Abstract
Closely related Escherichia coli B2 strains O1:K1, O2:K1, O18:K1, and O45:K1 constitute a major subgroup causing extraintestinal infections. A DNA pathoarray analysis was used to develop a PCR specific for this subgroup that was included in the multiplex phylogenetic-grouping PCR method. Our PCR may serve to identify this virulent subgroup among different ecological niches.
Escherichia coli is the main bacterial constituent of mammalian and avian gut aerobic microflora and a major cause of extra-intestinal infections. E. coli strains causing extraintestinal infections (extraintestinal pathogenic E. coli [ExPEC]) belong mainly to the phylogenetic group B2 (4, 5, 17). Among the B2 ExPEC group, strains harboring serotypes O2:K1, O18:K1, and O1:K1 have been shown to predominate (2, 21, 22) and to be closely related, according to several methods, including, recently, multilocus sequence typing (MLST) (1, 32), and they therefore represent a major human ExPEC phylogenetic subgroup. This subgroup includes, notably, the worldwide O18:K1:H7 strains causing cystitis and neonatal meningitis (18). We have previously shown that B2 strains belonging to these serotypes share the same ribotype that we designated B21 (7). The B21 ribotype also contains the neonatal meningitis O45:K1:H7 serotype strains that predominate in France and that are also present elsewhere in Europe (14, 29). Finally, analyzing several clinical collections of E. coli strains, we found that B21 was the ribotype most frequently encountered among urosepsis strains infecting non-host-compromised adults and young infants as well as among meningitis strains (5, 9).
Serogroup strains O2:K1, O18:K1, and O1:K1, similar to those of human ExPEC, also cause invasive diseases in animals, notably fatal avian septicemia (27, 33). Moreover, O45:K1:H7 strains resembling human neonatal meningitis strains have been sporadically described in cases of severe avian dermatitis (29). Thus, it has been suggested that poultry may be a vehicle for human ExPEC infection (27, 29).
All these observations imply that ribotype B21 strains might provide a key to understanding the pathogenetic mechanisms of invasive E. coli infections. However, ribotyping and MLST are costly and time consuming and are not suited to large-scale studies of the prevalence of this subgroup among human and animal ExPEC or to its detection within complex microflora.
In this study, we first applied MLST to published collections of B21 strains (O1:K1, O2:K1, O18:K1, and O45:K1) in order to determine the sequence type(s) (ST) to which ribotype B21 corresponds. We then used subtractive DNA pathoarray analysis to develop a PCR-based tool for the rapid identification of this highly virulent clonal group. Finally, we tested the capacity of this PCR method to detect these strains directly in human stools.
Highly virulent strains harboring ribotype B21 share a unique ST.
To establish the correspondence between ribotype B21 and MLST data, we selected 23 and 16 E. coli strains of ribotype B21 and non-B21, respectively, from clinical and reference collections (Table 1) (2, 6, 7, 9, 24, 31). MLST was performed as described by Whittam et al. at the EcMLST website, using seven housekeeping genes (http://www.shigatox.net) (26).
TABLE 1.
E. coli strains belonging to ribotype B21 or to other ribotypes used for MLST and DNA pathoarray analyses
| Ribotype and strain categorya | Sourceb | Country | Phylogenetic group | Serotype | Ribotype/sequence typec |
|---|---|---|---|---|---|
| Ribotype B21 | |||||
| S14 | NM | Finland | B2 | O18:K1 | B21/ST29 |
| C5 | NM | United States | B2 | O18:K1 | B21/ST29 |
| S26 | NM | United States | B2 | O18:K1 | B21/ST29 |
| S67 | NM | France | B2 | O18:K1 | B21/ST29 |
| S69 | NM | France | B2 | O18:K1 | B21/ST29 |
| RS218 | NM | United States | B2 | O18:K1 | B21/ST29 |
| S111 | NM | United States | B2 | O18:K1 | B21/ST29 |
| S126 | NM | France | B2 | O18:K1 | B21/ST29 |
| S32 | NM | France | B2 | O45:K1 | B21/ST29 |
| S50 | NM | France | B2 | O45:K1 | B21/ST29 |
| S53 | NM | France | B2 | O45:K1 | B21/ST29 |
| S72 | NM | France | B2 | O45:K1 | B21/ST29 |
| S88 | NM | France | B2 | O45:K1 | B21/ST29 |
| S132 | NM | France | B2 | O45:K1 | B21/ST29 |
| S174 | NM | France | B2 | O45:K1 | B21/ST29 |
| S136 | NM | France | B2 | O1:K1 | B21/ST29 |
| S158 | NM | France | B2 | O1:K1 | B21/ST29 |
| ECOR62 | ECOR, P | B2 | O2:K1 | B21/ST29 | |
| HN7 | P | France | B2 | O2:K1 | B21/ST29 |
| HN30 | P | France | B2 | O2:K1 | B21/ST29 |
| HN50 | P | France | B2 | O2:K1 | B21/ST29 |
| NNC28 | NC | France | B2 | O2:K1 | B21/ST29 |
| NNC59 | NC | France | B2 | O2:K1 | B21/ST29 |
| Ribotype non-B21 | |||||
| S114 | NM | United States | A | O12:K1 | A01/ST171 |
| S122 | NM | United States | A | O12:K1 | A01/ST171 |
| S82 | NM | France | A | ONT:K1 | A01/ST171 |
| K-12 (MG1655) | United States | A | O16:K− | A01/ST171 | |
| ECOR4 | ECOR | A | ONT | A01/ST167 | |
| ECOR15 | ECOR | A | O25 | A01/STND | |
| CFT073 | P | United States | B2 | O6:K2 | B26/ST27 |
| S107 | NM | United States | B2 | O16:K1 | B27/ST304 |
| S108 | NM | United States | B2 | O16:K1 | B28/ST304 |
| ECOR56 | ECOR | B2 | O6 | B26/STND | |
| ECOR59 | ECOR | B2 | O4 | B2J96/STND | |
| ECOR35 | ECOR | D | O1 | D10/STND | |
| ECOR41 | ECOR | D | O7 | D04/STND | |
| S16 | NM | United States | D | O7:K1 | D04/ST301 |
| S39 | NM | France | D | O7:K1 | D04/ST301 |
| S18 | NM | United States | D | O7:K1 | D08/ST301 |
All B21 strains had identical DNA sequences in the seven genes studied, corresponding to ST 29 of the EcMLST database, an ST previously attributed to only two strains, including the E. coli O18:K1:H7 neonatal meningitis (ECNM) strain RS218. This showed that the “O1, O2, O18:K1” clonal group (3, 30, 32) also encompasses a fourth serotype, 045:K1. This clonal group is designated B21/ST29 throughout this report.
DNA pathoarray-based identification of an svg open reading frame specific for the B21/ST29 clonal group.
Open reading frames (ORFs) specific for O18:K1, without homologs on the E. coli K-12 MG1655 chromosome, were amplified by PCR with primers based on the incomplete chromosomal sequence of the O18:K1 ECNM strain RS218 (www.genome.wisc.edu) (2, 6, 28), because the UTI89 (uropathogenic E. coli isolate O18:K1:H7) sequence (11) was not yet available. DNA fragments specific for strain RS218 were sought in silico by using sequences of clones (GenBank accession numbers AF222070 to AF222307) generated by subtractive hybridization between O18:K1:H7 ECNM and nonpathogenic E. coli strains (10). Specific ORFs were amplified in sections of about 500 bp. The amplicons of 300 known or putative ORFs generated by PCR from chromosomal DNA were spotted in duplicate by a robot (Eurogentec, Belgium) on nylon membranes which were then hybridized and analyzed as previously described (25).
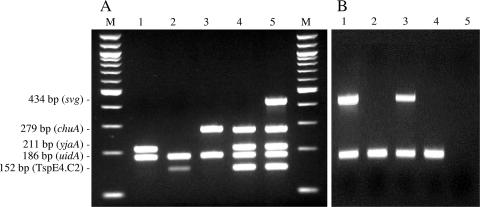
On our DNA array, only one ORF, of unknown function and coding for a hypothetical protein 277 amino acids long with no significant homology in the databases (now corresponding to GenBank accession number ABE08649 for E. coli strain UTI89) (11), hybridized with all the B21 strains and with none of the strains belonging to other groups and subgroups. We then assessed the specificity of this ORF, which we designated specific for virulent subgroup (svg) ORF, by using a PCR method. A pair of primers (svg.1, 5′-TCCGGCTGATTACAAACCAAC-3′; and svg.2, 5′-CTGCACGAGGTTGTAGTCCTG-3′) were designed to amplify a 434-bp fragment of the svg ORF. This PCR was then included, together with uidA (a β-glucuronidase gene) as a control amplification (16), in our triplex PCR method for phylogenetic group affiliation (12), with a modified protocol. Briefly, PCR was carried out in a 50-μl volume with 25 μl of 2× QIAGEN Multiple PCR Master Mix (QIAGEN, Courtaboeuf, France), 5 μl of 5× Q-solution, 1 μM of each primer, and 5 μl of bacterial lysate. PCR was performed as follows: DNA denaturation and polymerase activation for 15 min at 95°C; 30 cycles of 30 s at 94°C, 90 s at 55°C, and 90 s at 72°C; and a final extension step for 10 min at 72°C. Samples were electrophoresed as previously described (8) (Fig. 1A). This PCR screening test was then applied to 340 E. coli strains (including 97 strains of ribotype B21) belonging to previously published collections (5, 7, 9) (Table 2). In addition, 13 avian pathogenic E. coli (APEC) strains were assayed by PCR. Eight of them were B2 strains belonging to ribotype B21: one O1:K1 strain (LDA 6042253), three O2:K1 strains (LDA 5063391, LDA 5067912, and LDA 6081105), and four O45:K1 strains (BEN1068, BEN1082, BEN1090, and BEN1354). The five other APEC strains were group D strains belonging to serogroup O1 (LDA 6072791) and to serogroup O45 (BEN0058, BEN0214, BEN0289, and BEN0456). Strains “LDA” and strains “BEN” were kindly provided by Hervé Morvan (Laboratoire de développement et d'analyses des Côtes d'Armor, Ploufragan, France) and Maryvonne Moulin-Schouleur (INRA, Centre de Tours, UR1282 IASP, Pathogenie Bacterienne, Nouzilly, France), respectively. The results showed perfect specificity and sensitivity for the svg PCR for the identification of B21/ST29 strains (Table 2).
FIG. 1.
(A) Pentaplex PCR combining the amplification of a 434-bp fragment of the svg ORF specific for highly virulent B21/ST29 E. coli strains; uidA (β-glucuronidase gene) was used as a control (16), and chuA (outer membrane hemin receptor gene) and yjaA (unknown function) and DNA fragment TspE4.C2 were used for phylogenetic group affiliation (12). M, molecular weight marker (100-bp DNA ladder; New England BioLabs); lanes 1, 2 and 3, strains belonging to phylogenetic groups A, B1, and D, respectively; lane 4, reference strain belonging to the phylogenetic group B2 but not to B21/ST29 (strain CFT073); lane 5, meningitis strain belonging to B21/ST29 (strain S88). (B) Biplex PCR combining the amplification of a 434-bp fragment of ORF svg (upper band) together with uidA (β-glucuronidase gene, lower band), used as a control (16), for the detection of highly virulent B21/ST29 E. coli strains directly in stool samples. Lanes 1 and 3, stool samples harboring B21/ST29 E. coli strains (svg-positive PCR); lane 2 and 4, stool samples harboring E. coli strains not belonging to B21/ST29 subgroup; lane 5, negative control.
TABLE 2.
Results of svg ORF PCR specific for highly virulent B21/ST29 E. coli strains, applied to E. coli collections and other Enterobacteriaceae species
| E. coli strain and phylogenetic group (subgroup) (reference) | No. of strains | svg ORF-positive PCR |
|---|---|---|
| ECOR strains (n = 72) (13) | ||
| A | 25 | 0 |
| B1 | 16 | 0 |
| B2 (B21) | 2 | 2 |
| B2 (other than B21) | 13 | 0 |
| D | 12 | 0 |
| E | 4 | 0 |
| Meningitis strains (n = 132) (7) | ||
| A | 11 | 0 |
| B1 | 2 | 0 |
| B2 (B21) | 69 | 69 |
| B2 (other than B21) | 30 | 0 |
| D | 20 | 0 |
| Young infant urinary tract infection strains (n = 36) (9) | ||
| A | 1 | 0 |
| B1 | 1 | 0 |
| B2 (B21) | 7 | 7 |
| B2 (other than B21) | 25 | 0 |
| D | 2 | 0 |
| Adult uropathogenic strains (n = 100) (5) | ||
| A | 11 | 0 |
| B1 | 1 | 0 |
| B2 (B21) | 19 | 19 |
| B2 (other than B21) | 42 | 0 |
| D | 27 | 0 |
| APEC strains (n = 13) | ||
| B2 (B21) | 8 | 8 |
| D | 5 | 0 |
| Other Enterobacteriaceae species (n = 52) | ||
| Shigella (S. flexneri, n = 2; S. sonnei, n = 3) | 5 | 0 |
| Salmonella enterica | 6 | 0 |
| Enterobacter (E. cloacae, n = 5; E. aerogenes, n = 1) | 6 | 0 |
| Escherichia (E. hermanii, n = 1; E. vulneris, n = 1) | 2 | 0 |
| Hafnia alvei | 2 | 0 |
| Yersinia (Y. pseudotuberculosis, n = 3; Y. enterocolitica, n = 1) | 4 | 0 |
| Serratia (S. liquefaciens, n = 1; S. marcescens, n = 2) | 3 | 0 |
| Proteus mirabilis | 4 | 0 |
| Providencia stuartii | 1 | 0 |
| Morganella morganii | 1 | 0 |
| Citrobacter (C. freundii, n = 3; C. koseri, n = 3; C. youngae, n = 1) | 7 | 0 |
| Klebsiella (K. pneumoniae, n = 4; K. oxytoca, n = 3) | 7 | 0 |
| Raoultella terrigena | 1 | 0 |
| Leclercia adecarboxylata | 1 | 0 |
| Pantoea agglomerans | 2 | 0 |
svg amplification for B21/ST29 strain identification in a complex microflora.
We then tested the capacity of the svg ORF simplex PCR to detect B21/ST29 strains in a complex microflora. Specificity was first tested with 52 strains belonging to different Enterobacteriaceae species and with 30 stool samples that were culture negative for E. coli, as described below (Table 2). None of these strains or stool samples was positive, suggesting that the svg ORF is restricted to E. coli. We then applied the new method to 92 stool samples from healthy children who were culture positive for E. coli on two different types of samples: (i) 10 colonies of E. coli strains obtained by stool cultured on Uriselect 4 chromogenic medium (Bio-Rad, Marnes-la-Coquette, France) and (ii) whole-stool samples, as previously described (23). Briefly, about 100 μg of stool was mixed with 9 ml of peptone water and incubated at 37°C for 4 h. After centrifugation (1500 rpm for 10 min), the supernatant was boiled and 5 μl was used as template DNA for the PCR. Amplification of the gene uidA was used to check for PCR inhibitors (Fig. 1B). The svg PCR was positive for 10% of cases (9/92) of the predominant isolates and 14% (13/92) of the stool samples. Four specimens were thus PCR positive only for stool samples, suggesting that B21/ST29 strains may be a subdominant E. coli population in the microflora of some healthy children.
Serotype strains O1:K1, O2:K1, and O18:K1 encountered in human extraintestinal infections were recently shown to cluster within a single sequence type (ST95), based on the MLST method described by Achtman et al. (www.mlst.net) (19, 30, 32). However, several other sets of MLST target genes have been published, and no reference set of genes has yet been established (15, 26). In our study, using the set of genes cited by Whittam et al., we confirmed that strains O1:K1, O2:K1, and O18:K1 (all ribotype B21 in our study) clustered in a single ST (EcMLST, ST29) and that this clonal group includes meningitis strains of serotype O45:K1. Considering that (i) ST29 corresponds perfectly to ribotype B21, the leading ribotype causing septicemia in non-host-compromised humans (5, 7, 9) and (ii) since serotype strains O1:K1, O2:K1, O18:K1, and O45:K1 are major causes of fatal bacteremia in birds (29, 33), it would be useful to be able to detect this highly virulent ExPEC subgroup both rapidly and simply. By using DNA array technology, we developed a PCR test for inclusion in our multiplex phylogenetic grouping PCR (12), one of the most widely used methods for determining the main phylogenetic groups (A, B1, B2, and D). The resulting pentaplex PCR test will be helpful for determining in a unique reaction both the main phylogenetic group of a strain and its affiliation with the highly virulent subgroup B21/ST29.
We also showed that an svg PCR applied to stool samples is more sensitive than culture for B21/ST29 E. coli strains. These results are consistent with previous reports suggesting that E. coli strains harboring virulence factors may represent a minor subpopulation of the fecal microflora that might not be detected by colony sampling (20). To our knowledge, this is the first PCR-based approach to permit the detection of E. coli strains belonging to a particular phylogenetic subgroup directly within a complex bacterial population. In addition to its value as an epidemiological tool, our svg PCR method may prove useful in several fields of human and veterinary medicine. For example, it could serve for early identification (and treatment) of human neonates colonized by such E. coli strains and to screen avian hosts in industrial animal husbandry. Finally, this method could serve as a cost-effective first-line screening test in an E. coli serogrouping assay, as B21/ST29 strains belong mainly to only four serogroups.
Acknowledgments
We thank Colin Tinsley for helpful discussions.
Footnotes
Published ahead of print on 9 February 2007.
REFERENCES
- 1.Achtman, M., M. Heuzenroeder, B. Kusecek, H. Ochman, D. Caugant, R. K. Selander, V. Väisanen-Rhen, T. K. Korhonen, S. Stuart, F. Ørskov, et al. 1986. Clonal analysis of Escherichia coli O2:K1 isolated from diseased humans and animals. Infect. Immun. 51:268-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Achtman, M., A. Mercer, B. Kusecek, A. Pohl, M. Heuzenroeder, W. Aaronson, A. Sutton, and R. P. Silver. 1983. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect. Immun. 39:315-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Achtman, M., and G. Pluschke. 1986. Clonal analysis of descent and virulence among selected Escherichia coli. Annu. Rev. Microbiol. 40:185-210. [DOI] [PubMed] [Google Scholar]
- 4.Bingen, E., B. Picard, N. Brahimi, S. Mathy, P. Desjardins, J. Elion, and E. Denamur. 1998. Phylogenetic analysis of Escherichia coli strains causing neonatal meningitis suggests horizontal gene transfer from a predominant pool of highly virulent B2 group strains. J. Infect. Dis. 177:642-650. [DOI] [PubMed] [Google Scholar]
- 5.Bingen-Bidois, M., O. Clermont, S. Bonacorsi, M. Terki, N. Brahimi, C. Loukil, D. Barraud, and E. Bingen. 2002. Phylogenetic analysis and prevalence of urosepsis strains of Escherichia coli bearing pathogenicity island-like domains. Infect. Immun. 70:3216-3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 7.Bonacorsi, S., O. Clermont, V. Houdouin, C. Cordevant, N. Brahimi, A. Marecat, C. Tinsley, X. Nassif, M. Lange, and E. Bingen. 2003. Molecular analysis and experimental virulence of French and North American Escherichia coli neonatal meningitis isolates: identification of new virulent clone. J. Infect. Dis. 187:1895-1906. [DOI] [PubMed] [Google Scholar]
- 8.Bonacorsi, S., V. Houdouin, P. Mariani-Kurkdjian, F. Mahjoub-Messai, and E. Bingen. 2006. Comparative prevalence of virulence factors in Escherichia coli causing urinary tract infection in male infants with and without bacteremia. J. Clin. Microbiol. 44:1156-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonacorsi, S., S. Lefevre, O. Clermont, V. Houdouin, A. Bourrillon, C. Loirat, Y. Aujard, and E. Bingen. 2005. Escherichia coli strains causing urinary tract infection in uncircumcised infants resemble urosepsis-like adult strains. J. Urol. 173:195-197. [DOI] [PubMed] [Google Scholar]
- 10.Bonacorsi, S. P. P., O. Clermont, C. Tinsley, I. Le Gall, J.-C. Beaudoin, J. Elion, X. Nassif, and E. Bingen. 2000. Identification of regions of the Escherichia coli chromosome specific for neonatal meningitis-associated strains. Infect. Immun. 68:2096-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, S. L., C. S. Hung, J. Xu, C. S. Reigstad, V. Magrini, A. Sabo, D. Blasiar, T. Bieri, R. R. Meyer, P. Ozersky, J. R. Armstrong, R. S. Fulton, J. P. Latreille, J. Spieth, T. M. Hooton, E. R. Mardis, S. J. Hultgren, and J. I. Gordon. 2006. Identification of genes subject to positive selection in uropathogenic strains of Escherichia coli: a comparative genomics approach. Proc. Natl. Acad. Sci. USA 103:5977-5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clermont, O., S. Bonacorsi, and E. Bingen. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66:4555-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clermont, O., C. Cordevant, S. Bonacorsi, A. Marecat, M. Lange, and E. Bingen. 2001. Automated ribotyping provides rapid phylogenetic subgroup affiliation of clinical extraintestinal pathogenic Escherichia coli strains. J. Clin. Microbiol. 39:4549-4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Czirok, E., M. Herpay, and H. Milch. 1993. Computerized complex typing of Escherichia coli strains from different clinical materials. Acta Microbiol. Hung. 40:217-237. [PubMed] [Google Scholar]
- 15.Escobar-Paramo, P., O. Clermont, A. B. Blanc-Potard, H. Bui, C. Le Bouguenec, and E. Denamur. 2004. A specific genetic background is required for acquisition and expression of virulence factors in Escherichia coli. Mol. Biol. Evol. 21:1085-1094. [DOI] [PubMed] [Google Scholar]
- 16.Heininger, A., M. Binder, S. Schmidt, K. Unertl, K. Botzenhart, and G. Döring. 1999. PCR and blood culture for detection of Escherichia coli bacteremia in rats. J. Clin. Microbiol. 37:2479-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson, J. R., P. Delavari, M. Kuskowski, and A. L. Stell. 2001. Phylogenetic distribution of extraintestinal virulence-associated traits in Escherichia coli. J. Infect. Dis. 183:78-88. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, J. R., P. Delavari, and T. T. O'Bryan. 2001. Escherichia coli O18:K1:H7 isolates from patients with acute cystitis and neonatal meningitis exhibit common phylogenetic origins and virulence factor profiles. J. Infect. Dis. 183:425-434. [DOI] [PubMed] [Google Scholar]
- 19.Johnson, J. R., K. L. Owens, C. R. Clabots, S. J. Weissman, and S. B. Cannon. 2006. Phylogenetic relationships among clonal groups of extraintestinal pathogenic Escherichia coli as assessed by locus sequence analysis. Microbes Infect. 8:1702-1713. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, J. R., T. A. Russo, J. J. Brown, and A. Stapleton. 1998. papG alleles of Escherichia coli strains causing first-episode or recurrent acute cystitis in adult women. J. Infect. Dis. 177:97-101. [DOI] [PubMed] [Google Scholar]
- 21.Johnson, J. R., and A. L. Stell. 2000. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J. Infect. Dis. 181:261-272. [DOI] [PubMed] [Google Scholar]
- 22.Korhonen, T. K., M. V. Valtonen, J. Parkkinen, V. Väisänen-Rhen, J. Finne, F. Ørskov, I. Ørskov, S. B. Svenson, and P. H. Mäkelä. 1985. Serotypes, hemolysin production, and receptor recognition of Escherichia coli strains associated with neonatal sepsis and meningitis. Infect. Immun. 48:486-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mariani-Kurkdjian, P., H. Cave, J. Elion, C. Loirat, and E. Bingen. 1997. Direct detection of verotoxin genes in stool samples by polymerase chain reaction in hemolytic uremic syndrome patients in France. Clin. Microbiol. Infect. 3:117-119. [DOI] [PubMed] [Google Scholar]
- 24.Ochman, H., and R. K. Selander. 1984. Standard reference strains of Escherichia coli from natural populations. J. Bacteriol. 157:690-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perrin, A., S. Bonacorsi, E. Carbonnelle, D. Talibi, P. Dessen, X. Nassif, and C. Tinsley. 2002. Comparative genomics identifies the genetic islands that distinguish Neisseria meningitidis, the agent of cerebrospinal meningitis, from other Neisseria species. Infect. Immun. 70:7063-7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reid, S. D., C. J. Herbelin, A. C. Bumbaugh, R. K. Selander, and T. S. Whittam. 2000. Parallel evolution of virulence in pathogenic Escherichia coli. Nature 406:64-67. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Siek, K. E., C. W. Giddings, C. Doetkott, T. J. Johnson, M. K. Fakhr, and L. K. Nolan. 2005. Comparison of Escherichia coli isolates implicated in human urinary tract infection and avian colibacillosis. Microbiology 151:2097-2110. [DOI] [PubMed] [Google Scholar]
- 28.Silver, R. P., W. Aaronson, A. Sutton, and R. Schneerson. 1980. Comparative analysis of plasmids and some metabolic characteristics of Escherichia coli K1 from diseased and healthy individuals. Infect. Immun. 29:200-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tschäpe, H., H. Steinrück, P. Buchholz, R. Prager, E. Tietze, G. Seltmann, and J. Hacker. 1990. Molecular analysis of Escherichia coli from neonatal infections and its epidemiological implication, p. 224-234. In E. Gravel, L. Stern, I. Syllim-Rapoport, and R. Waver (ed.), Research in perinatal medicine II. Verlag Gesundheit, Berlin, Germany.
- 30.Weissman, S. J., S. Chattopadhyay, P. Aprikian, M. Obata-Yasuoka, Y. Yarova-Yarovaya, A. Stapleton, W. Ba-Thein, D. Dykhuizen, J. R. Johnson, and E. V. Sokurenko. 2006. Clonal analysis reveals high rate of structural mutations in fimbrial adhesins of extraintestinal pathogenic Escherichia coli. Mol. Microbiol. 59:975-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welch, R. A., V. Burland, G. Plunkett III, P. Redford, P. Roesch, D. Rasko, E. L. Buckles, S. R. Liou, A. Boutin, J. Hackett, D. Stroud, G. F. Mayhew, D. J. Rose, S. Zhou, D. C. Schwartz, N. T. Perna, H. L. Mobley, M. S. Donnenberg, and F. R. Blattner. 2002. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 99:17020-17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wirth, T., D. Falush, R. Lan, F. Colles, P. Mensa, L. H. Wieler, H. Karch, P. R. Reeves, M. C. Maiden, H. Ochman, and M. Achtman. 2006. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol. Microbiol. 60:1136-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wittig, W., R. Prager, E. Tietze, G. Seltmann, and H. Tschape. 1988. Aerobactin-positive Escherichia coli as causative agents of extra-intestinal infections among animals. Arch. Exp. Veterinarmed. 42:221-229. [PubMed] [Google Scholar]