a multifunctional polyketide synthase gene. Chem. Biol. 6:429-439. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman, W. F., A. W. Alberts, P. S. Anderson, J. S. Chen, R. L. Smith, and A. K. Willard. 1986. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors. 4. Side chain ester derivatives of mevinolin. J. Med. Chem. 29:849-852. [DOI] [PubMed] [Google Scholar]
- 6.Hong, C. I., J. W. Kim, H. J. Shin, T. W. Kang, and D. O. Cho. December 2004. Process for preparing simvastatin. U.S. patent 6,833,461B2.
- 7.Jacobsen, J. R., C. R. Hutchinson, D. E. Cane, and C. Khosla. 1997. Precursor-directed biosynthesis of erythromycin analogs by an engineered polyketide synthase. Science 277:367-369. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy, J., K. Auclair, S. G. Kendrew, C. Park, J. C. Vederas, and C. R. Hutchinson. 1999. Modulation of polyketide synthase activity by accessory proteins during lovastatin biosynthesis. Science 284:1368-1372. [DOI] [PubMed] [Google Scholar]
- 9.Reference deleted.
- 10.Manzoni, M., and M. Rollini. 2002. Biosynthesis and biotechnological production of statins by filamentous fungi and application of these cholesterol-lowering drugs. Appl. Microbiol. Biotechnol. 58:555-564. [DOI] [PubMed] [Google Scholar]
- 11.Masi, M., J. M. Pages, and E. Pradel. 2003. Overexpression and purification of the three components of the Enterobacter aerogenes AcrA-AcrB-TolC multidrug efflux pump. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 786:197-205. [DOI] [PubMed] [Google Scholar]
- 12.Reference deleted.
- 13.Murakami, S., R. Nakashima, E. Yamashita, T. Matsumoto, and A. Yamaguchi. 2006. Crystal structures of a multidrug transporter reveal a functionally rotating mechanism. Nature 443:173-179. [DOI] [PubMed] [Google Scholar]
- 14.Pfeifer, B., Z. Hu, P. Licari, and C. Khosla. 2002. Process and metabolic strategies for improved production of Escherichia coli-derived 6-deoxyerythronolide B. Appl. Environ. Microbiol. 68:3287-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reference deleted.
- 16.Schimmel, T. G., W. S. Borneman, and M. J. Conder. 1997. Purification and characterization of a lovastatin esterase from Clonostachys compactiuscula. Appl. Environ. Microbiol. 63:1307-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tobert, J. A. 2003. Lovastatin and beyond: the history of the HMG-CoA reductase inhibitors. Nat. Rev. Drug Discov. 2:517-526. [DOI] [PubMed] [Google Scholar]
- 18.Xie, X., K. Watanabe, W. A. Wojcicki, C. C. Wang, and Y. Tang. 2006. Biosynthesis of lovastatin analogs with a broadly specific acyltransferase. Chem. Biol. 13:1161-1169. [DOI] [PubMed] [Google Scholar]