Abstract
The ever-increasing industrial demand for biocatalysis necessitates innovations in the preparation and stabilization of biocatalysts. In this study, we demonstrated that β-galactosidase (β-Gal) displayed on Bacillus spores by fusion to the spore coat proteins (CotG) may be used as a whole-cell immobilized biocatalyst for transgalactosylation in water-solvent biphasic reaction systems. The resulting spores had a specific hydrolytic activity of 5 × 103 U/g (dry weight) of spores. The β-Gal was tightly attached to the spore surface and was more stable in the presence of various organic solvents than its native form was. The thermostability of the spore-displayed enzyme was also increased, and the enzyme was further stabilized by chemically cross-linking it with glutaraldehyde. With spore-displayed β-Gal, octyl-β-d-galactopyranoside was synthesized at concentrations up to 27.7 mM (8.1 g/liter) with a conversion yield of 27.7% (wt/wt) after 24 h from 100 mM lactose and 100 mM octanol dissolved in phosphate buffer and ethyl ether, respectively. Interestingly, the spores were found to partition mainly at the interface between the water and solvent phases, and they were more available to catalysis between the two phases, as determined by light microscopy and confocal fluorescence microscopy. We propose that spore display not only offers a new and facile way to construct robust biocatalysts but also provides a novel basis for phase transfer biocatalytic processes.
Biocatalysis carried out in organic solvents, as well as in aqueous environments, is being used increasingly in biochemical industries (22). Using organic solvents as the reaction media increases the solubility of hydrophobic substrates and/or changes the kinetic equilibrium, enhancing the productivity of the process, but it requires stabilization of the enzymes, such as immobilization (12, 15). Enzyme immobilization can frequently be used to improve product separation, operational stability, and reusability (1). However, during the immobilization process, the enzyme activity and natural properties are lost, and the reusability of the immobilized enzyme is also limited. As these issues can be overcome by using whole cells, whole-cell biocatalysis has been widely adopted for the commercial synthesis of a variety of compounds ranging from bulk chemicals to valuable pharmaceuticals (7, 22) and for bioremediation (23). Compared with the isolated enzymes, whole-cell biocatalysts can be much more readily and inexpensively prepared on an industrial scale. Owing to their diversity and ease of handling, microbial cells have been most commonly utilized for whole-cell biocatalysis. One of the technical problems in whole-cell biocatalysis is mass transfer limitation, since the cell membrane and wall act as a permeability barrier, which means that a permeabilization step is required. Chemical permeabilization processes may also result in other problems, such as enzyme inactivation and the release of cellular components. A more innovative solution to this problem would be to immobilize the target enzyme on the surface of bacteria. Microbial surface display of enzymes has been suggested as a cost-efficient alternative to whole-cell biocatalysts, which obviates the need for enzyme production and immobilization (3, 10, 26).
During our efforts to develop solvent-stable whole-cell biocatalysts (9), we became aware of the natural robustness of bacterial spores in the presence of heat, solvent, pH, oxidizing agents, salts, etc. (19), and we developed a spore surface display system (13, 14). Here, we demonstrate that β-galactosidase (β-Gal) displayed on spores may be used as a whole-cell immobilized biocatalyst for transgalactosylation in water-solvent biphasic reaction systems. β-Gal is an enzyme that hydrolyzes lactose to glucose and galactose, and it also catalyzes a transglycosylation reaction in water-organic solvent biphasic reaction systems (18). However, because of the low stability of the enzyme in solvents and a strong limitation in mass transfer between water and solvent phases separating enzymes and substrates, it was difficult to obtain a significant yield of glycosylation products (18). We addressed these problems with spore-displayed β-Gal and more efficiently produced alkyl-β-galactosides, a group of nonionic surfactants which exhibit antimicrobial activity and are used as drug intermediates (16). Interestingly, the spores were partitioned mainly at the interface between the water and solvent phases, and they were more available for catalysis between the two phases.
MATERIALS AND METHODS
Construction of plasmids and strains.
In order to display β-Gal on the spore surface, a genetic fusion of cotG from Bacillus subtilis and lacZ from Escherichia coli was constructed. By inserting BamHI and SalI sites and removing the stop codon, the cotG DNA was amplified from B. subtilis using two PCR primers (5′-GCCTTTGGATCCAGTGTCCCTAGCTCCGAG-3′ and 5′-AAAAGACGTCGACTTTGTATTTCTTTTTGACTA-3′). The amplified fragments were cleaved with restriction enzymes and inserted into pDG1728 (6). The lacZ gene was also PCR amplified and inserted in frame at the end of cotG to generate pCotG-LacZ, which could be integrated into the amyE sites by double-crossover homologous recombination events. The plasmids were transformed into the multiple-protease-deficient B. subtilis strains DB104 (nprR2 nprE18 ΔaprA3) (11) and WB700 (nprE nprB aprE mpr bpr vpr) (R. Ye, L. P. Yang, and S. L. Wong, presented at the International Symposium on Recent Advances in Bioindustry, 1996).
Sporulation and spore purification.
The recombinant B. subtilis cells were cultivated in GYS medium [2 g/liter (NH4)2SO4, 2 g/liter yeast extract, 0.5 g/liter K2HPO4, 1 g/liter glucose, 0.41 g/liter MgSO4·H2O, 0.08 g/liter CaCl2·2H2O, 0.07 g/liter MnSO4·5H2O] at 37°C and 250 rpm for 24 h. Spores were prepared as described previously (20). Spores were collected from 100 ml of a culture in the stationary phase by centrifugation (5,000 rpm) and were resuspended in 1 ml of 20% Renografin (sodium diatrizoate; Sigma-Aldrich). The suspension was gently layered on 10 ml of 50% Renografin in a 15-ml centrifuge tube and was then centrifuged for 30 min at 12,000 rpm. The resulting pellets contained very pure spores.
Determination of β-Gal activity.
β-Gal activity was determined spectrophotometrically at 405 nm, as previously described (20). In brief, the reaction mixture contained 3 μl of MgCl2 (1 mM) and β-mercaptoethanol (45 mM) and 267 μl of O-nitrophenyl-β-d-galactopyranoside (ONPG) (3 mM) dissolved in 0.1 M sodium phosphate buffer (pH 7.5). The purified spore-displayed or free form of β-Gal (30 μl) was then added to the reaction mixture. After incubation at 37°C for 30 min, the reaction was stopped by addition of 500 μl of 1 M Na2CO3, and the absorption at 405 nm of the liberated O-nitrophenol was determined. One unit of β-Gal activity was defined as the amount of enzyme that hydrolyzed 1 μmol of ONPG in 1 min at 37°C.
Confocal fluorescence microscopy of microemulsions.
The interfacial localization of β-Gal-displaying spores in two-phase reaction systems was analyzed by confocal fluorescence microscopy. For immunofluorescence staining of spores, the spores were probed first with anti-β-Gal antibody and then with fluorescein isothiocyanate-labeled secondary antibodies. Fluorescence-labeled spores were dispersed in a two-phase reaction system, which was followed by a short ultrasonic treatment to obtain microemulsions. Microemulsions were observed with a confocal fluorescence microscope (LSM510 META; Zeiss).
Biphasic transgalactosylation reaction system.
For the two-phase reaction system, lactose (100 mM) was dissolved in an aqueous buffer solution (200 μl; 20 mM phosphate, pH 7.0), and octanol (100 mM) was dissolved in organic solvents (500 μl). The reactions were carried out in capped vials or microtubes to avoid evaporation of volatile organic solvents and were initiated by addition of 1 mg (5 U) of spore-displayed enzyme or 5 U of free-form enzyme; this was followed by vigorous mixing in order to create a microemulsion at room temperature (25°C). Samples were removed and analyzed by reverse-phase high-performance liquid chromatography with isocratic elution with acetonitrile-water (1/1, vol/vol). An octadecyl silica column (25 cm by 4.6 mm [inside diameter]) was used with a flow rate of 0.8 ml/min, and the samples were detected with a refractive, index detector. The transgalactosylation product, octyl-β-d-galactopyranoside, was prepared from the reaction mixture by evaporation of the organic solvents and was purified by silica gel thin-layer chromatography (ethyl acetate-methanol, 20:1 [vol/vol]). The chemical structures of the synthesized products were confirmed by 1H and 13C nuclear magnetic resonance (NMR) spectroscopy. The octyl-β-d-galactopyranoside 1H NMR (CD3OD, 300 MHz) and 13C NMR (CD3OD, 75.4 MHz) spectra were as follows: for 1H NMR, δ 0.9 (3H, t), 1.3 (10H, m), 1.6 (2H, m), 3.5 (1H, dd), 3.6-3.8 (5H, m), 3.9 (2H, m), 4.4 (1H, d, J 7.2 Hz); and for 13C NMR, δ 14.4 (C-8′), 23.7 (C-7′), 27.1 (C-6′), 30.4 (C-5′), 30.6 (C-4′), 30.8 (C-3′), 33.0 (C-2′), 70.8 (C-1′), 62.5 (C-6), 76.6 (C-5), 70.3 (C-4), 75.1 (C-3), 72.6 (C-2), 105.0 (C-1, β-bond). The molecular weight was determined by electrospray ionization mass spectrometry [m/z 315.3 (M+Na)+, 607.5 (2 M+Na)+].
RESULTS AND DISCUSSION
Spore display and stability of β-Gal in solvents.
For display of β-Gal on the surfaces of B. subtilis spores, we used the previously selected CotG protein (14), a 23.9-kDa outer coat protein, as an anchoring motif. The expression vector containing the cotG-specific promoter was used to express β-Gal as a CotG-β-Gal fusion protein. Because proteases may destabilize target enzymes, we used the multiple-protease-deficient strain B. subtilis DB104 (11) or WB700 (Ye et al., presented at the International Symposium on Recent Advances in Bioindustry, 1996). The recombinant B. subtilis cells were cultivated for efficient sporulation in GYS medium at 37°C and 250 rpm for 24 h. Sporulation was initiated about 9 h after inoculation, and a sporulation efficiency of >95% was typically obtained for both recombinant B. subtilis strains. The concentrations of spores in the cultures were in the range from 5 × 108 to 7 × 108 spores/ml. The spores were collected from the cultures in the stationary phase by centrifugation, and levels of purity up to 99.4% were obtained by centrifugation using Renografin (see Materials and Methods). The enzyme activity of β-Gal displayed on the spores was 5 × 103 U/g (dry weight) of spores based on the hydrolysis of ONPG (20). Notably, once the CotG-β-Gal fusion proteins were expressed and assembled on the spore surface, after incubation for several days they were not detached or released into the buffer solution. Several physicochemical treatments, such as vigorous mixing, ultrasonic vibration, and detergent treatment, did not detach the displayed enzymes. The enzymes were extracted only by treatment with a hot alkaline sodium dodecyl sulfate solution (data not shown).
Because the stability of spore-displayed β-Gal enzymes in the presence of organic solvents is critical, we first evaluated the stability of enzymes with the commonly used organic solvents (Table 1). Equal volumes of various organic solvents, such as n-hexane, ethyl ether, toluene, ethyl acetate, acetonitrile, and ethanol, were mixed with phosphate-buffered saline (pH 7.5) solutions containing purified spores displaying β- Gal at a level of 20 U/ml. After 1 h of incubation at 37°C, the remaining β-Gal activities were determined. The results showed that the spore-displayed β-Gal was stable in the presence of nonpolar hydrophobic organic solvents. β-Gal displayed on spores of multiple-protease-deficient strain B. subtilis WB700 was more stable than β-Gal displayed on DB104 spores. Even though β-Gal was displayed, it was unstable in the presence of water-miscible polar solvents, such as acetonitrile and ethanol. Throughout this work, ethyl ether was used.
TABLE 1.
Solvent stabilities of spore-displayed β-Gal on different B. subtilis spores and free-form enzymes in various organic solvents after 1 h of incubation at 37°C
| Solvent | Solvent hydrophobicity (log P value) | Relative activities of β-Gal
|
||
|---|---|---|---|---|
| Displayed on DB104 spores | Displayed on WB700 spores | Free form | ||
| Control | 100 | 100 | 100 | |
| n-Hexane | 3.5 | 100 | 100 | 84.3 |
| Ethyl ether | 0.8 | 77.2 | 97.0 | 48.2 |
| Toluene | 2.5 | 51.6 | 78.8 | 4.2 |
| Ethyl acetate | 0.68 | 9.6 | 10.6 | 0.1 |
| Acetonitrile | −0.33 | 0.8 | 8.3 | 0.0 |
| Ethanol | −0.24 | 0.0 | 0.0 | 0.0 |
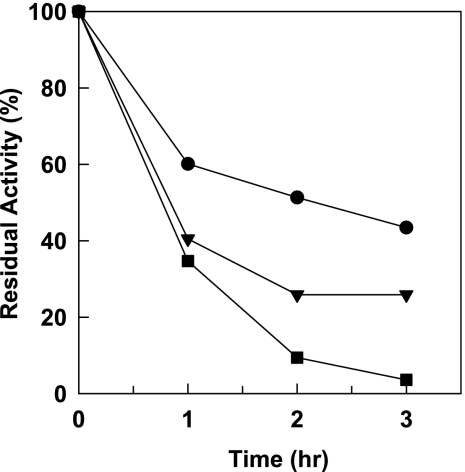
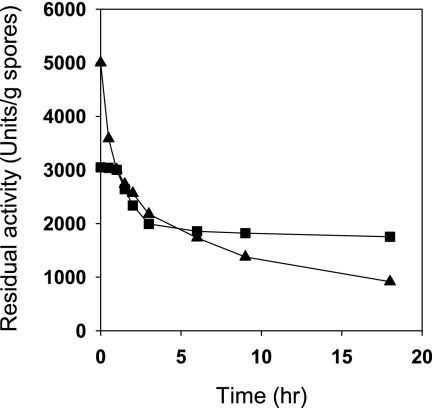
Not only was the stability in the presence of organic solvents improved by displaying enzymes on spores (Table 1), but the thermal stability was also improved. Incubation for 2 h at 40°C completely inactivated free-form β-Gal, whereas more than 45% of the initial activity of the spore-displayed enzyme remained (Fig. 1) when it was expressed in B. subtilis WB700 strain. The thermostability was also affected by the host protease background, as shown in Fig. 1. We tried to further increase the stability of displayed enzymes by chemical cross-linking with glutaraldehyde. After spores of B. subtilis WB700 were cross-linked with 2% (wt/vol) glutaraldehyde at room temperature for 24 h, an activity of 3,050 U/g of spores was obtained for a preparation that initially had an activity of 5,000 U/g of spores (enzyme activity yield, 61%). Then the residual enzyme activities of spore-displayed enzymes were determined during incubation at 37°C in a phosphate-buffered saline solution, as shown in Fig. 2. The residual enzyme activity of cross-linked spore-displayed β-Gal on spores was stably maintained for the first 2 h and slowly decreased to 1,754 U/g (57.5% of the initial activity) over 18 h, while the activity of spore-displayed β-Gal decreased rapidly to 916 U/g (18.3% of the initial activity). These analyses revealed that chemical modification combined with genetic immobilization on spores could be an excellent method for enzyme stabilization.
FIG. 1.
Thermostability of free-form β-Gal (▪) and spore-displayed β-Gal on spores of B. subtilis DB104 (▾) and WB700 (•). The residual hydrolytic activities of the enzymes were determined after incubation for 1 h at 42°C.
FIG. 2.
Stability of displayed enzymes on spores of B. subtilis WB700 due to chemical cross-linking with glutaraldehyde (0.5%). ▴, spore-displayed β-Gal; ▪, cross-linked spore-displayed β-Gal. The residual hydrolytic activities of the enzymes were determined.
Transgalactosylation with spore-displayed β-Gal.
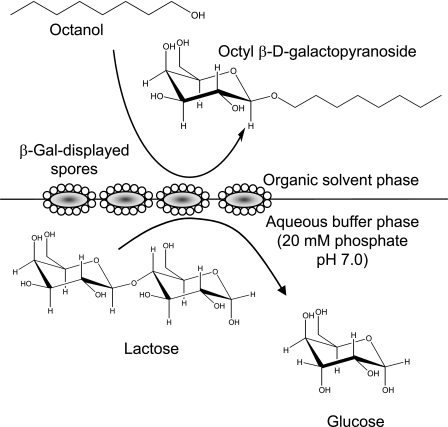
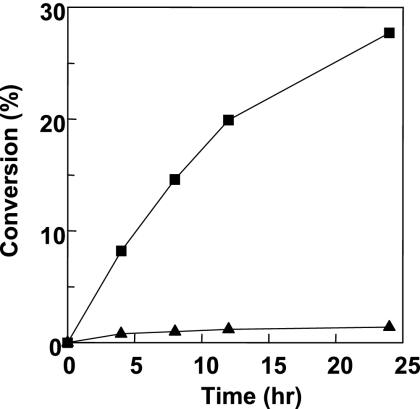
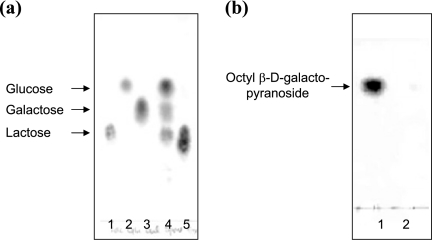
Figure 3 shows a schematic diagram of the biphasic transgalactosylation system, in which lactose and spore-displayed enzymes are dissolved in the phosphate-saline buffer phase (pH 7.0), while octanol is dissolved in the ethyl ether solvent phase. Vigorous mixing or a short ultrasonication resulted in a microemulsion and completely dispersed the spores, probably because of the spores' hydrophobicity. The microemulsion, which was stably maintained for an overnight reaction, should have increased the interfacial area, thus enhancing the transgalactosylation reaction at the interface. For the initial trial, the water content (water activity) was not optimized for transgalactosylation. The general water content (10 to 30%, vol/vol) for transgalactosylation with β-Gal in the water-solvent biphasic reaction system was used in our experiments (24). It is obvious that this water content is desirable to increase the equilibrium of the hydrolase-based transglycosylation synthetic reaction. However, the minimum amount of water is usually added since the hydrolase enzymes are not stable at low water contents and the sugar substrate should be dissolved. Figure 4 shows a typical conversion profile obtained with 5 U/100 μl of each of the enzymes. With spore-displayed β-Gal, up to 27.7 mM (8.1 g/liter) octyl-β-d-galactopyranoside was synthesized with a 27.7% (wt/wt) conversion yield from 100 mM lactose and 100 mM octanol in 24 h, but with the free β-Gal lactose neither was hydrolyzed nor produced octyl-galactopyranoside. We analyzed the reaction mixtures by a thin-layer chromatography, as shown in Fig. 5. In the aqueous phase of the spore-displayed β-Gal reaction, glucose, galactose, and lactose were observed, while only lactose was present in the aqueous phase of the free enzyme reaction. The end product was also confirmed by 1H and 13C NMR spectroscopy, which revealed the β-configuration anomeric structure of octyl-galactopyranoside (data not shown).
FIG. 3.
Schematic diagram of the biphasic transgalactosylation reaction system. The water-to-solvent phase volume ratio was 2:5, and the reaction was carried out in capped vials at room temperature (25°C).
FIG. 4.
Time course of transgalactosylation with free-form β-Gal (▴) and spore-displayed β-Gal (▪).
FIG. 5.
Thin-layer chromatography analysis of reaction mixtures after 24 h of incubation. (a) Lanes 1 to 3, standard sugars, including lactose (lane 1), glucose (lane 2), and galactose (lane 3); lanes 4 and 5, reaction mixtures from aqueous phases with spore-displayed β-Gal (lane 4) and free β-Gal (lane 5). (b) Lane 1, reaction mixture from organic phase with spore-displayed β-Gal; lane 2, reaction mixture from organic phase with free β-Gal.
Localization of spores at water-solvent interfaces.
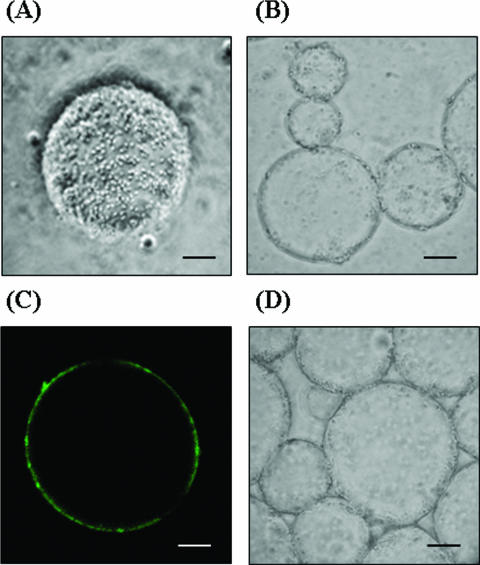
Enhanced reactions were expected not only due to the enzyme stabilization with spore display but also due to the localization of spore enzymes at the water-solvent interface. Interface-binding enzymes are highly desirable for two-phase reactions because they can provide maximum accessibility to substrates dissolved in both phases across the interface (25, 28). We observed spores of the B. subtilis WB700 control strain without expression of β-Gal and spores of the strain displaying β-Gal in microemulsions with a light microscope to determine how they were dispersed in the reaction mixture. Quite surprisingly, spores were found to almost adhere on the surfaces of solvent droplets (Fig. 6A) and were found to be localized at the interfaces in water-solvent systems (Fig. 6B and D). To observe the interface partitioning more closely with a confocal fluorescence microscope, the spores were fluorescently stained by using primary anti-β-Gal antibodies and secondary fluorescein isothiocyanate-labeled anti-mouse immunoglobulin G antibodies. As shown in Fig. 6C, the spores were clearly localized at the interface. However, no fluorescence signals of control spores were observed with the fluorescence microscope after they were stained with primary and secondary antibodies as described above (data not shown). The hydrophobicity of the spore surface should have facilitated localization at the interface between the two phases or adherence to the surfaces of solvent droplets. Once present at the interphase either through localization or through surface adherence, spore-displayed enzymes should be more available for catalysis than the biocatalysts dissolved in either the water or solvent phase. The findings are remarkable because the transgalactosylation by β-Gal is essentially an interphase catalytic reaction which transfers the galactosyl moiety from lactose present in the water phase to the octanol acceptor dissolved in the solvent phase. Thus, the biocatalysis reaction enhanced by spore display resulted not only from the stabilization of β-Gal by spore display but also from the increased availability of biocatalysts localized at the interface. Despite the fact that the orientation of spore-displayed β-Gal at the interface of the emulsion could not be determined precisely, spores might be freely mobile at the interface of the emulsion, which might enhance their chance to face both phases. In this connection, it is notable that in vitro interfacial self-assembly of β-Gal, when β-Gal was conjugated with polystyrene polymer, improved the catalytic efficiency more than 145-fold for a transgalactosylation between water and toluene phases (28). Such polymer-conjugated β-Gal can efficiently self-localize at the interfaces mainly due to the hydrophobicity of the polymer, resulting a very high rate of production of hexyl galatoside from hexanol and lactose. Thus, spore-displayed β-Gal and polymer-conjugated β-Gal have technological advantages in common, including self-binding to the interface in a solvent-water reaction system, high specific activity, high transgalactosylation activity, increased stability of the enzymes, etc. Spore-displayed β-Gal is comparable to polymer-conjugated β-Gal in terms of industrial-scale applications of enzymes, as described below. Chemical processes under harsh conditions are inevitable for immobilization of β-Gal on synthetic resins or by polymer conjugation. Spore-displayed β-Gal, however, can be made available by simple fermentation at a mild temperature and atmospheric pressure, resulting in a naturally folded enzyme. One possible drawback is that display of β-Gal on spores requires a specific protein expression system and a low-protease background for the Bacillus host cells in order to avoid enzyme degradation during sporulation of the culture, which could be solved by using protease-negative strains.
FIG. 6.
Determining the localization of spores at the interfacial surfaces of microemulsions by observing them with various microscopes. Scale bars = 10 μm. (A) Light microscopy of spore of B. subtilis WB700 displaying β-Gal adhering on the surface of microemulsions. (B) Light microscopy of spores of B. subtilis WB700 displaying β-Gal localized at the interface of the water and solvent phases. (C) Confocal fluorescence microscopy of microemulsion of fluorescently labeled spore of B. subtilis WB700 displaying β-Gal using primary rabbit anti-β-Gal antibodies and secondary fluorescein isothiocyanate-labeled anti-rabbit immunoglobulin G antibodies. (D) Light microscopy of control spores of B. subtilis WB700 localized at the interface of the water and solvent phases.
Factors affecting process development.
The economic feasibility of biocatalytic processes depends on biocatalyst availability, volumetric productivity, and stability (22). Spore-displayed biocatalysts can be prepared much more readily and inexpensively, like a whole-cell biocatalyst, because methods for industrial-scale production of bacterial spores by conventional high-cell-density fermentation have already been established. High volumetric productivity (space-time yield), which influences the capital costs of the process, requires a high specific activity of the biocatalyst (U/g biocatalyst) (24). Quite encouragingly, we obtained a specific hydrolytic activity of 5 × 103 U/g (dry weight) of spores, which is higher than the activity of β-Gal immobilized on phenol-formaldehyde resins, on which the specific hydrolytic activities are 6 to ∼160 U/g (27). The high specific activities of spore-displayed enzymes are best explained by the high ratio of surface area to volume of spores, which reflects their extremely small size (diameter, ca. 1 μm). It was observed that the smaller the particle size of the support, the greater the activity of the immobilized enzyme, which is typical of heterogeneous catalysts due to a decrease in diffusion resistance and an increase in specific volume as the particle size decreases (1, 2). The enzyme loading capacity for spore display may be further improved by constructing fusions to multiple coat proteins or by modification, overcoming the limited productivity due to the low enzyme loading in a conventional immobilization technique (1, 5).
Another notable aspect of the spore display immobilization system is its reusability. After the reaction, spores can be separated using standard microfiltration processes. At each cycle of the transgalactosylation reaction, more than 87% of the initial activity was recovered. Furthermore, in the presence of nutrients, including the so-called germinants such as sugars and amino acids, spores can germinate into vegetative cells and sporulate again upon nutrient deprivation. Thus, when denatured or deactivated, spores displaying enzymes can be simply regenerated by regrowth of the culture, followed by sporulation, as shown in our previous work (21).
In this study, we carried out the experiments on a small scale (reaction volume, 1 ml). However, with the high specific activity of spore-displayed enzymes, as described above, we expect high productivity for production of alkyl galactoside with spore-displayed β-Gal. We are currently trying to optimize the production at the kilogram scale.
Conclusions.
Based on the results obtained here, we propose that spore display not only offers a new and facile way for constructing robust biocatalysts but also provides a basis for the novel phase transfer biocatalytic processes. Since our previous results showed that diverse enzymes, such as cellulase, subtilisin, lipase, etc., can be displayed (14), the use of spore-displayed enzymes is not specific for the β-Gal reaction in phase transfer catalysis but is more generally applicable to other biocatalysis reactions. Furthermore, by expanding spore display motifs, such as CotB (8), CotC (17), and CotG (13) of B. subtilis and InhA (21) and Cry1A (4) of Bacillus thuringiensis, and broadening displayable target proteins for antigen (17), streptavidin (13), green fluorescent protein (4), antibody (4), and a fragment of tetanus toxin (8), spore display technology can be used in a wide array of biotechnological fields in addition to biocatalysis, including biosensors, alternative vaccines, nanobiomaterials, and high-throughput library screening.
Acknowledgments
This research was financially supported by the National Research Laboratory program of the Ministry of Commerce, Industry, and Energy and the Ministry of Science and Technology, Republic of Korea, and by the Cooperative Research Program of the Korea Research Council of Fundamental Science and Technology.
We thank S. H. Park and S. K. Choi for providing Bacillus strains and plasmids and for discussions. We also thank B. K. Kim and J. H. Kim for their collaboration in the early phase of development of spore display technology.
Footnotes
Published ahead of print on 22 December 2006.
REFERENCES
- 1.Cao, L. 2005. Immobilised enzymes: science or art? Curr. Opin. Chem. Biol. 9:217-226. [DOI] [PubMed] [Google Scholar]
- 2.Cao, L., U. T. Bornscheuer, and R. D. Schmid. 1999. Lipase-catalyzed solid-phase synthesis of sugar esters. Influence of immobilization on productivity and stability of the enzyme. J. Mol. Catal. B Enzym. 6:279-285. [Google Scholar]
- 3.Chen, W., and G. Georgiou. 2002. Cell-surface display of heterologous proteins: from high-throughput screening to environmental applications. Biotechnol. Bioeng. 79:496-503. [DOI] [PubMed] [Google Scholar]
- 4.Du, C., W. C. Chan, T. W. McKeithan, and K. W. Nickerson. 2005. Surface display of recombinant proteins on Bacillus thuringiensis spores. Appl. Environ. Microbiol. 71:3337-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gill, I., and A. Ballesteros. 1998. Encapsulation of biologicals within silicate, siloxane, and hybrid Sol-Gel polymers: an efficient and generic approach. J. Am. Chem. Soc. 120:8587-8598. [Google Scholar]
- 6.Guerout-Fleury, A. M., N. Frandsen, and P. Stragier. 1996. Plasmids for ectopic integration in Bacillus subtilis. Gene 180:57-61. [DOI] [PubMed] [Google Scholar]
- 7.Ishige, T., K. Honda, and S. Shimizu. 2005. Whole organism biocatalysis. Curr. Opin. Chem. Biol. 9:174-180. [DOI] [PubMed] [Google Scholar]
- 8.Isticato, R., G. Cangiano, H. T. Tran, A. Ciabattini, D. Medaglini, M. R. Oggioni, M. De Felice, G. Pozzi, and E. Ricca. 2001. Surface display of recombinant proteins on Bacillus subtilis spores. J. Bacteriol. 183:6294-6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung, H. C., S. J. Kwon, and J. G. Pan. 2006. Display of a thermostable lipase on the surface of a solvent-resistant bacterium, Pseudomonas putida GM730, and its applications in whole-cell biocatalysis. BMC Biotechnol. 6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung, H. C., J. M. Lebeault, and J. G. Pan. 1998. Surface display of Zymomonas mobilis levansucrase by using the ice-nucleation protein of Pseudomonas syringae. Nat. Biotechnol. 16:576-580. [DOI] [PubMed] [Google Scholar]
- 11.Kawamura, F., and R. H. Doi. 1984. Construction of a Bacillus subtilis double mutant deficient in extracellular alkaline and neutral proteases. J. Bacteriol. 160:442-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khmelnitsky, Y. L., and J. O. Rich. 1999. Biocatalysis in nonaqueous solvents. Curr. Opin. Chem. Biol. 3:47-53. [DOI] [PubMed] [Google Scholar]
- 13.Kim, J. H., C. S. Lee, and B. G. Kim. 2005. Spore-displayed streptavidin: a live diagnostic tool in biotechnology. Biochem. Biophys. Res. Commun. 331:210-214. [DOI] [PubMed] [Google Scholar]
- 14.Kim, J. H., H. Yun, B. G. Kim, H. C. Jung, S. K. Choi, and J. G. Pan. December. 2001. Methods for expression of proteins on spore surface. International patent WO2002046388.
- 15.Klibanov, A. M. 2001. Improving enzymes by using them in organic solvents. Nature 409:241-246. [DOI] [PubMed] [Google Scholar]
- 16.Matsumura, S., K. Imai, S. Yoshikawa, K. Kawada, and T. Uchibori. 1990. Surface activities, biodegradability and antimicrobial properties of n-alkyl glucosides, mannosides and galactosides. J. Am. Oil Chem. Soc. 67:996-1001. [Google Scholar]
- 17.Mauriello, E. M., H. Duc le, R. Isticato, G. Cangiano, H. A. Hong, M. De Felice, E. Ricca, and S. M. Cutting. 2004. Display of heterologous antigens on the Bacillus subtilis spore coat using CotC as a fusion partner. Vaccine 22:1177-1187. [DOI] [PubMed] [Google Scholar]
- 18.Mori, T., S. Fujita, and Y. Okahata. 1997. Transglycosylation in a two-phase aqueous-organic system with catalysis by a lipid-coated beta-d-galactosidase. Carbohydr. Res. 298:65-73. [DOI] [PubMed] [Google Scholar]
- 19.Nicholson, W. L., N. Munakata, G. Horneck, H. J. Melosh, and P. Setlow. 2000. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 64:548-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination and outgrowth, p. 391-450. In C. Horwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & Sons, Ltd., Chichester, United Kingdom.
- 21.Park, T. J., K. B. Lee, S. J. Lee, J. P. Park, Z. W. Lee, S. K. Choi, H. C. Jung, J. G. Pan, S. Y. Lee, and I. S. Choi. 2004. Micropatterns of spores displaying heterologous proteins. J. Am. Chem. Soc. 126:10512-10513. [DOI] [PubMed] [Google Scholar]
- 22.Schmid, A., J. S. Dordick, B. Hauer, A. Kiener, M. Wubbolts, and B. Witholt. 2001. Industrial biocatalysis today and tomorrow. Nature 409:258-268. [DOI] [PubMed] [Google Scholar]
- 23.Timmis, K. N., and D. H. Pieper. 1999. Bacteria designed for bioremediation. Trends Biotechnol. 17:200-204. [DOI] [PubMed] [Google Scholar]
- 24.van Rantwijk, F., M. Woudenberg-van Oosterom, and R. A. Sheldon. 1999. Glycosidase-catalysed synthesis of alkyl glycosides. J. Mol. Catal. B Enzym. 6:511-532. [Google Scholar]
- 25.Wang, L., G. Zhu, P. Wang, and B. M. Zhang Newby. 2005. Self-assembling of polymer-enzyme conjugates at oil/water interfaces. Biotechnol. Prog. 21:1321-1328. [DOI] [PubMed] [Google Scholar]
- 26.Wernerus, H., and S. Stahl. 2004. Biotechnological applications for surface-engineered bacteria. Biotechnol. Appl. Biochem. 40:209-228. [DOI] [PubMed] [Google Scholar]
- 27.Woudenberg-van Oosterom, M., H. J. A. van Belle, F. van Rantwijk, and R. A. Sheldon. 1998. Immobilised β-galactosidases and their use in galactoside synthesis. J. Mol. Catal. A Chem. 134:267-274. [Google Scholar]
- 28.Zhu, G., and P. Wang. 2004. Polymer-enzyme conjugates can self-assemble at oil/water interfaces and effect interfacial biotransformations. J. Am. Chem. Soc. 126:11132-11133. [DOI] [PubMed] [Google Scholar]