Abstract
The vgb gene, encoding Vitreoscilla hemoglobin (VHb), was introduced into a specific desulfurization bacterium, Rhodococcus erythropolis LSSE8-1. The VHb-specific spectrum was observed for the recombinant. Compared to the wild type, the strain bearing vgb showed a higher biomass yield and desulfurizing activity.
Organic sulfur compounds in petroleum have become one of the main sources of air pollution. Petroleum contains heterocyclic sulfur compounds, such as dibenzothiophene (DBT) and its derivatives, which are difficult to remove by conventional hydrodesulfurization. Biodesulfurization (BDS) is being studied as an effective method of sulfur removal (5, 9). Immobilized-cell or free-cell biocatalyst was used to remove sulfur from oil in two-liquid phase systems (7, 14, 17). The desulfurization genes, dszABC, and their encoding enzymes, DszA, DszB, and DszC, have been elucidated clearly. Both DszC and DszA are monooxygenases in their oxidation pathways (6, 13). BDS strains utilize oxygen not only as a cosubstrate of monooxygenases but also in their endogenous metabolism. The Michaelis constant (Km) of the oxygenase for oxygen is relatively high, so it might be necessary to maintain significant oxygen pressure during bioconversion to allow the oxygenase to compete for oxygen with endogenous respiration (2, 18). However, the increase in air inlet results in some problems, such as volatilization of diesel oil or gasoline, resulting in an explosion hazard. It would be important to develop a biocatalyst with high desulfurization activity under hypoxic conditions.
Vitreoscilla hemoglobin (VHb) technology can be considered a promising strategy for improving the supply, transfer, and store of oxygen in vivo. The vgb gene, encoding VHb, has been expressed in heterologous hosts, including bacteria and plants, such as Escherichia coli and Nicotiana tabaccum (tobacco), to improve host growth, enhance the yield of antibiotics, or alter metabolite production (3, 8). Expression of VHb became an important inverse metabolic engineering approach for alleviating adverse effects of inadequate oxygen availability in bioprocess (1). However, there has been no report on the enhancement of desulfurization activity under low aeration in the two-liquid phase system.
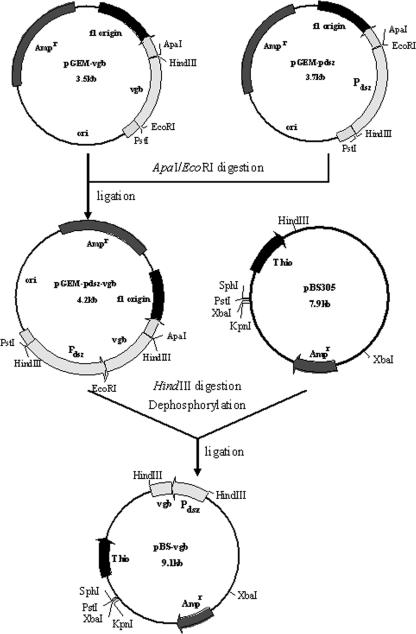
In this study, the vgb gene was introduced into a specific desulfurization bacterium, Rhodococcus erythropolis LSSE8-1 (4). The recombinant was designated LSSE8-1-vgb. The primers were designed based on the sequences of the dsz operon of LSSE8-1 (GenBank accession no. AY714058) and the vgb gene (GenBank accession no. L21670). The vgb gene was amplified with primers vgb1 and vgb2, using plasmid pSK-vgb as the template. Similarly, the promoter of the dsz operon was amplified with primers dsz1 and dsz2, using Rhodococcus sp. strain LSSE8-1 genomic DNA as the template. The sequences of these primers were as follows: vgb1, 5′-ATGAATTCCCAGCAAACCA-3′ (EcoRI restriction site underlined); vgb2, 5′-CCAAGCTTATTCAACCGC-3′ (HindIII restriction site underlined); dsz1, 5′-GACAAGCTTCAACGAACTCACCCAAACCAC-3′ (HindIII restriction site underlined); and dsz2, 5′-CCGGAATTCATCGCGTATGCGTCCTTTA-3′ (EcoRI restriction site underlined). The PCR products were ligated into pGEM-T. The orientations of the inserted fragments were identified by digestion of plasmids pGEM-vgb and pGEM-pdsz with EcoRI and PstI. Plasmid pGEM-vgb was cut by EcoRI and ApaI, and then the 0.5-kb vgb digestion product was inserted into pGEM-pdsz digested with the same restriction endonucleases to produce pGEM-pdsz-vgb. Finally, the vgb gene fragment driven by the native dsz promoter was ligated into pBS305 to form the resulting plasmid, pBS-vgb (Fig. 1). Electroporation of Rhodococcus sp. strain LSSE8-1 with pBS-vgb was done using the method of Shao et al. (16). A maximal absorbance of 419 nm was observed in crude extracts of the recombinants in the CO difference spectrum, while no such peak could be detected with the control strain LSSE8-1 (12). This typical peak demonstrated that active VHb was expressed in LSSE8-1-vgb.
FIG. 1.
Construction of vgb expression plasmid pBS-vgb based on the E. coli-R. erythropolis shuttle vector pBS305. Abbreviations of all plasmids as shown: Ampr, ampicillin resistance phenotype; ori, origin of replication; Thio, thiostrepton resistance phenotype; P, promoter.
The cultures were incubated in basal salt medium (BSM; for LSSE8-1-vgb, containing thiostrepton) at 30°C and 200 rpm until late log phase (7). Equal units of each culture were harvested by centrifugation, washed twice with fresh medium to remove the antibiotic and metabolites, and inoculated into 100 ml of BSM in a 500-ml flask. Incubation was carried out at 30°C and 200 rpm for 72 h. The cell concentration at each time point was obtained by measuring optical density at 600 nm (absorbance at a wavelength of 600 nm). One unit of optical density was equal to 0.38 mg of cells (dry weight) ml−1. The growth curve is shown in Fig. 2. The recombinant and LSSE8-1 showed similar growth patterns in the BSM. However, LSSE8-1-vgb showed a higher biomass yield at the end. The control displayed a lower cell density, about 10.4, and the maximal optical density at 600 nm was 12.6 at 66 h for the recombinant.
FIG. 2.
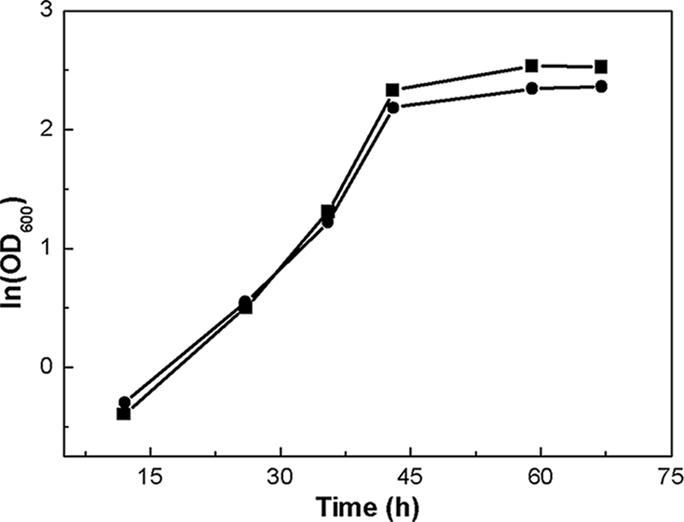
Growth of LSSE8-1 and recombinant cells in BSM at 30°C and 200 rpm containing 0.2 mM DBT. •, LSSE8-1; ▪, LSSE8-1-vgb.
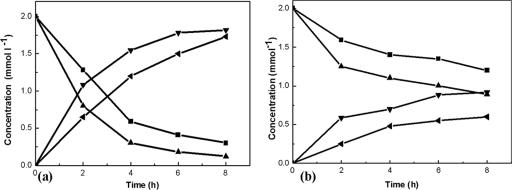
The effect of vgb expression on desulfurization activity was studied. BDS was carried out with resting cells. The harvested cells were resuspended in 0.85% NaCl solution, and the concentration was regulated to about 8.5 mg of cells (dry weight) ml−1. We prepared model oil consisting of 2.0 mM DBT in n-dodecane. Then, the cell suspensions were incubated with the model oil at 30°C on a rotary shaker. The shaker was set at 70 rpm or 200 rpm to explore the diversity of the desulfurization activities under different aerations. Desulfurization of diesel oil was also tested. High-performance liquid chromatography was used to determine the desulfurization product of DBT. The total sulfur content (by weight) of diesel oil was determined in triplicate using a microcoulomb analyzer (15). Figure 3 shows the consumption of DBT and the production of HBP by recombinant and wild-type cells at 200 rpm and 70 rpm, respectively. Compared with that for LSSE8-1, the specific rate of desulfurization for recombinant cells was increased remarkably. When the revolution of the shaker was decreased to 70 rpm, LSSE8-1-vgb cells indicated much higher activities than LSSE8-1 cells. The desulfurization ratios of LSSE8-1-vgb and LSSE8-1 were 37.5% and 20.5%, respectively, at 70 rpm. At a low aeration rate, the BDS activity of LSSE8-1-vgb indicated a lower influence on the desulfurization activities of the cells. This is presumably due to the greater advantage afforded by heterogeneous expression of vgb. The desulfurization of diesel oil was similar to the removal of DBT. As shown in Fig. 4, the sulfur content of diesel oil was reduced from 261.3 mg liter−1 to 70.1 mg liter−1 by the recombinant.
FIG. 3.
Time function of DBT desulfurization by resting cells of LSSE8-1 and LSSE8-1-vgb. The reaction was carried out in a 100-ml flask containing 5 ml model oil and 10 ml cell suspension with a cell content of 8.5 mg of cells (dry weight) ml−1. Desulfurization of model oil was carried out in rotary shakers at 200 rpm (a) or 70 rpm (b). ▪, DBT(LSSE8-1); ◂, HBP(LSSE8-1); ▴, DBT(LSSE8-1-vgb); ▾, HBP(LSSE8-1-vgb).
FIG. 4.
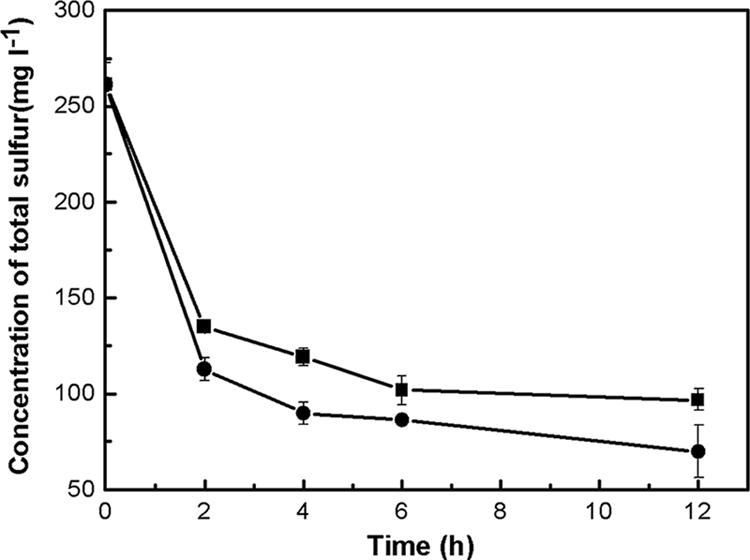
Time function of diesel oil desulfurization by resting cells. The cell content was 8.5 mg of cells (dry weight) ml−1. The desulfurization was carried out at 30°C and 200 rpm. The volume ratio of oil phase to aqueous phase was 1:2. Each data point indicated is the average for three independent experiments, and the error bars represent the standard deviations. ▪, LSSE8-1; •, LSSE8-1-vgb.
Although Rhodococcus spp. play important roles in biodegradation and bioremediation, there have been no reports of vgb expression in Rhodococcus (19). An expression vector, pBS-vgb, was constructed. A higher biomass yield of LSSE8-1-vgb suggested that the recombinant strain would be beneficial to cells of high-density culture. VHb improved the desulfurization activities of R. erythropolis LSSE8-1 in two-liquid systems not only under hypoxic conditions. Similar results were previously observed in the degradation of 2,4-dinitrotoluene (2,4-DNT), which was catalyzed by dioxygenase in Burkholderia spp. (10). Moreover, the Dsz enzymes are soluble and found in the cytoplasm (5, 9). This is different from what occurs with most of the oxygenases for the biodegradation of other hydrophobic molecules located in the cell membrane (11). The oxygen transfer issues are quite important. So vgb expression is a favorable approach for developing biocatalysts used in two-liquid systems, and it might be promotive for BDS in commercial applications.
Acknowledgments
We acknowledge Chen Jiayong for revising the manuscript. We thank Ning Jiang (Institute of Microbiology, Chinese Academy of Sciences, Beijing, China) for the gift of plasmid pSK-vgb and Warren A. Dick (Centre for Land and Biological Resources Research, Research Branch, Agriculture Canada, Ottawa, Canada) for the gift of plasmid pBS305.
This work was supported by the State Major Basic Research Development Program of China (grant no. 2006CB202507) and the National Natural Science Foundation of China (no. 30370046).
Footnotes
Published ahead of print on 9 February 2007.
REFERENCES
- 1.Bailey, J. E., A. Sburlati, V. Hatzimanikatis, K. Lee, W. A. Renner, and P. S. Tsai. 2002. Inverse metabolic engineering: a strategy for directed genetic engineering of useful phenotypes. Biotechnol. Bioeng. 79:568-579. [DOI] [PubMed] [Google Scholar]
- 2.Duetz, W. A., J. B. van Beilen, and B. Witholt. 2001. Using proteins in their natural environment: potential and limitations of microbial whole-cell hydroxylations in applied biocatalysis. Curr. Opin. Biotechnol. 12:419-425. [DOI] [PubMed] [Google Scholar]
- 3.Frey, A. D., J. Farres, C. J. Bollinger, and P. T. Kallio. 2002. Bacterial hemoglobins and flavohemoglobins for alleviation of nitrosative stress in Escherichia coli. Appl. Environ. Microbiol. 68:4835-4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gou, Z. X., H. Z. Liu, M. F. Luo, S. Li, J. M. Xing, and J. Y. Chen. 2002. Isolation and identification of nondestructive desulfurization bacterium. Sci. China Ser. B 45:521-531. [Google Scholar]
- 5.Gray, K. A., G. T. Mrachko, and C. H. Squires. 2003. Biodesulfurization of fossil fuels. Curr. Opin. Microbiol. 6:229-235. [DOI] [PubMed] [Google Scholar]
- 6.Gray, K. A., O. S. Pogrebinsky, G. T. Mrachko, L. Xi, D. J. Monticello, and C. H. Squires. 1996. Molecular mechanisms of biocatalytic desulfurization of fossil fuels. Nat. Biotechnol. 14:1705-1709. [DOI] [PubMed] [Google Scholar]
- 7.Guobin, S., Z. Huaiying, C. Weiquan, X. Jianmin, and L. Huizhou. 2005. Improvement of biodesulfurization rate by assembling nanosorbents on the surfaces of microbial cells. Biophys. J. 89:L58-L60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holmberg, N., G. Lilius, J. E. Bailey, and L. Bulow. 1997. Transgenic tobacco expressing Vitreoscilla hemoglobin exhibits enhanced growth and altered metabolite production. Nat. Biotechnol. 15:244-247. [DOI] [PubMed] [Google Scholar]
- 9.Kilbane, J. J., II. 2006. Microbial biocatalyst developments to upgrade fossil fuels. Curr. Opin. Biotechnol. 17:305-314. [DOI] [PubMed] [Google Scholar]
- 10.Lin, J. M., B. C. Stark, and D. A. Webster. 2003. Effects of Vitreoscilla hemoglobin on the 2,4-dinitrotoluene (2,4-DNT) dioxygenase activity of Burkholderia and on 2,4-DNT degradation in two-phase bioreactors. J. Ind. Microbiol. Biotechnol. 30:362-368. [DOI] [PubMed] [Google Scholar]
- 11.Maruyama, T., M. Ishikura, H. Taki, K. Shindo, H. Kasai, M. Haga, Y. Inomata, and N. Misawa. 2005. Isolation and characterization of o-xylene oxygenase genes from Rhodococcus opacus TKN14. Appl. Environ. Microbiol. 71:7705-7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minas, W., P. Brunker, P. T. Kallio, and J. E. Bailey. 1998. Improved erythromycin production in a genetically engineered industrial strain of Saccharopolyspora erythraea. Biotechnol. Prog. 14:561-566. [DOI] [PubMed] [Google Scholar]
- 13.Piddington, C. S., B. R. Kovacevich, and J. Rambosek. 1995. Sequence and molecular characterization of a DNA region encoding the dibenzothiophene desulfurization operon of Rhodococcus sp. strain IGTS8. Appl. Environ. Microbiol. 61:468-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shan, G. B., J. M. Xing, H. Y. Zhang, and H. Z. Liu. 2005. Biodesulfurization of dibenzothiophene by microbial cells coated with magnetite nanoparticles. Appl. Environ. Microbiol. 71:4497-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shan, G. B., H. Y. Zhang, J. M. Xing, C. Guo, W. L. Li, and H. Z. Liu. 2006. Biodesulfurization of hydrodesulfurized diesel oil with Pseudomonas delafieldii R-8 from high density culture. Biochem. Eng. J. 27:305-309. [Google Scholar]
- 16.Shao, Z., W. A. Dick, and R. M. Behki. 1995. An improved Escherichia coli-Rhodococcus shuttle vector and plasmid transformation in Rhodococcus spp. using electroporation. Lett. Appl. Microbiol. 21:261-266. [DOI] [PubMed] [Google Scholar]
- 17.Tao, F., B. Yu, P. Xu, and C. Q. Ma. 2006. Biodesulfurization in biphasic systems containing organic solvents. Appl. Environ. Microbiol. 72:4604-4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Beilen, J. B., W. A. Duetz, A. Schmid, and B. Witholt. 2003. Practical issues in the application of oxygenases. Trends Biotechnol. 21:170-177. [DOI] [PubMed] [Google Scholar]
- 19.van der Geize, R., and L. Dijkhuizen. 2004. Harnessing the catabolic diversity of rhodococci for environmental and biotechnological applications. Curr. Opin. Microbiol. 7:255-261. [DOI] [PubMed] [Google Scholar]