Abstract
The alternative sigma factor (RpoN-RpoS) pathway controls the expression of key virulence factors in Borrelia burgdorferi. However, evidence to support whether RpoN controls rpoS directly or, perhaps, indirectly via a transactivator has been lacking. Herein we provide biochemical and genetic evidence that RpoN directly controls rpoS in B. burgdorferi.
Lyme disease, caused by Borrelia burgdorferi, is the most commonly reported arthropod-borne disease in both the United States and Europe (32). At the molecular level, certain membrane lipoproteins of B. burgdorferi are vital for maintaining the spirochete in its zoonotic transmission cycle between ticks and mammals. For example, the reciprocal regulation of outer surface (lipo)proteins A (OspA) and C (OspC) is the best-studied paradigm of the dramatic alterations in protein expression patterns that ensue as the spirochete transitions from the arthropod vector into mammalian tissues (8, 22, 30, 31). Our lab determined previously that expression of OspC is regulated by the alternative sigma factor RpoS (σS) (15, 40). RpoS, though, must first be activated by another alternative transcription factor, RpoN (σ54/σN), resulting in an RpoN-RpoS regulatory network (10, 15). However, the precise mechanism(s) by which RpoN activates rpoS has not been determined. RpoN could control rpoS expression directly by binding to a region near the rpoS open reading frame (ORF). Alternatively, another transactivator induced by RpoN might activate rpoS expression.
We have been most attracted to the notion that RpoN binds directly to a region upstream of the rpoS ORF to facilitate RpoS expression in B. burgdorferi. This is because nucleotides −78 to −63 upstream of the rpoS ORF comprise a theoretical, RpoN-dependent consensus −24/−12 promoter (33, 34). Many studies with various bacteria have indicated close contact of RpoN with such a −24/−12 promoter region (5, 6, 21, 33). However, as yet, there have been no experimental data to substantiate this prediction for B. burgdorferi. The purpose of this study was to garner additional evidence that would either substantiate or refute the hypothesis that rpoS is regulated directly by RpoN.
Identification of rpoS initiation of transcription.
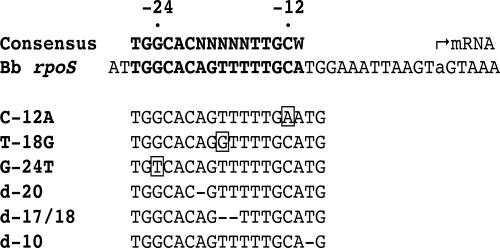
Determination of the initiation of transcription for a given gene can provide strategic information for identifying a gene's nearby promoter. rpoS transcripts of B. burgdorferi BbAH130 (41) were reverse transcribed in BD SMART-RACE (switching mechanism at 5′ end of RNA transcript-rapid amplification of cDNA ends) reactions (BD Biosciences, San Jose, CA) using two rpoS-specific primers (rpoSR422 and rpoSR232) (Table 1), which are 422 and 232 bases, respectively, downstream of the rpoS translation start site. The BD SMART IIA primer, which anneals to the BD SMART IIA oligonucleotide linked to the 3′ end of the first-strand cDNA, and an rpoS-specific primer upstream of the gene-specific primers used in first-strand cDNA synthesis (rpoSR125) (Table 1) were used to amplify the resulting cDNAs. PCR-amplified inserts cloned into the pGEM-T Easy vector (Promega, Madison, WI) were digested with the restriction endonuclease DraI (New England Biolabs, Ipswich, MA) to verify that they represented rpoS. Seventeen of 25 clones contained rpoS-specific sequences, as determined by the DraI restriction pattern (not shown). Eight of these 17 clones were selected for further analysis. For five of these eight clones, residue −50 upstream of the rpoS ORF represented the transcript start site by RACE analysis, whereas there was no consensus residue for the other three sequences. The identified transcription start site was 14 nucleotides downstream of the conserved GC dinucleotide (Fig. 1). This result correlates well with the putative −24/−12 region representing the promoter for rpoS, inasmuch as initiation of transcription from a −24/−12 promoter occurs between 8 and 21 nucleotides downstream of the GC dinucleotide (2).
TABLE 1.
Oligonucleotides used in this study
| Name | Sequence (5′-3′) | Experiment(s) |
|---|---|---|
| rpoSR422 | TTGGGCGATTTTTCTTCTTC | Transcript initiation |
| rpoSR232 | ATCCCAAGTTGCCTTCTTCA | Transcript initiation, qRT-PCR |
| rpoSR125 | TTTGCTTTTGCATTGCCTCT | Transcript initiation |
| EMSA_rpoS1a | ACTTAATTTCCATGCAAAAACTGTGCCAATTTAATTTAAA | EMSA |
| EMSA_rpoS2a | TTTAAATTAAATTGGCACAGTTTTTGCATGGAAATTAAGT | EMSA |
| rpoS promoterFa | CCTATTTAGTTTAAAACCATTTTTAAATTAAATTGGCACAGTTTTTGCATGGAAATTAAGTAG | EMSA |
| rpoS promoterRa | CTACTTAATTTCCATGCAAAAACTGTGCCAATTTAATTTAAAAATGGTTTTAAACTAAATAGG | EMSA |
| rpoS_C-12Aa | CCTATTTAGTTTAAAACCATTTTTAAATTAAATTGGCACAGTTTTTGAATGGAAATTAAGTAG | Promoter mutants, EMSA |
| rpos_G-12Ta | CTACTTAATTTCCATTCAAAAACTGTGCCAATTTAATTTAAAAATGGTTTTAAACTAAATAGG | Promoter mutants, EMSA |
| rpoS_T-18Ga | CCTATTTAGTTTAAAACCATTTTTAAATTAAATTGGCACAGGTTTTGCATGGAAATTAAGTAG | EMSA |
| rpoS_A-18Ca | CTACTTAATTTCCATGCAAAACCTGTGCCAATTTAATTTAAAAATGGTTTTAAACTAAATAGG | EMSA |
| rpoS_G-24Ta | CCTATTTAGTTTAAAACCATTTTTAAATTAAATTGTCACAGTTTTTGCATGGAAATTAAGTAG | Promoter mutants, EMSA |
| rpoS_C-24Aa | CTACTTAATTTCCATGCAAAAACTGTGACAATTTAATTTAAAAATGGTTTTAAACTAAATAGG | Promoter mutants, EMSA |
| rpoS_d-20Fa | CTACTTAATTTCCATGCAAAAAC-GTGCCAATTTAATTTAAAAATGGTTTTAAACTAAATAGG | Promoter mutants, EMSA |
| rpoS_d-20Ra | CCTATTTAGTTTAAAACCATTTTTAAATTAAATTGGCAC-GTTTTTGCATGGAAATTAAGTAG | Promoter mutants, EMSA |
| rpoS_d-17/18Fa | CTACTTAATTTCCATGCAAA-CTGTGCCAATTTAATTTAAAAATGGTTTTAAACTAAATAGG | Promoter mutants, EMSA |
| rpoS_d-17/18Ra | CCTATTTAGTTTAAAACCATTTTTAAATTAAATTGGCACAG-TTTGCATGGAAATTAAGTAG | Promoter mutants, EMSA |
| rpoS_d-10Fa | CTACTTAATTTCC-TGCAAAAACTGTGCCAATTTAATTTAAAAATGGTTTTAAACTAAATAGG | EMSA |
| rpoS_d-10Ra | CCTATTTAGTTTAAAACCATTTTTAAATTAAATTGGCACAGTTTTTGCA-GGAAATTAAGTAG | EMSA |
| flgB promoterFb | AAAATACAAATTAACGAACAAAAAGATTTGAATTATTATATAATTTAGTTAAAAGCATTTTAA | EMSA |
| flgB promoterRb | TTAAAATGCTTTTAACTAAATTATATAATAATTCAAATCTTTTTGTTCGTTAATTTGTATTTT | EMSA |
| rpoSF30 | CATATATTTAAAATCAGTAAGAGAACACAAGCTAATTACTCACGAAGAAG | qRT-PCR |
| flaBF9 | CAATCATAATACATCAGCTATTAATGCTTCAAG | qRT-PCR |
| flaBR82 | CTTGAGTTTTACTAAGATTAGCAGCGTTAATG | qRT-PCR |
The putative −24/−12 region of the rpoS promoter is underlined, and nucleotides deleted are shown with dashes.
The −35 and −10 promoter sequences are underlined.
FIG. 1.
B. burgdorferi (Bb) rpoS promoter region. The top line indicates the consensus −24/−12 RpoN-binding site (34), which is highlighted in bold in the rpoS promoter region beneath it. The lowercase letter “a” indicates the transcript start site (mRNA) experimentally determined by 5′-RACE analysis in this study. The relevant sequence changes in double-stranded oligonucleotides designed for this study are shown below the consensus region. The boxes highlight base substitutions, whereas the dashes identify base deletions.
Assessing RpoN binding to the rpoS promoter.
Ideally, direct binding of the B. burgdorferi RpoN protein to the rpoS promoter sequence would provide the most compelling evidence that the rpoS promoter in B. burgdorferi is RpoN dependent. However, numerous attempts to produce soluble recombinant forms of B. burgdorferi RpoN were unsuccessful. This was true for either the full-length protein or a truncated recombinant that preserved the predicted DNA-binding domain. We also employed lysates generated from B. burgdorferi cultures, grown under conditions in which RpoN is active (15, 41), in electrophoretic mobility shift assays (EMSAs). We were unable to demonstrate an interaction of rpoS sequences with borrelial cell lysates in EMSAs.
Previous studies have shown functional homology between RpoN proteins from different bacterial species. For example, Escherichia coli RpoN recognizes Caulobacter crescentus flbG and flaN promoters in an E. coli cell-free transcription system (25), and Klebsiella pneumoniae RpoN recognizes the nifH promoter sequence of Rhizobium meliloti (4). For this reason, as an alternative heterologous approach, we performed experiments to determine whether E. coli RpoN was capable of binding to the B. burgdorferi rpoS promoter region.
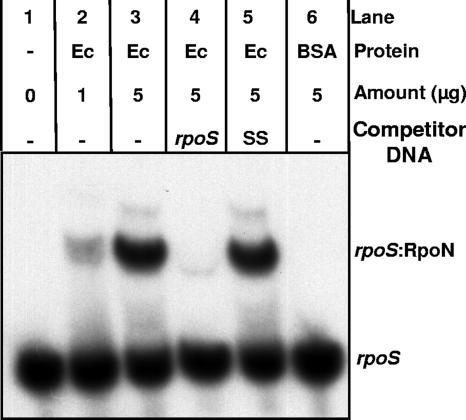
A 40-bp oligonucleotide encoding the predicted −24/−12 region of B. burgdorferi rpoS was end labeled with 32P and mixed with graded amounts of purified recombinant E. coli RpoN (16) in binding buffer (19). Resulting DNA-protein complexes were resolved by electrophoresis on 4.5% polyacrylamide and visualized by autoradiography. These EMSAs (Fig. 2) showed that E. coli RpoN bound to the rpoS target in a dose-dependent fashion. In addition, unlabeled rpoS promoter DNA could compete for binding, but sheared salmon sperm DNA had no competing effect. No shift was observed when bovine serum albumin was added as a nonbinding protein in place of E. coli RpoN. These data constitute strong evidence that the rpoS promoter region contains a binding site for RpoN.
FIG. 2.
EMSAs demonstrating binding of E. coli RpoN to the B. burgdorferi rpoS promoter region (40-bp oligonucleotide). Lanes 1, 2, and 3 represent increasing amounts (μg) of E. coli RpoN protein (Ec); lane 4 contains a 50-fold excess of unlabeled double-stranded oligonucleotide (rpoS); lane 5 contains a 50-fold excess of sheared salmon sperm DNA (SS); and lane 6 contains 5 μg of bovine serum albumin (BSA) in place of E. coli RpoN.
Site-directed mutagenesis of key residues in the proposed RpoN-binding site upstream of rpoS.
The −24 (GG region) and −12 (GC region) regions are highly conserved among RpoN-dependent promoters (2); removal of these dinucleotides in C. crescentus, for example, eliminates transcription of flbG and flaN (23). Promoter function is also essentially abolished by deleting one or more nucleotides between the −24/−12 regions of the 4521 gene in Myxococcus xanthus (18), nifH in K. pneumoniae (3), and flbG and flaN in C. crescentus (23, 24). The preponderance of information thus indicates that there is a stringent requirement for these motifs to be located on the same face of the DNA helix (2).
Double-stranded oligonucleotides with single-base-pair substitutions (C-12A and G-24T) or 1 (d-20)- or 2 (d-17/18)-bp deletions (Fig. 1 and Table 1) in the rpoS promoter region were used as competing DNAs in EMSAs. A mutation downstream of the −24/−12 region (d-10), which theoretically should have no effect on RpoN binding, and a base change in a less-conserved area of the −24/−12 region (T-18G) were also included. A 63-bp oligonucleotide carrying the predicted rpoS promoter was 3′ digoxigenin-11-dUTP labeled (DIG gel shift kit, second generation; Roche Applied Science, Mannheim, Germany) and incubated with a lysate from RpoN-overproducing E. coli LCA7 (1). When quantitation was desired, X-ray film was exposed for various times, and relative units of chemiluminescence were determined using a Gel Logic 200 imaging system (Kodak Scientific Imaging Systems, New Haven, CT).
Incubation of a cell lysate with labeled double-stranded DNA (dsDNA) and resolution by EMSA resulted in two shifted bands, both of which were supershifted when a monoclonal anti-σ54 antibody (W0005; NeoClone Biotechnology International, Madison, WI) was added to the reaction mixture (not shown). The observed supershifts confirmed that both bands represented E. coli RpoN bound to the labeled dsDNA. It is known that the DNA-bound holoenzyme has reduced mobility compared to the RpoN-DNA complex (4). Therefore, the two bands visible in the gel are a reflection of RpoN bound to the DNA with (upper band) and without (lower band) the core RNA polymerase.
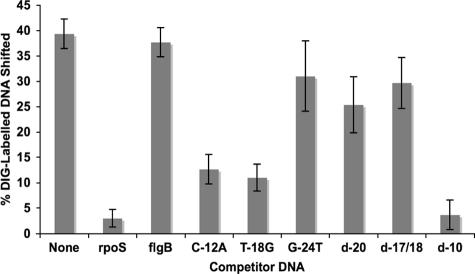
A 125-fold excess of unlabeled dsDNA corresponding to the σ70 promoter from B. burgdorferi flgB (13) could not compete with the labeled rpoS dsDNA for RpoN binding (Fig. 3), confirming that binding of E. coli RpoN was sequence dependent. In addition, a 125-fold excess of dsDNA containing deletions (d-17/18 and d-20) or the G-24T base pair substitution could not compete for E. coli RpoN binding (Fig. 3). However, there was a reduction of labeled RpoN-bound DNA that shifted when a 125-fold excess of the dsDNA containing the C-12A or T-18G base pair substitution was added as competitor DNA. These reductions, however, were substantially less than those when the unlabeled rpoS promoter was the competing dsDNA (Fig. 3). As expected, a deletion outside the −24/−12 region (d-10) could readily compete with the rpoS promoter double-stranded oligonucleotide. The combined competitive EMSA results indicate that E. coli RpoN has a greater affinity for the B. burgdorferi rpoS −24/−12 region than for dsDNA containing a C-12A or T-18G substitution and has little to no affinity for dsDNA with the G-24T substitution or for sequences containing deletions in the −24/−12 region.
FIG. 3.
Quantitative competitive EMSAs demonstrating that the affinity of E. coli RpoN for dsDNA is sequence dependent. Proportions of dsDNA shifted by the binding of E. coli RpoN to the labeled rpoS promoter are shown for the absence and presence of a 125-fold excess of unlabeled competitor dsDNA (n = 3). Competitor dsDNAs consisted of the unlabeled rpoS promoter, a σ70-specific flgB promoter, and rpoS promoters with the base pair substitutions and deletions noted. EMSAs were performed with a 63-bp digoxigenin (DIG)-labeled target of the rpoS promoter mixed with a lysate from a strain of E. coli that overexpresses E. coli RpoN.
As in other RpoN systems (3, 18, 23, 24), there seems to be a requirement for the −24 and −12 regions to be on the same face of the DNA helix for recognition, as deleting one or two nucleotides impeded RpoN binding. The −24 region of the glnAP2 promoter is more important than the −12 region for recognition and binding by E. coli RpoN (39); this was corroborated in our study in that a dsDNA with a base substitution at position −24 could not compete with the borrelial rpoS dsDNA for RpoN binding. A dsDNA with a base pair substitution in a less-conserved residue (T-18G) could compete somewhat with the wild-type promoter, but the binding affinity was markedly lower. The affinity was similar to that for the C-12A base pair substitution, which is a key conserved residue. The −12 element not only contributes to binding affinity, although it is of secondary importance relative to the −24 region, but also determines the level of basal transcription (14, 36, 37). Substitutions of the −12 C nucleotide result in deregulated transcription in vitro (37), although these substitutions do not result in stronger transcription under activating conditions in vivo (36). RpoN binds to DNA in the absence of RNA polymerase, but the holoenzyme binds tighter than RpoN alone, except to early melted DNA, where both bind equally well (7, 12, 38). The spatial relationship between RpoN, the core RNA polymerase, and the −12 promoter element changes when the closed promoter complex becomes an open one (6). The weaker base pairing that results from the C-12A substitution may cause the DNA conformation to be more similar to that of the early melted DNA, thereby still allowing recognition even though there is a base pair change in a conserved region of the −24/−12 promoter.
Mutations in the predicted RpoN-dependent promoter influence expression of RpoS and OspC.
The results of quantitative competitive EMSAs showed that double-stranded oligonucleotides containing mutations in the putative RpoN-binding site upstream of rpoS were partially (C-12A and T-18G) or almost totally (G-24T, d-20, and d-17/18) impeded in the ability to compete with the wild-type promoter sequence for binding by E. coli RpoN. By extrapolation, these same mutations should reduce or prevent RpoN-dependent expression of rpoS in B. burgdorferi. As an initial investigation of this possibility, the mutations in the key conserved residues (C-12A, G-24T, d-20, and d-17/18) were introduced by site-directed mutagenesis (QuikChange II site-directed mutagenesis kit; Stratagene, La Jolla, CA) of plasmid pXY240. Plasmid pXY240 contains a 4.2-kb region of B. burgdorferi strain 297 DNA, including 1.9 kb upstream and 1.5 kb downstream of rpoS, cloned into the shuttle vector pJD7, derived from pKFSS1 (11). The erythromycin-resistant, RpoS-deficient mutant B. burgdorferi BbAH206 (15) was made electrocompetent and transformed with sequence-verified plasmids (28) containing either a wild-type or mutated rpoS promoter. Three transformants for each plasmid, selected by streptomycin resistance, were chosen for further studies. Desired plasmid sequences in B. burgdorferi transformants were confirmed by specific digestion patterns obtained after treatment with pertinent restriction enzymes.
Cultures of transformants were grown at 34°C in complete Barbour-Stoenner-Kelly (BSK-H) medium (Sigma-Aldrich, St. Louis, MO) at pH 6.8 to a high cell density (>1 × 108 bacteria/ml). Complementation of rpoS in the RpoS-deficient mutant was determined at the protein level by immunoblot detection of RpoS and OspC, which is under the control of RpoS (9, 15, 42), utilizing the SuperSignal West Pico chemiluminescent substrate (Pierce Biotechnology, Inc., Rockford, IL). To confirm that the numbers of spirochetes loaded into the gel lanes were substantially equivalent, immunoblotting for the flagellar protein FlaB was performed. RpoS was detected with rat polyclonal antibodies, generated according to previously published protocols (20). Chicken immunoglobulin Y anti-FlaB antibody was a gift from Kayla Hagman (University of Texas Southwestern Medical Center, Dallas). A monoclonal antibody directed against OspC, 1B2-105, was generated by immunizing BALB/c mice with the corresponding fusion proteins according to previously published protocols (29). The wild-type B. burgdorferi strain BbAH130 and an RpoN-deficient mutant, BbJSB18-B2, were included as positive and negative controls, respectively. The 297M3 rpoN::aadA insertion mutant, BbJSB18-2, was generated as previously described (15), except that the erythromycin resistance marker (ermC) in the mutagenesis construct pALH394 was exchanged for a cassette conferring resistance to streptomycin (aadA) (11).
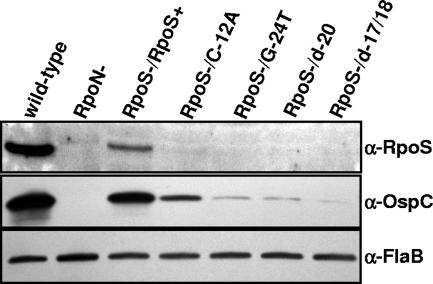
In immunoblots, RpoS and OspC were not detected in RpoS-deficient (not shown) or RpoN-deficient (Fig. 4) mutants of B. burgdorferi, as expected (15). In the rpoS-complemented RpoS-deficient strain, RpoS expression was restored, albeit to a lesser degree than the level of wild-type expression (Fig. 4). RpoS was below chemiluminescence immunoblot detection limits in all promoter mutant complements. Given its low abundance, RpoS is difficult to detect even by sensitive chemiluminescence immunoblotting. However, OspC is highly abundant when induced in B. burgdorferi strain 297. OspC expression was thus detectable in the promoter mutant complements (Fig. 4), and the relative levels of OspC expression in the promoter mutant complements corresponded to the relative binding affinities of E. coli RpoN (Fig. 3). Elevated levels of OspC were present in the C-12A promoter complement compared to those in the G-24T and deletion mutants (d-17/18 and d-20), which had little to no binding affinity for E. coli RpoN. In other words, in the three cases where the double-stranded oligonucleotide mutations G-24T, d-20, and d-17/18 failed to compete with the native rpoS sequence (i.e., those mutations that adversely affected RpoN binding) (Fig. 3), the cognate B. burgdorferi complemented stains barely expressed OspC, if at all (Fig. 4). Similarly the C-12A mutation, which only partially competed with native rpoS in EMSAs (Fig. 3), allowed more robust OspC production in the borrelial complemented strain (Fig. 4).
FIG. 4.
Mutations in the rpoS −24/−12 region influence the rescue of OspC expression in an RpoS-deficient background. Whole-cell lysates from B. burgdorferi grown in BSK-H medium at pH 6.8 to a high density (>1 × 108 bacteria/ml) were loaded at ∼5 × 107 cells per well for chemiluminescence immunoblot detection of FlaB and OspC. About 108 cells per well were loaded for the detection of RpoS. Immunoblots of an RpoN-deficient strain (RpoN−) and an RpoS-deficient strain (RpoS−) complemented with the wild-type rpoS promoter or promoter mutants are depicted.
It has been reported that cultivation of B. burgdorferi in the presence of whole blood can induce the upregulation of a number of genes, including ospC and other genes governed by the RpoN-RpoS pathway (35). Therefore, B. burgdorferi strains were cultivated in BSK-H medium with 6% whole blood as described previously (35), except that fresh heparinized rabbit blood with the buffy coat removed was used as an alternative to citrated human blood. Under these cultivation conditions, RpoS protein levels were below the limits of chemiluminescence immunoblot detection in the rpoS-complemented strain (not shown). OspC protein was detected in the rpoS-complemented strain, but OspC protein was barely detectable, if at all, in the promoter mutant-complemented strains (not shown).
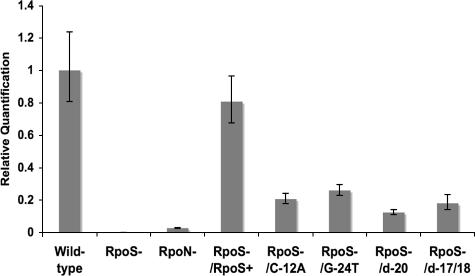
Because RpoS was not detectable in immunoblots of complemented strains, further assessment of rpoS transcription was accomplished by quantifying rpoS transcripts with SYBR green one-step quantitative reverse transcription-PCR (qRT-PCR), using the flaB gene as an internal control (Applied Biosystems 7500 real-time PCR system). qRT-PCR confirmed that rpoS transcription was restored in an rpoS-complemented strain to about 80% of the wild-type level (Fig. 5). For the promoter mutants, rpoS transcription was reduced to 12 to 26% of the wild-type level, with no significant differences between them. This level of induction is slightly higher than the levels measured by galactosidase activity for similar mutations in an M. xanthus RpoN-dependent promoter (18), which were just below 10% of wild-type activity. The RpoN-deficient strain exhibited rpoS transcript levels with <10% of wild-type activity. Based on many studies (3, 18, 23, 24) and our EMSA results, one might predict that rpoS transcription would be abolished, especially in the deletion mutants. However, if there is excess RpoN present in the cell, even low-affinity promoters may be significantly occupied in vivo prior to activation. For example, in E. coli there is a larger number of RpoN molecules in the cell (close to 100) than the number of promoters (fewer than 20) (5, 17), and a similar ratio of RpoN molecules to promoters may be present in B. burgdorferi.
FIG. 5.
Complementation of an RpoS-deficient strain with plasmids containing mutations in key residues of the rpoS −24/−12 region influences transcript levels of rpoS. B. burgdorferi cells were harvested at ∼1 × 108 bacteria/ml from BSK-H medium containing 6% rabbit blood, and rpoS transcript levels relative to flaB (flagellar protein) levels were determined by qRT-PCR. Results for RpoN-deficient (RpoN−) and RpoS-deficient (RpoS−) strains and for the RpoS-deficient strain complemented with the wild-type rpoS promoter or promoter mutant are presented compared to wild-type rpoS expression (n = 3, except for d-17/18, where n = 2).
Summary and implications.
It is now generally accepted that the alternative sigma factor regulatory network, the RpoN-RpoS pathway (10, 15), controls virulence factor expression in B. burgdorferi. However, evidence has been lacking as to whether RpoN controls rpoS expression directly or possibly via a transactivator induced by RpoN. The transcription start site of rpoS was determined to be 14 nucleotides downstream of the conserved GC element of a putative RpoN-dependent −24/−12 promoter. EMSAs indicated that E. coli RpoN affinity was higher for a wild-type B. burgdorferi rpoS promoter sequence than for rpoS promoter sequences in which essential bases were replaced or deleted. Complementation of an RpoS-deficient strain of B. burgdorferi indicated that mutation of key conserved residues in the −24/−12 region reduced rpoS transcription and protein expression as well as the expression of OspC, which is controlled by an RpoS-dependent promoter (9, 15, 42). These results provide compelling evidence that RpoN directly controls rpoS expression in B. burgdorferi by binding to the −24/−12 sequence upstream of the rpoS ORF. In addition, RpoN-mediated transcription is a tightly controlled process, inasmuch as the promoter-bound RpoN holoenzyme remains inactive until it is activated by an enhancer-binding protein (26, 27, 38). The response regulator Rrp2, the only enhancer-binding protein yet identified in B. burgdorferi, is essential for activation of the RpoN-RpoS regulatory network (41). Therefore, we also predict that there is a requisite Rrp2 enhancer-binding site in the vicinity of the B. burgdorferi rpoS gene.
Acknowledgments
We thank Larry Reitzer and Farol Tomson for constructive criticism of the manuscript.
This work was supported by grant AI-59602 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health. J.S.B. was supported by National Institutes of Health training grant T32-AI07520 and a Ruth L. Kirschstein National Research Service Award (F32-AI058487) from the National Institutes of Health.
Footnotes
Published ahead of print on 8 December 2006.
REFERENCES
- 1.Anthony, L. C., K. M. Foley, N. E. Thompson, and R. R. Burgess. 2003. Expression, purification of, and monoclonal antibodies to σ factors from Escherichia coli. Methods Enzymol. 370:181-192. [DOI] [PubMed] [Google Scholar]
- 2.Barrios, H., B. Valderrama, and E. Morett. 1999. Compilation and analysis of σ54-dependent promoter sequences. Nucleic Acids Res. 27:4305-4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buck, M. 1986. Deletion analysis of the Klebsiella pneumoniae nitrogenase promoter: importance of spacing between conserved sequences around positions −12 and −24 for activation by the nifA and ntrC (glnG) products. J. Bacteriol. 166:545-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buck, M., and W. Cannon. 1992. Specific binding of the transcription factor σ54 to promoter DNA. Nature 358:422-424. [DOI] [PubMed] [Google Scholar]
- 5.Buck, M., M. T. Gallegos, D. J. Studholme, Y. Guo, and J. D. Gralla. 2000. The bacterial enhancer-dependent σ54 (σN) transcription factor. J. Bacteriol. 182:4129-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burrows, P. C., K. Severinov, A. Ishihama, M. Buck, and S. R. Wigneshweraraj. 2003. Mapping σ54-RNA polymerase interactions at the −24 consensus promoter element. J. Biol. Chem. 278:29728-29743. [DOI] [PubMed] [Google Scholar]
- 7.Cannon, W., M. T. Gallegos, and M. Buck. 2001. DNA melting within a binary σ54-promoter DNA complex. J. Biol. Chem. 276:386-394. [DOI] [PubMed] [Google Scholar]
- 8.de Silva, A. M., S. R. Telford III, L. R. Brunet, S. W. Barthold, and E. Fikrig. 1996. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J. Exp. Med. 183:271-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eggers, C. H., M. J. Caimano, and J. D. Radolf. 2004. Analysis of promoter elements involved in the transcriptional initiation of RpoS-dependent Borrelia burgdorferi genes. J. Bacteriol. 186:7390-7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher, M. A., D. Grimm, A. K. Henion, A. F. Elias, P. E. Stewart, P. A. Rosa, and F. C. Gherardini. 2005. Borrelia burgdorferi σ54 is required for mammalian infection and vector transmission but not for tick colonization. Proc. Natl. Acad. Sci. USA 102:5162-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank, K. L., S. F. Bundle, M. E. Kresge, C. H. Eggers, and D. S. Samuels. 2003. aadA confers streptomycin resistance in Borrelia burgdorferi. J. Bacteriol. 185:6723-6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallegos, M. T., and M. Buck. 2000. Sequences in σ54 region I required for binding to early melted DNA and their involvement in sigma-DNA isomerisation. J. Mol. Biol. 297:849-859. [DOI] [PubMed] [Google Scholar]
- 13.Ge, Y., I. G. Old, I. Saint Girons, and N. W. Charon. 1997. Molecular characterization of a large Borrelia burgdorferi motility operon which is initiated by a consensus σ70 promoter. J. Bacteriol. 179:2289-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo, Y., C. M. Lew, and J. D. Gralla. 2000. Promoter opening by σ54 and σ70 RNA polymerases: sigma factor-directed alterations in the mechanism and tightness of control. Genes Dev. 14:2242-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hübner, A., X. Yang, D. M. Nolen, T. G. Popova, F. C. Cabello, and M. V. Norgard. 2001. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc. Natl. Acad. Sci. USA 98:12724-12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunt, T. P., and B. Magasanik. 1985. Transcription of glnA by purified Escherichia coli components: core RNA polymerase and the products of glnF, glnG, and glnL. Proc. Natl. Acad. Sci. USA 82:8453-8457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jishage, M., A. Iwata, S. Ueda, and A. Ishihama. 1996. Regulation of RNA polymerase sigma subunit synthesis in Escherichia coli: intracellular levels of four species of sigma subunit under various growth conditions. J. Bacteriol. 178:5447-5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keseler, I. M., and D. Kaiser. 1995. An early A-signal-dependent gene in Myxococcus xanthus has a σ54-like promoter. J. Bacteriol. 177:4638-4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knight, S. W., and D. S. Samuels. 1999. Natural synthesis of a DNA-binding protein from the C-terminal domain of DNA gyrase A in Borrelia burgdorferi. EMBO J. 18:4875-4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lahdenne, P., S. F. Porcella, K. E. Hagman, D. R. Akins, T. G. Popova, D. L. Cox, L. I. Katona, J. D. Radolf, and M. V. Norgard. 1997. Molecular characterization of a 6.6-kilodalton Borrelia burgdorferi outer membrane-associated lipoprotein (lp6.6) which appears to be downregulated during mammalian infection. Infect. Immun. 65:412-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merrick, M. J. 1993. In a class of its own—the RNA polymerase sigma factor σ54 (σN). Mol. Microbiol. 10:903-909. [DOI] [PubMed] [Google Scholar]
- 22.Montgomery, R. R., S. E. Malawista, K. J. Feen, and L. K. Bockenstedt. 1996. Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J. Exp. Med. 183:261-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mullin, D. A., and A. Newton. 1989. Ntr-like promoters and upstream regulatory sequence ftr are required for transcription of a developmentally regulated Caulobacter crescentus flagellar gene. J. Bacteriol. 171:3218-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mullin, D. A., and A. Newton. 1993. A σ54 promoter and downstream sequence elements ftr2 and ftr3 are required for regulated expression of divergent transcription units flaN and flbG in Caulobacter crescentus. J. Bacteriol. 175:2067-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ninfa, A. J., D. A. Mullin, G. Ramakrishnan, and A. Newton. 1989. Escherichia coli σ54 RNA polymerase recognizes Caulobacter crescentus flbG and flaN flagellar gene promoters in vitro. J. Bacteriol. 171:383-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Popham, D. L., D. Szeto, J. Keener, and S. Kustu. 1989. Function of a bacterial activator protein that binds to transcriptional enhancers. Science 243:629-635. [DOI] [PubMed] [Google Scholar]
- 27.Reitzer, L. J., and B. Magasanik. 1986. Transcription of glnA in E. coli is stimulated by activator bound to sites far from the promoter. Cell 45:785-792. [DOI] [PubMed] [Google Scholar]
- 28.Revel, A. T., J. S. Blevins, C. Almazan, L. Neil, K. M. Kocan, J. de la Fuente, K. E. Hagman, and M. V. Norgard. 2005. bptA (bbe16) is essential for the persistence of the Lyme disease spirochete, Borrelia burgdorferi, in its natural tick vector. Proc. Natl. Acad. Sci. USA 102:6972-6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robertson, S. M., J. R. Kettman, J. N. Miller, and M. V. Norgard. 1982. Murine monoclonal antibodies specific for virulent Treponema pallidum (Nichols). Infect. Immun. 36:1076-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwan, T. G., and J. Piesman. 2000. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 38:382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steere, A. C., J. Coburn, and L. Glickstein. 2004. The emergence of Lyme disease. J. Clin. Investig. 113:1093-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Studholme, D. J., and M. Buck. 2000. The biology of enhancer-dependent transcriptional regulation in bacteria: insights from genome sequences. FEMS Microbiol. Lett. 186:1-9. [DOI] [PubMed] [Google Scholar]
- 34.Studholme, D. J., and M. Buck. 2000. Novel roles of σN in small genomes. Microbiology 146:4-5. [DOI] [PubMed] [Google Scholar]
- 35.Tokarz, R., J. M. Anderton, L. I. Katona, and J. L. Benach. 2004. Combined effects of blood and temperature shift on Borrelia burgdorferi gene expression as determined by whole genome DNA array. Infect. Immun. 72:5419-5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang, L., and J. D. Gralla. 1998. Multiple in vivo roles for the −12-region elements of σ54 promoters. J. Bacteriol. 180:5626-5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang, L., Y. Guo, and J. D. Gralla. 1999. Regulation of σ54-dependent transcription by core promoter sequences: role of −12 region nucleotides. J. Bacteriol. 181:7558-7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wedel, A., and S. Kustu. 1995. The bacterial enhancer-binding protein NTRC is a molecular machine: ATP hydrolysis is coupled to transcriptional activation. Genes Dev. 9:2042-2052. [DOI] [PubMed] [Google Scholar]
- 39.Wong, C., Y. Tintut, and J. D. Gralla. 1994. The domain structure of σ54 as determined by analysis of a set of deletion mutants. J. Mol. Biol. 236:81-90. [DOI] [PubMed] [Google Scholar]
- 40.Yang, X., M. S. Goldberg, T. G. Popova, G. B. Schoeler, S. K. Wikel, K. E. Hagman, and M. V. Norgard. 2000. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol. Microbiol. 37:1470-1479. [DOI] [PubMed] [Google Scholar]
- 41.Yang, X. F., S. M. Alani, and M. V. Norgard. 2003. The response regulator Rrp2 is essential for the expression of major membrane lipoproteins in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 100:11001-11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang, X. F., M. C. Lybecker, U. Pal, S. M. Alani, J. Blevins, A. T. Revel, D. S. Samuels, and M. V. Norgard. 2005. Analysis of the ospC regulatory element controlled by the RpoN-RpoS regulatory pathway in Borrelia burgdorferi. J. Bacteriol. 187:4822-4829. [DOI] [PMC free article] [PubMed] [Google Scholar]