Abstract
Comparative genomic analysis has revealed limited strain diversity between Salmonella pathogenic and nonpathogenic isolates. Thus, some of the relative virulence and host-immune response disparities may be credited to differential gene regulation rather than gross differences in genomic content. Here we show that altered levels of Salmonella DNA adenine methylase (Dam) resulted in acute defects in virulence-associated gene expression, motility, flagellin synthesis, and bile resistance in the Salmonella pathogenic strain 14028 but not in avirulent laboratory strain LT2. The defects in motility exhibited by 14028 in response to altered Dam levels was not dependent on the presence of the regulatory protein, RpoS. The transitioning between flagellar types (phase variation) was also differentially regulated in 14028 versus LT2 in response to dam levels, resulting in distinct differences in flagellin expression states. These data suggest that differential gene regulation may contribute to the relative virulence disparities observed between Salmonella serovars that are closely related at the DNA level.
Salmonella enterica is a significant pathogen of reptiles, birds, and mammals and is an important food-borne pathogen of humans, wherein a wide variety of infections can occur, ranging from gastroenteritis to bacteremia and typhoid fever (53). More than 2,500 serovars of S. enterica have been identified and classified typically by serotyping, based on antigenic variation in the lipopolysaccharide (O-antigen) and phase 1 (H1) and phase 2 (H2) flagella (33, 56, 57). Although serotyping has been a versatile, convenient, and epidemiologically useful tool for classifying isolates, comparative genomic analysis has provided much of our insight regarding bacterial diversity, evolutionary relatedness, and pathogenicity between species and between serovars (10, 17, 27, 40, 79, 84).
Surprisingly, limited strain diversity has emerged from comparative genomic analyses between pathogenic Salmonella serovars (4, 84), as well as within pathogenic and nonpathogenic isolates of the same serovar (68; http://www.sanger.ac.uk). Accordingly, some of the relative differences in virulence may be attributed to differential gene regulation, which is not revealed by standard genomic comparisons (17). For example, the avirulent laboratory Typhimurium strain, LT2, harbors the principal pathogenicity islands and other known functions associated with virulence but remains defective in the ability to cause disease in animal models of infection (68; http://www.sanger.ac.uk). The principal known virulence difference at the genomic level between Salmonella pathogenic strains and avirulent laboratory strain LT2 resides within the alternative sigma factor rpoS, wherein replacement of the mutant rpoSLT2 allele with that of an rpoS allele from a pathogenic strain results in a significant, but incomplete, restoration of virulence to LT2 (55, 97, 102). Since pathogenic Typhimurium strains and LT2 are closely related at the genomic level, some of the rpoS-independent virulence disparities may also be regulatory in nature.
DNA adenine methylase (Dam) is a regulatory protein that directly controls bacterial virulence gene expression (5, 11, 15, 48). In Salmonella, dam mutants ectopically express multiple genes that are preferentially expressed during infection, modulate host immune responses, are attenuated for virulence, and confer heightened immunity in vaccinated hosts (35, 44, 45, 62, 92). We show here that altered levels of Dam differentially affected several virulence-associated phenotypes, including bacterial virulence gene expression, motility, flagellar synthesis, bile resistance, and phase variation in Salmonella pathogenic strain 14028 compared to the avirulent laboratory strain, LT2.
MATERIALS AND METHODS
Bacterial strains, phage, and media.
The Salmonella pathogenic strains used in the present study were derived from S. enterica serovar Typhimurium strain ATCC 14028 (CDC 6516-60), UK-1 (43), and F98 (6, 43); the pathogenic strains Typhimurium TY1212 and S. enterica O6,14,24:e,h- monophasic K00-670 (29, 30) were recovered from recent virulent calf and poultry outbreaks, respectively, and were obtained from the California Animal Health and Food Safety Laboratory; all Salmonella field isolates were obtained from U.S. Department of Agriculture-Agricultural Research Service (USDA-ARS). Typhimurium avirulent laboratory strains derived from LT2 (25, 89) and LT7 (54, 89) were obtained from John Roth and Tom Cebula, respectively. Dam-overproducing (DamOP) strains contained Escherichia coli dam on a recombinant plasmid (pTP166) (67); introduction of pTP166 into all Salmonella isolates tested resulted in ∼50- to 100-fold increased Dam activity (as observed in E. coli [59, 67]). dam derivatives contained a dam102::Mud-Cm insertion or damΔ232, a nonpolar in-frame deletion (45); dam+ and dam mutant derivatives contained an empty plasmid vector, pTP166-Δdam, in which the dam gene was removed from pTP166. lacZ transcriptional fusions to flagellar genes were obtained from Kelly Hughes and transduced into strains 14028 and LT2 (flhC5213::MudA [TH4314]; fliA::lacZ [TH5597]; flgM5207::MudJ [TH2507]; fliC5050::MudJ [TH1077]; fljB5001::MudJ [TH714]; motA5457::MudJ [TH3929]; and cheY5458::MudJ [TH3930]) (21, 39). spvB, pmrB, mgtA, entF, and fdnG lacZ transcriptional fusion strains were derived from in vivo expression technology (45, 65). A nonpolar in-frame deletion ΔflgM8041 was constructed by using internal oligonucleotides that serve as PCR primers designed to construct an in-frame 240-bp deletion of defined flgM sequence, which was confirmed by sequencing. The rpoSLT2 allele was introduced into virulent strain 14028 by standard allelic replacement, generating strain MT2892 (28). rpoS1221::MudJ was constructed by standard genetic methods (16).
The high-frequency generalized transducing bacteriophage P22 mutant HT105/1, int-201 was used for all transductional crosses (90), and phage-free, phage-sensitive transductants were isolated as previously described (18). Unless otherwise specified, Luria-Bertani (LB) broth (25) was the laboratory media used in these studies. The final concentrations of antibiotics (Sigma) were as follows: ampicillin (100 μg/ml), chloramphenicol (20 μg/ml), kanamycin (50 μg/ml), and carbenicillin (100 μg/ml).
Motility assays.
dam+, dam mutant, and DamOP derivatives of Salmonella were inoculated into the center of soft-agar motility plates (38), incubated for 7 h at 37°C, and the motility area (in square centimeters) was calculated by the formula πr2, where r is the growth radius of the swarm. Motility assays were conducted in the presence of ampicillin to maintain the Dam-overproducing plasmid, pTP166 (67) in DamOP strains; dam+ and dam mutant derivatives contained an empty plasmid vector, pTP166-Δdam, in which the dam gene was removed from pTP166. FlhC− (flhC5456::MudJ) strains TH3928 and MT2425 were used as nonmotile controls (21). For each strain, the assay was performed in triplicate, and the average growth diameter of the swarm was determined (standard deviation of <10% of the mean).
Western blot analysis.
Whole-cell protein extracts prepared from ∼107 cells were processed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (∼20 μg of protein/well), transferred to polyvinylidene difluoride (PVDF) membrane (Pierce), and probed with Salmonella primary antibody H antiserum i (anti-FliC) or H antiserum 1 complex (anti-FljB) for Typhimurium or H antiserum eh (anti-FliC) for S. enterica O6,14,24:e,h− monophasic (Difco); for Salmonella field isolates, E. coli flagella monoclonal antibody 15D8 (IgG1; BioVeris), which recognizes a conserved flagellar epitope that cross-reacts with other flagellum-expressing Enterobacteriaceae, was used as the primary antibody. Peroxidase-conjugated donkey anti-rabbit immunoglobulin G (Amersham Biosciences) and goat anti-mouse immunoglobulin G (Pierce) were used as secondary antibody for Salmonella specific and Escherichia coli nonspecific flagellar primary antibodies, respectively. Signal was detected by chemiluminescence using Supersignal West Femto maximum sensitivity substrate (Pierce), followed by exposure to film. Cultures used for Western analysis were grown in the presence of carbenicillin to maintain the Dam-overproducing plasmid, pTP166 (67) in DamOP strains; dam+ and dam mutant derivatives contained an empty plasmid vector, pTP166-Δdam, in which the dam gene was removed from pTP166. FlhC− (flhC5456::MudJ) strains TH3928 and MT2425 were used as nonflagellated controls (21).
β-galactosidase assays.
Salmonella cultures containing dam+, dam mutant, and DamOP derivatives of lacZ transcriptional fusions were grown for 16 h in Luria-Bertani medium (25) at 37°C (13, 14) or 30°C (MudA::lacZ fusions) and assayed for β-galactosidase activities as described previously (94). Dam-overproducing strains contained E. coli dam on a recombinant plasmid (pTP166) (67); dam+ and dam mutant derivatives contained an empty plasmid vector, pTP166-Δdam, in which the dam gene was removed from pTP166. Units refer to β-galactosidase activities (micromoles of o-nitrophenol [ONP] formed per minute per A600 unit per milliliter of cell suspension × 103). Values are an average of at least two triplicates performed on separate days; the standard deviation was <10% of the mean.
Bile sensitivity assays.
Bile sensitivity assays were performed as a modification of methods described previously (101). Salmonella cultures containing dam+, dam mutant, and DamOP derivatives of strains 14028 and LT2 were grown overnight in LB medium (25) at 37°C. Approximately 5 × 102 cells from overnight grown cultures were added to individual wells in 96-well Polystyrene microtiter plates (Becton Dickinson) containing 150 μl of LB medium with the indicated concentrations of ox bile (sodium choleate [Sigma]) and incubated for 16 h at 37°C without shaking. Growth was assessed by measurement of the optical density at 600 nm (OD600). Assays were conducted in the presence of ampicillin to maintain the Dam-overproducing plasmid, pTP166 (67), in DamOP strains; dam+ and dam mutant derivatives contained an empty plasmid vector, pTP166-Δdam, in which the dam gene was removed from pTP166. The values given are an average of the OD600 values from at least three triplicates; the standard deviation was <20% of the mean. Values of <0.02 represent no detectable growth under the condition tested.
Flagellar-phase transition rates.
The fljB::lacZ transition rates (per cell per generation) of fljBOn to fljBOff and of fljBOff to fljBOn were calculated from a single blue colony (Lac+) or a single white colony (Lac−) from dam+, dam mutant, and DamOP derivatives of Typhimurium fljB5001::MudJ fusion strain (38) grown on minimal E medium agar (25) containing 0.2% glycerol and 40 to 80 μg of 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal; US Biologicals)/ml. Colonies exhibiting a Lac+ or Lac− phenotype (no sectors) were excised from the agar and plated to determine the total number of organisms in the colony and to score the Lac phenotype after incubation for 48 h at 37°C. Transition rates represent the weighted average of five independent colonies as described previously (8, 32). The transition rates were calculated by the formula (M/N)/g, where M/N is the ratio of Lac+ or Lac− cells to total cells, and g is the number of generations of growth from a single cell to the total number of cells in the colony. The weighted average of the transition rates was calculated by the formula [(M1/g1) + (M2/g2) + (Mn/gn)]/(N1 + N2 + Nn), where M, N, and g are as described above and n depicts each individual transition rate calculation. In order to calculate the transition rates, the assumption was made that Lac+ colonies arose from a single Lac+ parent cell and Lac− colonies arose from a single Lac− parent cell.
RESULTS
Dam overproduction results in acute defects in motility and flagellar synthesis in Salmonella pathogenic strains but not in the avirulent laboratory strain LT2. Flagella are an important virulence factor for a wide variety of pathogens, engaging in required roles in bacterial adhesion to epithelial cell surfaces, colonization, biofilm formation, and invasion of host tissues (reviewed in reference 88). Although Salmonella flagellin and motility are dispensable in the mouse model (61, 91), there are several reports indicating that flagella are important for the establishment of Salmonella infection. Salmonella flagella are required for efficient attachment and transport through rabbit appendix M cells in vivo (66), for Salmonella invasiveness in a cell culture model, and for induction of polymorphonuclear leukocyte infiltration in a calf intestinal model of infection (91). In addition, Salmonella flagellins are principal antigens that are recognized by the innate immune system via flagellin pathogen associated molecular patterns (2, 51, 98), have the capacity to elicit different host responses (20), and are trafficked through eukaryotic cells in advance of infecting organisms (63).
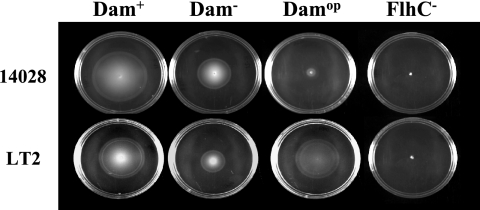
To further understand the virulence disparity between the pathogenic strain 14028 and the relatively pathogenic strain LT2, we examined whether motility and flagellar synthesis were differentially regulated in response to altered Dam levels. Note that the growth rates of dam mutant and DamOP derivatives did not significantly differ from that of wild type. As was shown in another pathogenic strain (5), the lack of dam was associated with relatively mild defects in all Salmonella strains tested (Table 1) . In contrast, Dam overproduction resulted in severe defects in motility (Fig. 1) and flagellar synthesis (Fig. 2) in 14028 and in three other pathogenic Typhimurium strains that have been associated with acute disease in livestock, as well as in one field isolate of Typhimurium var. Copenhagen that has been associated with asymptomatic colonization and/or persistence in chickens (Table 1). Growth under DamOP conditions did not significantly alter motility or flagellar synthesis in avirulent laboratory Typhimurium strain LT2 or LT7.
TABLE 1.
Motility and flagellar synthesis are defective in DamOP derivatives of Salmonella clinical isolates
| Straina | Serovar (serogroup) | Motilityb (area in cm2)
|
Flagellar synthesisc (mutant/wild-type)
|
||||
|---|---|---|---|---|---|---|---|
| dam+ | dam mutant | DamOP | dam+ | dam mutant | DamOP | ||
| MT2425 (FlhC−) | Typhimurium (B) | 0.031 | 0.031 | 0.031 | 0.0078 | 0.0078 | 0.0078 |
| Laboratory strains | |||||||
| LT2 | Typhimurium (B) | 11.0 | 2.3 | 10.1 | 1 | 1 | 1 |
| LT7 | Typhimurium (B) | 17.3 | 2.3 | 12.3 | 1 | 1 | 1 |
| Pathogenic strains | |||||||
| ATCC 14028 | Typhimurium (B) | 16.3 | 5.9 | 0.34 | 1 | 1 | 0.0156 |
| UK-1 | Typhimurium (B) | 16.8 | 5.1 | 0.38 | 1 | 1 | 0.0078 |
| F98 | Typhimurium (B) | 17.5 | 5.9 | 0.72 | 1 | 1 | 0.0078 |
| TY1212 | Typhimurium (B) | 14.9 | ND | 0.28 | 1 | ND | 0.0078 |
| 03-721 | Newport (C2) | 16.6 | ND | 1.81 | 1 | ND | 0.25 |
| K00-670 | O6,14,24:e,h− monophasic (H) | 14.2 | ND | 0.31 | 1 | ND | 0.0156 |
| Field isolates | |||||||
| EPIMD142 | Typhimurium var. Copenhagen (B) | 14.51 | ND | 0.45 | 1 | ND | 0.0156 |
| EPIMD144 | Istanbul (C3) | 16.61 | ND | 1.91 | 1 | ND | 0.0156 |
| BL9W2FL | Thompson (C1) | 15.19 | ND | 0.67 | 1 | ND | 0.0312 |
| CH10W4WI | Montevideo (C1) | 5.55 | ND | 0.34 | 1 | ND | 0.0156 |
| NM 1-41 | Kentucky (C3) | 16.10 | ND | 0.41 | 1 | ND | 0.0312 |
| NM 26-71 | Anatum (E1) | 16.10 | ND | 2.54 | 1 | ND | 0.0312 |
| NM 27-07 | Montevideo (C1) | 9.23 | ND | 2.08 | 1 | ND | 0.0312 |
| NM 25-06 | Meleagridis (E1) | 10.51 | ND | 0.83 | 1 | ND | 0.0312 |
| NM 25-46 | Sandiego (B) | 17.78 | ND | 7.06 | 1 | ND | 1 |
| NM 28-54 | Cerro (K) | 10.34 | ND | 0.83 | 1 | ND | 0.0625 |
| NM 30-31 | Paratyphi B var. Java (B) | 17.56 | ND | 2.16 | 1 | ND | 0.0312 |
Salmonella pathogenic strains used in this study were derived from Typhimurium strain ATCC 14028 (CDC 6516-60), UK-1 (43); F98 (6, 43); Typhimurium TY1212 and O6,14,24:e,h− monophasic K00-670 (29, 30) were recovered from recent virulent calf and poultry outbreaks, respectively, and were obtained from the California Animal Health and Food Safety Laboratory; Newport 03-721 was recovered from a recent calf outbreak and was obtained from Veterinary Medical Teaching Hospital Microbiology Lab at the University of California, Davis. All Salmonella field isolates were obtained from the USDA-ARS. Typhimurium avirulent laboratory strains were derived from LT2 (25, 89) and LT7 (54, 89). ND, not determined.
dam+, dam mutant, and DamOP derivatives of Salmonella were inoculated into the center of soft agar motility plates (38) and incubated for 7 h at 37°C, and the motility area of the swarm was determined. For each strain, the assay was performed in triplicate and the average growth diameter of the swarm was determined; the standard deviation was <10% of the mean. FlhC− (flhC5456::MudJ) strain MT2425 was used as a nonmotile control (21).
Whole-cell protein extracts prepared from ∼107 cells were processed by SDS-PAGE (∼20 μg of protein/well), transferred to PVDF membrane (Pierce), and probed with Salmonella primary antibody H antiserum i (anti-FliC) or H antiserum 1 complex (anti-FljB) for Typhimurium or H antiserum eh (anti-FliC) for S. enterica O6,14,24:e,h− monophasic (Difco); for Salmonella field isolates, E. coli flagellum monoclonal antibody 15D8 (IgG1; BioVeris) was used as a primary antibody. Signal was detected as described in Materials and Methods. Units refer to relative mutant/wild-type levels of flagellin determined by Western analysis using flagellar antibodies. Protein extracts of dam+ strains were diluted 4- to 128-fold before the signal was equal to that observed in DamOP cells; values given are representative of at least three independent Western blots. FlhC− (flhC5456::MudJ) strain MT2425 was used as a nonmotile control (21).
FIG. 1.
Dam overproduction (DamOP) results in motility defects in Salmonella strain 14028 but not in strain LT2. dam+, dam mutant, and DamOP derivatives of the Typhimurium pathogenic strain, 14028, and the avirulent laboratory strain, LT2, were inoculated into the center of soft agar motility plates (38). Motility was assessed by measuring the growth diameter of the swarm after 7 h of incubation at 37°C. Cells recovered from the outermost motility zone of the swarm generated by DamOP cells were shown to be motile escape mutants selected during the assay (data not shown). FlhC− (flhC5456::MudJ) strains TH3928 and MT2425 were used as nonmotile controls (21).
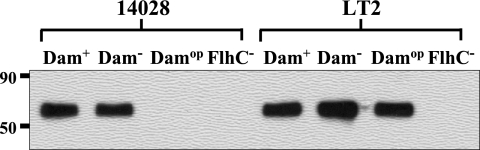
FIG. 2.
Dam overproduction results in flagellar synthesis defects in Salmonella strain 14028 but not in strain LT2. Whole-cell protein extracts corresponding to ∼107 Salmonella cells from dam+, dam mutant, and DamOP derivatives of the Typhimurium pathogenic strain, 14028, and the avirulent laboratory strain, LT2, were subjected to SDS-PAGE and transferred to a PVDF membrane. Membranes were probed with Salmonella H antiserum 1 complex (anti-FljB). Signal was detected as described in Materials and Methods. Extracts of dam+ and dam mutant 14028 and LT2 strains were diluted 64-fold before the flagellar signal was undetectable. FlhC− (flhC5456::MudJ) strains TH3928 (LT2 background) and MT2425 (14028 background) were used as nonflagellated controls (21).
To assess whether such differential regulation extended to other Salmonella serovars, motility and flagellar synthesis were examined in two other pathogenic Salmonella serovars that are associated with acute disease in chickens and cattle and in ten field isolates that are associated with asymptomatic colonization and/or persistence without acute disease manifestation in these animals. Similar to Typhimurium, growth under DamOP conditions resulted in defects in motility and flagellar synthesis in nearly all (11 of 12) of the non-Typhimurium pathogenic and field isolates tested (Table 1). These data suggest that the differential regulation of motility and flagellar synthesis in response to Dam levels extends to other Salmonella serovars, including pathogenic isolates as well as field isolates that are associated with asymptomatic colonization or persistence.
The defect in motility exhibited by pathogenic strain 14028 in response to altered Dam levels is not dependent on the presence of RpoS.
The alternative sigma factor, RpoS, is involved in Salmonella virulence and virulence-associated gene expression (34). In addition, allelic replacement of rpoS from a pathogenic strain results in the partial restoration of virulence to LT2 (55, 97, 102). Here we examined whether the differential regulation of motility under DamOP conditions was dependent on the presence of RpoS. An rpoS mutation (rpoS1221::MudJ) did not significantly affect the motility of strain 14028 or LT2 (Table 2), a finding consistent with the observation that the lack of RpoS results in only mild defects in flagellin production in a pathogenic strain (1). However, neither rpoS mutation nor the introduction of the rpoSLT2 allele into strain 14028 alleviated the acute motility defect inherent to 14028 DamOP cells, although a mild derepression of flagellin synthesis was observed (Table 2 and data not shown). In addition, sequence analysis of avirulent Typhimurium strain, LT7, revealed a wild-type rpoS, indicating that the inability of LT7 to respond to Dam overproduction (Table 1) was not attributable to a mutant rpoS allele. Taken together, these data indicate that differential regulation of motility exhibited by 14028 and LT2 in response to altered Dam levels was not dependent on the presence of RpoS or a mutant allele of rpoS.
TABLE 2.
Differential regulation of motility exhibited by 14028 and LT2 grown under DamOP conditions was not dependent on the presence of RpoS or mutant allele of rpoS
| Relevant strain and genotype | Motilitya (area in cm2)
|
||
|---|---|---|---|
| dam+ | dam mutant | DamOP | |
| 14028 | 16.4 | 4.1 | 0.50 |
| 14028 rpoS1221::MudJ | 14.2 | 3.4 | 0.31 |
| 14028 rpoSLT2 | 18.2 | 6.6 | 0.58 |
| LT2 | 11.1 | 2.4 | 11.7 |
| LT2 rpoS1221::MudJ | 8.5 | 1.8 | 8.1 |
dam+, dam mutant, and DamOP derivatives of Salmonella were inoculated into the center of soft agar motility plates (38) and incubated for 7 h at 37°C, and the motility area of the swarm was determined. For each strain, the assay was performed in triplicate, and the average growth diameter of the swarm was determined; the standard deviation was <10% of the mean.
Dam overproduction leads to enhanced bile sensitivity in pathogenic strain 14028 but not in strain LT2.
Enteric bacteria are inherently resistant to bile and utilize bile concentrations as a signal for the temporal and spatial production of virulence factors and for the induction of other adaptive mechanisms, including multidrug resistance (41, 78). Bile has been shown to repress Salmonella flagellar gene expression and motility (81). In addition, mutants that lack or overproduce dam are highly sensitive to bile (44, 82). Here we examined whether bile sensitivity was differentially regulated in pathogenic strain 14028 and LT2 in response to altered Dam levels. Although the lack of dam was associated with bile sensitivity in both strains, growth under DamOP conditions resulted in enhanced bile sensitivity specifically in 14028 cells over a range of physiologically relevant bile concentrations (3 to 5%) (41); the bile sensitivity of LT2 DamOP cells did not significantly differ from that exhibited by wild type (dam+) (Table 3). Thus, growth under DamOP conditions is associated with acute defects in motility, flagellar synthesis, and bile resistance in pathogenic strain 14028 but not in the laboratory strain LT2.
TABLE 3.
Dam overproduction leads to enhanced bile sensitivity in Salmonella sp. strain 14028 but not in LT2
| Bile content (%) | OD600a
|
|||||
|---|---|---|---|---|---|---|
| 14028
|
LT2
|
|||||
| MT2461 dam+ | MT2462 dam mutant | MT2128 DamOP | MT2582 dam+ | MT2583 dam mutant | MT2584 DamOP | |
| 5 | 0.097 | <0.02 | <0.02 | 0.084 | <0.02 | 0.074 |
| 4.5 | 0.125 | <0.02 | 0.048 | 0.100 | <0.02 | 0.093 |
| 4 | 0.170 | 0.084 | 0.069 | 0.128 | 0.036 | 0.112 |
| 3 | 0.228 | 0.156 | 0.127 | 0.168 | 0.117 | 0.159 |
| 0 | 0.299 | 0.267 | 0.257 | 0.264 | 0.249 | 0.247 |
Salmonella cultures containing dam+, dam mutant, and DamOP derivatives of strains 14028 and LT2 were grown for 16 h in Luria-Bertani media (25). Approximately 5 ∞ 102 of the overnight grown cells were added to individual microtiter wells containing LB in addition to the listed final concentration of ox bile (sodium choleate). Cells were incubated for an additional 16 h without shaking at 37°C. Values given are an average of OD600 values from at least three triplicates; the standard deviation was <20% of the mean. Values of <0.02 represent no detectable growth under the condition tested.
Salmonella gene expression is differentially regulated in dam mutant derivatives of strains 14028 and LT2.
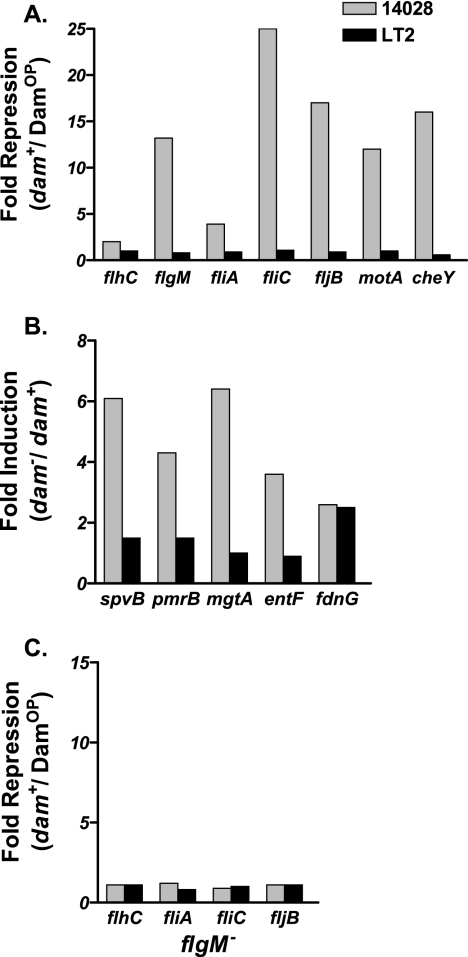
Greater than 40 genes are required for the proper morphogenic development of a functional flagellum, and they are classified with respect to the timing of their expression as early, middle, and late genes (reviewed in reference 19). Here we examined whether altered levels of the Dam regulatory protein differentially affected the transcription of Typhimurium flagellar genes (21, 38, 39), which encode products that contribute to pathogenicity and the elicitation of host immune responses (2, 22, 50, 86) in pathogenic strain 14028 versus laboratory strain LT2. Although the lack of dam did not significantly affect flagellar gene expression in either strain, Dam overproduction in pathogenic strain 14028 resulted in a 2- to 25-fold reduction in the transcription of early and/or middle regulatory genes (flhC, flgM, and fliA) and late structural genes encoding FliC and FljB flagellins, a motor-force-generating protein (MotA), and a chemosensory protein (CheY) with respect to wild-type (dam+) levels (Fig. 3A) . In contrast, Dam overproduction in avirulent laboratory strain LT2 did not significantly affect the transcription of these genes compared to that observed in wild type (dam+). These data indicate that flagellar gene expression is differentially regulated in 14028 versus LT2 in response to DamOP conditions.
FIG. 3.
Altered levels of Dam result in acute differences in bacterial gene expression in strain 14028 compared to strain LT2. (A) Salmonella cultures containing dam+ and DamOP derivatives of flagellar gene transcriptional lacZ fusions in strain 14028 and strain LT2 were grown for 16 h in Luria-Bertani media (25) and assayed for β-galactosidase activity as described previously (94). Units reflect the relative fold repression (dam+/DamOP). B. dam+ and dam mutant derivatives of strains 14028 and LT2 containing spvB, pmrB, mgtA, entF, and fdnG lacZ transcriptional fusions were cultured and assayed as described above. Units reflect the relative fold induction (dam mutant/dam+). C. dam+ and DamOP derivatives of flgM mutant and flgM+ isolates of strains 14028 and LT2 containing flagellar transcriptional fusions were cultured and assayed as described above. Units reflect the relative fold repression (dam+/DamOP).
Dam represses the expression of several Salmonella genes that are preferentially expressed during infection (designated as in vivo-induced genes [ivi]) in strain 14028 (45, 65). In addition, microarray analysis of another Typhimurium pathogenic strain indicates that many genes are either activated or repressed in response to dam (5). To determine whether differential regulation affected genes other than those of the flagellar regulon, we assessed whether the lack of dam differentially affected ivi gene expression in strain 14028 versus strain LT2. As reported earlier (45), the lack of dam resulted in the derepression of several ivi genes in strain 14028, including spvB, encoding an actin cytotoxin (58); pmrB, involved in resistance to antimicrobial peptides (87); and mgtA and entF, involved in the transport of magnesium and iron, respectively (31, 36) (Fig. 3B and data not shown). In contrast, only 5 of 26 ivi genes that were previously shown to be dam regulated in strain 14028 were derepressed in dam mutant strains of LT2, one of which is fdnG, encoding formate dehydrogenase, involved in anaerobic metabolism (96). These data indicate that differential regulation observed between strains 14028 and LT2 in response to altered Dam levels is not limited to genes of the flagellar regulon.
flgM contributes to the differential gene regulation observed between strains 14028 and LT2 in response to altered Dam levels.
To further understand the molecular basis of flagellar differential regulation displayed by 14028 and LT2 in response to growth under DamOP conditions, we assessed the role of FlgM, a negative regulator of flagellar gene expression (37, 38). flgM mutation in DamOP 14028 cells resulted in the derepression of all (four of four) flagellar genes tested under DamOP conditions (compared to the relative flagellar gene expression in flgM+ [Fig. 3A] versus flgM mutant [Fig. 3C] cells). Accordingly, the flgM mutation partially relieved the defects in FliC and FljB synthesis (Fig. 4) and the associated defect in motility inherent to DamOP 14028 cells relative to that observed in dam+ ΔflgM 14028 cells (data not shown). Although flgM mutation does not fully restore flagellar synthesis and motility to wild-types levels under DamOP conditions, these data indicate that significant aspects of flagellar differential gene regulation exhibited by 14028 relative to LT2 occur in an FlgM-dependent fashion.
FIG. 4.
Differential regulation of the strain 14028 flagellar synthesis in response to altered Dam levels occurs in a FlgM-dependent fashion. Whole-cell protein extracts corresponding to ∼107 Salmonella cells from dam+, dam mutant, and DamOP derivatives of ΔflgM and flgM+ Typhimurium pathogenic strain 14028 were subjected to SDS-PAGE and transferred to PVDF membrane. Membranes were probed with Salmonella primary antibody H antiserum i (anti-FliC). Signal was detected as described in Materials and Methods. Extracts of dam+ and dam mutant derivatives of ΔflgM 14028 were diluted fourfold to obtain a FliC signal intensity similar to that observed in DamOP derivatives. FlhC− (flhC5456::MudJ) strain MT2425 was used as a nonflagellated control (21).
Salmonella flagellar-phase variation is differentially affected in Dam-overproducing derivatives of strains 14028 and LT2.
Typhimurium strains oscillate between two flagellar expression states, consisting of either FliC (H1) or FljB (H2) flagellin subunits—a process termed phase variation (9, 93). The frequency of switching between flagellar types and magnitude of flagellar synthesis can be modulated by environmental and genetic signals, so that the pool of infecting organisms can be comprised of antigenically distinct populations that are altered in their capacity for virulence and elicitation of host immune responses (reviewed in reference 19 and 100). The flagellar-phase transition rate is controlled by a reversible genetic switch comprising the site-specific inversion of a promoter fragment that results in the mutually exclusive expression of either FliC or FljB (9, 93).
To assess whether altered Dam levels differentially affected flagellar phase variation, transition rates of fljBOn to fljBOff and fljBOff to fljBOn expression states were evaluated in pathogenic and nonpathogenic strains. In agreement with previous reports (38, 50), both 14028 and LT2 dam+ strains favored the fljBOff expression state; i.e., the ratio of fljBOn to fljBOff/fljBOff to fljBOn was >1.0 (Table 4). However, under DamOP conditions, the inherent bias toward the fljBOff expression state was increased from 3.8- to 8.0-fold in strain 14028 and decreased from 2.5- to 1.3-fold in LT2 relative to the transition rates observed in the respective dam+ strains. Due to the reversible nature of the phase-variable switch, the increased and decreased frequency of the fljBOff expression state was also accompanied by a concomitant increased and decreased frequency of the fliCOn expression state in 14028 and LT2, respectively (data not shown). Thus, phase variation was differentially regulated in 14028 and LT2 in response to altered Dam levels, resulting in distinct differences in flagellin expression states.
TABLE 4.
Flagellar-phase transition rates are differentially affected in DamOP derivatives of strains 14028 and LT2
| Straina | Genotype | Switching frequency (10−3)
|
fljBOn to fljBOff/fljBOff to fljBOn | |
|---|---|---|---|---|
| fljBOn to fljBOff | fljBOff to fljBOn | |||
| 14028 | dam+ | 1.69 | 0.446 | 3.8 |
| dam mutant | 2.79 | 0.820 | 3.4 | |
| DamOP | 1.90 | 0.238 | 8.0 | |
| LT2 | dam+ | 1.19 | 0.469 | 2.5 |
| dam mutant | 2.23 | 0.463 | 4.8 | |
| DamOP | 1.65 | 1.26 | 1.3 | |
fljB::lacZ transition rates (per cell per generation) of fljBOn to fljBOff and fljBOff to fljBOn were calculated from a single blue colony (Lac+) or a single white colony (Lac−) from dam+, dam mutant, and DamOP derivatives of fljB5001::MudJ fusions (38) in Typhimurium pathogenic strain, 14028, and avirulent laboratory strain, LT2, grown on minimal E medium agar (25) containing 0.2% glycerol and 40 to 80 μg of X-Gal/ml. Colonies exhibiting a Lac+ or Lac− phenotype (no sectors) were excised from the agar and plated to determine the total number of organisms in the colony and to score the Lac phenotype after incubation for 48 h at 37°C. Transition rates represent the weighted average of five independent colonies as described in Materials and Methods.
DISCUSSION
The fundamental principles that distinguish a pathogenic serovar from a nonpathogenic serovar are often obscure since some nonpathogenic serovars contain virulence genes that could encode the capacity to enter into, replicate within, and persist at host sites that are inaccessible to commensal species. However, many nonpathogenic strains remain impaired for these virulence activities and cannot sustain a productive infection. Pathogenicity is further complicated by the fact that, among pathogenic isolates, some strains are capable of asymptomatic colonization or persistence in a particular animal species while causing acute disease in another animal species (84). In the present study, we show that altered Dam levels differentially affected virulence-associated bacterial gene expression, as well as flagellar synthesis, bacterial motility, bile resistance, and phase variation, in pathogenic strain 14028 compared to the closely related avirulent laboratory strain LT2. These data suggest that significant aspects of pathogenicity may be attributed to differential gene regulation rather than to major differences in genomic content.
Dam methylation coordinates many cellular processes, including gene expression, DNA mismatch repair, chromosomal replication, and nucleoid structure (15, 60, 62). dam expression is increased in bacterial cells grown under log-phase conditions in vitro, presumably to keep pace with the need of rapidly dividing cells to maintain the appropriate methylation state. Such growth rate control may be a reflection of a pathogen's life cycle whereby, for example, E. coli is thought to grow more rapidly in the colon than outside the host (60, 83). The capacity of pathogenic strain 14028 to sharply decrease flagellar synthesis, motility, and bile resistance in response to altered Dam levels (Tables 1 and 3) may mimic the in vivo condition, wherein bile-mediated repression of flagellin upon entry into the intestine may be favorable until Salmonella transits through the mucus layer to colonize the epithelial surface (3, 23, 24, 41, 80). Since the Dam-dependent differential regulation exhibited by 14028 and LT2 extends to a wide variety of genes (Fig. 3b), the integration of environmental cues into bacterial regulatory networks that are critical to pathogenicity (26, 64, 69, 72) may not be operational, or may be operational to the same extent, in nonpathogenic strains such as LT2. Consistent with this suggestion, allelic differences in the Salmonella regulatory protein, RpoS, have been associated with the avirulence phenotype of LT2 (55, 97, 102). However, the differential regulation of motility exhibited by pathogenic strain 14028 and LT2 under DamOP conditions is not dependent on the presence of RpoS, indicating that other functions contribute to the regulatory differences inherent to these closely related strains. Differential gene regulation may also contribute to some of the virulence, host range, and disease manifestation disparities exhibited within and between closely related pathogenic serovars. Indeed, the fliC gene of another pathogenic Typhimurium strain is also regulated by dam but in a reciprocal fashion from that exhibited by strain 14028 (5).
The transitioning between Typhimurium flagellar types (phase variation) comprises a reversible genetic switch, involving the site-specific inversion of a promoter fragment, which controls the expression of FliC and FljB flagellins such that an individual cell is limited to one specific type at any given time (9, 93). In response to Dam overproduction, pathogenic strains exhibit an enhanced bias toward the FliC expression state (Table 4). The capacity of Dam levels to influence flagellar expression states in vitro may reflect a mechanism by which pathogenic strains are able to augment the frequency of FliC expressing cells, which have a selective advantage over FljB-expressing cells in animal models of typhoid fever (50). Thus, the capacity to alter dam levels can result in marked differences in flagellin expression states.
Insights into the possible mechanism by which Dam contributes to differential gene regulation come from the regulatory analysis of the uropathogenic E. coli pap operon, which encodes pili that are essential for urinary tract infections (11, 15, 47-49). The regulatory mechanism involves the formation of heritable DNA methylation patterns (11, 42, 85, 99) that control gene expression by modulating the binding of regulatory proteins, similar to what has been observed in eukaryotes (7, 46, 52). Since epigenetic regulatory mechanisms involve DNA modifications (methylation) that do not alter the DNA sequence, the progeny expression state can be readily reversed to that of the parent once the selection stimulus imposed upon the progeny cells is removed. Thus, bacterial pathogens may utilize epigenetic control of specific virulence functions as a reversible and heritable mechanism by which to engender variability to the infecting population. Epigenetic modifications are not subject to the same constraints as genetic mutations that are, by nature, relatively stable and perhaps more restricted in their ability to respond to evolutionary pressures. For example, through host Toll-like receptor 5, the innate immune system targets a conserved site on flagellin that is essential for bacterial motility, precluding mutations that result in a nonfunctional flagellum (73, 95). Taken together, the present study suggests that significant aspects of pathogenicity may be attributable to differential gene regulation, perhaps via epigenetic modifications (DNA methylation) that may enhance microbial fitness by the augmentation of diversity at the phenotypic level without a concomitant augmentation of diversity at the genomic level.
Differential gene regulation coupled with classical genetic mutation may be vital to microbial fitness within the host since bacterial infections often originate from clonal expansion of a single cell (71, 75). Thus, pathogenic bacteria must generate diversity to adapt to host polymorphisms and immune clearance mechanisms, enabling them to evade immune defenses and to gain access to new sites within its natural host(s) (74, 76, 77). This is achieved by the generation of genetic variants with altered antigenic properties (antigenic variation) that arise by either classical genetic mutation or by gene regulatory mechanisms that facilitate the transitioning between expressed and unexpressed states (12, 24, 70) (e.g., phase variation of type 1 or type 2 flagella [100]). Such genetic plasticity may also have a profound effect on the emergence and/or evolution of pathogenic serovars as selective pressures give rise to genetic variants that may have altered virulence properties, e.g., maintaining the ability to cause acute disease in a given natural animal host while acquiring the ability to cause acute disease or asymptomatic colonization or persistence in a new animal host.
Acknowledgments
We thank C. Samuel and D. Morse for critically reviewing the manuscript, K. Hughes for kindly providing strains, and R. Werlin for technical assistance with the bile assay.
This study was supported by the G. Harold and Leila Y. Mathers Foundation; by National Institutes of Health grants AI 61399-01 and AI 59242-04A1; and by National Research Initiative of the USDA Cooperative State Research, Education, and Extension Service grant (2004-04574) (to M.J.M.).
Footnotes
Published ahead of print on 15 December 2006.
REFERENCES
- 1.Adams, P., R. Fowler, N. Kinsella, G. Howell, M. Farris, P. Coote, and C. D. O'Connor. 2001. Proteomic detection of PhoPQ- and acid-mediated repression of Salmonella motility. Proteomics 1:597-607. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. [DOI] [PubMed] [Google Scholar]
- 3.Alaniz, R. C., L. A. Cummings, M. A. Bergman, S. L. Rassoulian-Barrett, and B. T. Cookson. 2006. Salmonella typhimurium coordinately regulates FliC location and reduces dendritic cell activation and antigen presentation to CD4+ T cells. J. Immunol. 177:3983-3993. [DOI] [PubMed] [Google Scholar]
- 4.Amavisit, P., D. Lightfoot, G. F. Browning, and P. F. Markham. 2003. Variation between pathogenic serovars within Salmonella pathogenicity islands. J. Bacteriol. 185:3624-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balbontin, R., G. Rowley, M. G. Pucciarelli, J. Lopez-Garrido, Y. Wormstone, S. Lucchini, F. Garcia-Del Portillo, J. C. Hinton, and J. Casadesus. 2006. DNA adenine methylation regulates virulence gene expression in Salmonella enterica serovar Typhimurium. J. Bacteriol. 188:8160-8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrow, P. A., H. W. Smith, and J. F. Tucker. 1984. The effect of feeding diets containing avoparcin on the excretion of salmonellas by chickens experimentally infected with natural sources of salmonella organisms. J. Hyg. 93:439-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bird, A. 2002. DNA methylation patterns and epigenetic memory. Genes Dev. 16:6-21. [DOI] [PubMed] [Google Scholar]
- 8.Blyn, L. B., B. A. Braaten, C. A. White-Ziegler, D. H. Rolfson, and D. A. Low. 1989. Phase-variation of pyelonephritis-associated pili in Escherichia coli: evidence for transcriptional regulation. EMBO J. 8:613-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonifield, H. R., and K. T. Hughes. 2003. Flagellar phase variation in Salmonella enterica is mediated by a posttranscriptional control mechanism. J. Bacteriol. 185:3567-3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyd, E. F., S. Porwollik, F. Blackmer, and M. McClelland. 2003. Differences in gene content among Salmonella enterica serovar Typhi isolates. J. Clin. Microbiol. 41:3823-3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braaten, B. A., X. Nou, L. S. Kaltenbach, and D. A. Low. 1994. Methylation patterns in pap regulatory DNA control pyelonephritis-associated pili phase variation in Escherichia coli. Cell 76:577-588. [DOI] [PubMed] [Google Scholar]
- 12.Brown, N. F., B. A. Vallance, B. K. Coombes, Y. Valdez, B. A. Coburn, and B. B. Finlay. 2005. Salmonella pathogenicity island 2 is expressed prior to penetrating the intestine. PLoS Pathog. 1:e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casadaban, M. J., and J. Chou. 1984. In vivo formation of gene fusions encoding hybrid beta-galactosidase proteins in one step with a transposable Mu-lac transducing phage. Proc. Natl. Acad. Sci. USA 81:535-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casadaban, M. J., and S. N. Cohen. 1979. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc. Natl. Acad. Sci. USA 76:4530-4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casadesus, J., and D. Low. 2006. Epigenetic gene regulation in the bacterial world. Microbiol. Mol. Biol. Rev. 70:830-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castilho, B. A., P. Olfson, and M. J. Casadaban. 1984. Plasmid insertion mutagenesis and lac gene fusion with mini-mu bacteriophage transposons. J. Bacteriol. 158:488-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan, K., S. Baker, C. C. Kim, C. S. Detweiler, G. Dougan, and S. Falkow. 2003. Genomic comparison of Salmonella enterica serovars and Salmonella bongori by use of an S. enterica serovar Typhimurium DNA microarray. J. Bacteriol. 185:553-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan, R. K., D. Botstein, T. Watanabe, and Y. Ogata. 1972. Specialized transduction of tetracycline resistance by phage P22 in Salmonella typhimurium. II. Properties of a high-frequency-transducing lysate. Virology 50:883-898. [DOI] [PubMed] [Google Scholar]
- 19.Chilcott, G. S., and K. T. Hughes. 2000. Coupling of flagellar gene expression to flagellar assembly in Salmonella enterica serovar Typhimurium and Escherichia coli. Microbiol. Mol. Biol. Rev. 64:694-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciacci-Woolwine, F., I. C. Blomfield, S. H. Richardson, and S. B. Mizel. 1998. Salmonella flagellin induces tumor necrosis factor alpha in a human promonocytic cell line. Infect. Immun. 66:1127-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clegg, S., and K. T. Hughes. 2002. FimZ is a molecular link between sticking and swimming in Salmonella enterica serovar Typhimurium. J. Bacteriol. 184:1209-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cookson, B. T., and M. J. Bevan. 1997. Identification of a natural T-cell epitope presented by Salmonella-infected macrophages and recognized by T cells from orally immunized mice. J. Immunol. 158:4310-4319. [PubMed] [Google Scholar]
- 23.Cummings, L. A., S. L. Barrett, W. D. Wilkerson, I. Fellnerova, and B. T. Cookson. 2005. FliC-specific CD4+ T-cell responses are restricted by bacterial regulation of antigen expression. J. Immunol. 174:7929-7938. [DOI] [PubMed] [Google Scholar]
- 24.Cummings, L. A., W. D. Wilkerson, T. Bergsbaken, and B. T. Cookson. 2006. In vivo, fliC expression by Salmonella enterica serovar Typhimurium is heterogeneous, regulated by ClpX, and anatomically restricted. Mol. Microbiol. 61:795-809. [DOI] [PubMed] [Google Scholar]
- 25.Davis, R. W., D. Botstein, and J. R. Roth. 1980. Advanced bacterial genetics. Cold Spring Harbor Laboratory Press, Plainview, NY.
- 26.DiRita, V. J., and J. J. Mekalanos. 1989. Genetic regulation of bacterial virulence. Annu. Rev. Genet. 23:455-482. [DOI] [PubMed] [Google Scholar]
- 27.Dobrindt, U., B. Hochhut, U. Hentschel, and J. Hacker. 2004. Genomic islands in pathogenic and environmental microorganisms. Nat. Rev. Microbiol. 2:414-424. [DOI] [PubMed] [Google Scholar]
- 28.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dueger, E. L., J. K. House, D. M. Heithoff, and M. J. Mahan. 2001. Salmonella DNA adenine methylase mutants elicit protective immune responses to homologous and heterologous serovars in chickens. Infect. Immun. 69:7950-7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dueger, E. L., J. K. House, D. M. Heithoff, and M. J. Mahan. 2003. Salmonella DNA adenine methylase mutants elicit early and late onset protective immune responses in calves. Vaccine 21:3249-3258. [DOI] [PubMed] [Google Scholar]
- 31.Earhart, C. F. 1996. Uptake and metabolism of iron and molybdenum, 2nd ed. ASM Press, Washington, DC.
- 32.Eisenstein, B. I. 1981. Phase variation of type 1 fimbriae in Escherichia coli is under transcriptional control. Science 214:337-339. [DOI] [PubMed] [Google Scholar]
- 33.Ewing, E. H. 1986. Edwards and Ewing's identification of Enterobacteriaceae, 4th ed. Elsevier Science Publishing Co., Inc., New York, NY.
- 34.Fang, F. C., S. J. Libby, N. A. Buchmeier, P. C. Loewen, J. Switala, J. Harwood, and D. G. Guiney. 1992. The alternative sigma factor katF (rpoS) regulates Salmonella virulence. Proc. Natl. Acad. Sci. USA 89:11978-11982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Del Portillo, F., M. G. Pucciarelli, and J. Casadesus. 1999. DNA adenine methylase mutants of Salmonella typhimurium show defects in protein secretion, cell invasion, and M cell cytotoxicity. Proc. Natl. Acad. Sci. USA 96:11578-11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia Vescovi, E., F. C. Soncini, and E. A. Groisman. 1996. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell 84:165-174. [DOI] [PubMed] [Google Scholar]
- 37.Gillen, K. L., and K. T. Hughes. 1991. Molecular characterization of flgM, a gene encoding a negative regulator of flagellin synthesis in Salmonella typhimurium. J. Bacteriol. 173:6453-6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gillen, K. L., and K. T. Hughes. 1991. Negative regulatory loci coupling flagellin synthesis to flagellar assembly in Salmonella typhimurium. J. Bacteriol. 173:2301-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gillen, K. L., and K. T. Hughes. 1993. Transcription from two promoters and autoregulation contribute to the control of expression of the Salmonella typhimurium flagellar regulatory gene flgM. J. Bacteriol. 175:7006-7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Groisman, E. A., and J. Casadesus. 2005. The origin and evolution of human pathogens. Mol. Microbiol. 56:1-7. [DOI] [PubMed] [Google Scholar]
- 41.Gunn, J. S. 2000. Mechanisms of bacterial resistance and response to bile. Microbes. Infect. 2:907-913. [DOI] [PubMed] [Google Scholar]
- 42.Hale, W. B., M. W. van der Woude, and D. A. Low. 1994. Analysis of nonmethylated GATC sites in the Escherichia coli chromosome and identification of sites that are differentially methylated in response to environmental stimuli. J. Bacteriol. 176:3438-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hassan, J. O., and R. Curtiss III. 1994. Development and evaluation of an experimental vaccination program using a live avirulent Salmonella typhimurium strain to protect immunized chickens against challenge with homologous and heterologous Salmonella serotypes. Infect. Immun. 62:5519-5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heithoff, D. M., E. Y. Enioutina, R. A. Daynes, R. L. Sinsheimer, D. A. Low, and M. J. Mahan. 2001. Salmonella DNA adenine methylase mutants confer cross-protective immunity. Infect. Immun. 69:6725-6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heithoff, D. M., R. L. Sinsheimer, D. A. Low, and M. J. Mahan. 1999. An essential role for DNA adenine methylation in bacterial virulence. Science 284:967-970. [DOI] [PubMed] [Google Scholar]
- 46.Hendrich, B., and A. Bird. 2000. Mammalian methyltransferases and methyl-CpG-binding domains: proteins involved in DNA methylation. Curr. Top. Microbiol. Immunol. 249:55-74. [DOI] [PubMed] [Google Scholar]
- 47.Hernday, A., B. Braaten, and D. Low. 2004. The intricate workings of a bacterial epigenetic switch. Adv. Exp. Med. Biol. 547:83-89. [DOI] [PubMed] [Google Scholar]
- 48.Hernday, A., M. Krabbe, B. Braaten, and D. Low. 2002. Self-perpetuating epigenetic pili switches in bacteria. Proc. Natl. Acad. Sci. USA 99(Suppl. 4):16470-16476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hernday, A. D., B. A. Braaten, and D. A. Low. 2003. The mechanism by which DNA adenine methylase and PapI activate the pap epigenetic switch. Mol. Cell 12:947-957. [DOI] [PubMed] [Google Scholar]
- 50.Ikeda, J. S., C. K. Schmitt, S. C. Darnell, P. R. Watson, J. Bispham, T. S. Wallis, D. L. Weinstein, E. S. Metcalf, P. Adams, C. D. O'Connor, and A. D. O'Brien. 2001. Flagellar phase variation of Salmonella enterica serovar Typhimurium contributes to virulence in the murine typhoid infection model but does not influence Salmonella-induced enteropathogenesis. Infect. Immun. 69:3021-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Janeway, C. A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197-216. [DOI] [PubMed] [Google Scholar]
- 52.Jeltsch, A. 2002. Beyond Watson and Crick: DNA methylation and molecular enzymology of DNA methyltransferases. Chembiochem 3:274-293. [DOI] [PubMed] [Google Scholar]
- 53.Kennedy, M., R. Villar, D. J. Vugia, T. Rabatsky-Ehr, M. M. Farley, M. Pass, K. Smith, P. Smith, P. R. Cieslak, B. Imhoff, and P. M. Griffin. 2004. Hospitalizations and deaths due to Salmonella infections, FoodNet, 1996-1999. Clin. Infect. Dis. 38(Suppl. 3):S142-S148. [DOI] [PubMed] [Google Scholar]
- 54.Koch, W. H., E. Henrikson, E. Eisenstadt, and T. A. Cebula. 1995. Salmonella typhimurium LT7 and LT2 strains carrying the imp operon on colIa. J. Bacteriol. 177:1903-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee, I. S., J. Lin, H. K. Hall, B. Bearson, and J. W. Foster. 1995. The stationary-phase sigma factor sigma S (RpoS) is required for a sustained acid tolerance response in virulent Salmonella typhimurium. Mol. Microbiol. 17:155-167. [DOI] [PubMed] [Google Scholar]
- 56.Le Minor, L. 1984. Genus III, Salmonella, Lingniers 1900, 389AL, vol. 1. The Williams & Williams Co., Baltimore, MD.
- 57.Le Minor, L., and Bockemuhl. 1988. 1987 supplement (no 31) to the schema of Kauffmann-White. Ann. Inst. Pasteur Microbiol. 139:331-335. [DOI] [PubMed] [Google Scholar]
- 58.Lesnick, M. L., N. E. Reiner, J. Fierer, and D. G. Guiney. 2001. The Salmonella spvB virulence gene encodes an enzyme that ADP-ribosylates actin and destabilizes the cytoskeleton of eukaryotic cells. Mol. Microbiol. 39:1464-1470. [DOI] [PubMed] [Google Scholar]
- 59.Lobner-Olesen, A., M. G. Marinus, and F. G. Hansen. 2003. Role of SeqA and Dam in Escherichia coli gene expression: a global/microarray analysis. Proc. Natl. Acad. Sci. USA 100:4672-4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lobner-Olesen, A., O. Skovgaard, and M. G. Marinus. 2005. Dam methylation: coordinating cellular processes. Curr. Opin. Microbiol. 8:154-160. [DOI] [PubMed] [Google Scholar]
- 61.Lockman, H. A., and R. Curtiss III. 1990. Salmonella typhimurium mutants lacking flagella or motility remain virulent in BALB/c mice. Infect. Immun. 58:137-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Low, D. A., N. J. Weyand, and M. J. Mahan. 2001. Roles of DNA adenine methylation in regulating bacterial gene expression and virulence. Infect. Immun. 69:7197-7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lyons, S., L. Wang, J. E. Casanova, S. V. Sitaraman, D. Merlin, and A. T. Gewirtz. 2004. Salmonella typhimurium transcytoses flagellin via an SPI2-mediated vesicular transport pathway. J. Cell Sci. 117:5771-5780. [DOI] [PubMed] [Google Scholar]
- 64.Mahan, M. J., D. M. Heithoff, R. L. Sinsheimer, and D. A. Low. 2000. Assessment of bacterial pathogenesis by analysis of gene expression in the host. Annu. Rev. Genet. 34:139-164. [DOI] [PubMed] [Google Scholar]
- 65.Mahan, M. J., J. M. Slauch, and J. J. Mekalanos. 1993. Selection of bacterial virulence genes that are specifically induced in host tissues. Science 259:686-688. [DOI] [PubMed] [Google Scholar]
- 66.Marchetti, M., J. C. Sirard, P. Sansonetti, E. Pringault, and S. Kerneis. 2004. Interaction of pathogenic bacteria with rabbit appendix M cells: bacterial motility is a key feature in vivo. Microbes Infect. 6:521-528. [DOI] [PubMed] [Google Scholar]
- 67.Marinus, M. G., A. Poteete, and J. A. Arraj. 1984. Correlation of DNA adenine methylase activity with spontaneous mutability in Escherichia coli K-12. Gene 28:123-125. [DOI] [PubMed] [Google Scholar]
- 68.McClelland, M., K. E. Sanderson, J. Spieth, S. W. Clifton, P. Latreille, L. Courtney, S. Porwollik, J. Ali, M. Dante, F. Du, S. Hou, D. Layman, S. Leonard, C. Nguyen, K. Scott, A. Holmes, N. Grewal, E. Mulvaney, E. Ryan, H. Sun, L. Florea, W. Miller, T. Stoneking, M. Nhan, R. Waterston, and R. K. Wilson. 2001. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 413:852-856. [DOI] [PubMed] [Google Scholar]
- 69.Mekalanos, J. J. 1992. Environmental signals controlling expression of virulence determinants in bacteria. J. Bacteriol. 174:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Merighi, M., C. D. Ellermeier, J. M. Slauch, and J. S. Gunn. 2005. Resolvase-in vivo expression technology analysis of the Salmonella enterica serovar Typhimurium PhoP and PmrA regulons in BALB/c mice. J. Bacteriol. 187:7407-7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meynell, G. G., and B. A. Stocker. 1957. Some hypotheses on the etiology of fatal infections in partially resistant hosts and their application to mice challenged with Salmonella paratyphi-B or Salmonella typhimurium by intraperitoneal injection. J. Gen. Microbiol. 16:38-58. [DOI] [PubMed] [Google Scholar]
- 72.Miller, J. F., J. J. Mekalanos, and S. Falkow. 1989. Coordinate regulation and sensory transduction in the control of bacterial virulence. Science 243:916-922. [DOI] [PubMed] [Google Scholar]
- 73.Mizel, S. B., A. P. West, and R. R. Hantgan. 2003. Identification of a sequence in human Toll-like receptor 5 required for the binding of gram-negative flagellin. J. Biol. Chem. 278:23624-23629. [DOI] [PubMed] [Google Scholar]
- 74.Morschhauser, J., G. Kohler, W. Ziebuhr, G. Blum-Oehler, U. Dobrindt, and J. Hacker. 2000. Evolution of microbial pathogens. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355:695-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moxon, E. R., and P. A. Murphy. 1978. Haemophilus influenzae bacteremia and meningitis resulting from survival of a single organism. Proc. Natl. Acad. Sci. USA 75:1534-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moxon, E. R., and C. Tang. 2000. Challenge of investigating biologically relevant functions of virulence factors in bacterial pathogens. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355:643-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moxon, E. R., and C. Wills. 1999. DNA microsatellites: agents of evolution? Sci. Am. 280:94-99. [DOI] [PubMed] [Google Scholar]
- 78.Piddock, L. J. 2006. Multidrug-resistance efflux pumps: not just for resistance. Nat. Rev. Microbiol. 4:629-636. [DOI] [PubMed] [Google Scholar]
- 79.Porwollik, S., R. M. Wong, and M. McClelland. 2002. Evolutionary genomics of Salmonella: gene acquisitions revealed by microarray analysis. Proc. Natl. Acad. Sci. USA 99:8956-8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Prouty, A. M., I. E. Brodsky, S. Falkow, and J. S. Gunn. 2004. Bile-salt-mediated induction of antimicrobial and bile resistance in Salmonella typhimurium. Microbiology 150:775-783. [DOI] [PubMed] [Google Scholar]
- 81.Prouty, A. M., I. E. Brodsky, J. Manos, R. Belas, S. Falkow, and J. S. Gunn. 2004. Transcriptional regulation of Salmonella enterica serovar Typhimurium genes by bile. FEMS Immunol. Med. Microbiol. 41:177-185. [DOI] [PubMed] [Google Scholar]
- 82.Pucciarelli, M. G., A. I. Prieto, J. Casadesus, and F. Garcia-del Portillo. 2002. Envelope instability in DNA adenine methylase mutants of Salmonella enterica. Microbiology 148:1171-1182. [DOI] [PubMed] [Google Scholar]
- 83.Rasmussen, L. J., A. Lobner-Olesen, and M. G. Marinus. 1995. Growth-rate-dependent transcription initiation from the dam P2 promoter. Gene 157:213-215. [DOI] [PubMed] [Google Scholar]
- 84.Reen, F. J., E. F. Boyd, S. Porwollik, B. P. Murphy, D. Gilroy, S. Fanning, and M. McClelland. 2005. Genomic comparisons of Salmonella enterica serovar Dublin, Agona, and Typhimurium strains recently isolated from milk filters and bovine samples from Ireland, using a Salmonella microarray. Appl. Environ. Microbiol. 71:1616-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ringquist, S., and C. L. Smith. 1992. The Escherichia coli chromosome contains specific, unmethylated dam and dcm sites. Proc. Natl. Acad. Sci. USA 89:4539-4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Robertson, J. M., N. H. McKenzie, M. Duncan, E. Allen-Vercoe, M. J. Woodward, H. J. Flint, and G. Grant. 2003. Lack of flagella disadvantages Salmonella enterica serovar Enteritidis during the early stages of infection in the rat. J. Med. Microbiol. 52:91-99. [DOI] [PubMed] [Google Scholar]
- 87.Roland, K. L., L. E. Martin, C. R. Esther, and J. K. Spitznagel. 1993. Spontaneous pmrA mutants of Salmonella typhimurium LT2 define a new two-component regulatory system with a possible role in virulence. J. Bacteriol. 175:4154-4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Salazar-Gonzalez, R. M., and S. J. McSorley. 2005. Salmonella flagellin, a microbial target of the innate and adaptive immune system. Immunol. Lett. 101:117-122. [DOI] [PubMed] [Google Scholar]
- 89.Sanderson, K. E., and B. A. D. Stocker. 1987. Salmonella typhimurium strains used in genetic analysis, p. 1220-1224. In F. C. Neidhardt, J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC.
- 90.Schmeiger, H. 1972. Phage P22-mutants with increased or decreased transduction abilities. Mol. Gen. Genet. 119:75-88. [DOI] [PubMed] [Google Scholar]
- 91.Schmitt, C. K., J. S. Ikeda, S. C. Darnell, P. R. Watson, J. Bispham, T. S. Wallis, D. L. Weinstein, E. S. Metcalf, and A. D. O'Brien. 2001. Absence of all components of the flagellar export and synthesis machinery differentially alters virulence of Salmonella enterica serovar Typhimurium in models of typhoid fever, survival in macrophages, tissue culture invasiveness, and calf enterocolitis. Infect. Immun. 69:5619-5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shtrichman, R., D. M. Heithoff, M. J. Mahan, and C. E. Samuel. 2002. Tissue selectivity of interferon-stimulated gene expression in mice infected with Dam+ versus Dam− Salmonella enterica serovar Typhimurium strains. Infect. Immun. 70:5579-5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Silverman, M., J. Zieg, M. Hilmen, and M. Simon. 1979. Phase variation in Salmonella: genetic analysis of a recombinational switch. Proc. Natl. Acad. Sci. USA 76:391-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Slauch, J. M., and T. J. Silhavy. 1991. cis-acting ompF mutations that result in OmpR-dependent constitutive expression. J. Bacteriol. 173:4039-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Smith, K. D., E. Andersen-Nissen, F. Hayashi, K. Strobe, M. A. Bergman, S. L. Barrett, B. T. Cookson, and A. Aderem. 2003. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat. Immunol. 4:1247-1253. [DOI] [PubMed] [Google Scholar]
- 96.Stewart, V., J. T. Lin, and B. L. Berg. 1991. Genetic evidence that genes fdhD and fdhE do not control synthesis of formate dehydrogenase-N in Escherichia coli K-12. J. Bacteriol. 173:4417-4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Swords, W. E., B. M. Cannon, and W. H. Benjamin, Jr. 1997. Avirulence of LT2 strains of Salmonella typhimurium results from a defective rpoS gene. Infect. Immun. 65:2451-2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takeda, K., and S. Akira. 2004. Microbial recognition by Toll-like receptors. J. Dermatol. Sci. 34:73-82. [DOI] [PubMed] [Google Scholar]
- 99.Tavazoie, S., and G. M. Church. 1998. Quantitative whole-genome analysis of DNA-protein interactions by in vivo methylase protection in Escherichia coli. Nat. Biotechnol. 16:566-571. [DOI] [PubMed] [Google Scholar]
- 100.van der Woude, M. W., and A. J. Baumler. 2004. Phase and antigenic variation in bacteria. Clin. Microbiol. Rev. 17:581-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.van Velkinburgh, J. C., and J. S. Gunn. 1999. PhoP-PhoQ-regulated loci are required for enhanced bile resistance in Salmonella spp. Infect. Immun. 67:1614-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wilmes-Riesenberg, M. R., J. W. Foster, and R. Curtiss III. 1997. An altered rpoS allele contributes to the avirulence of Salmonella typhimurium LT2. Infect. Immun. 65:203-210. [DOI] [PMC free article] [PubMed] [Google Scholar]