Abstract
The Bordetella BvgAS virulence control system is prototypical of phosphorelays that use a polydomain sensor and a response regulator to control gene expression in response to environmental cues. BvgAS controls the expression of at least three distinct phenotypic phases (Bvg−, Bvgi, and Bvg+) by differentially regulating the expression of at least four classes of genes. Among the loci regulated by BvgAS is bvgAS itself. We investigated the role of autoregulation in the ability of BvgAS to control multiple gene expression patterns in a temporal and steady-state manner by constructing Bordetella bronchiseptica strains in which the bvgAS promoter was replaced with constitutively active promoters. Our results show that positive autoregulation of bvgAS transcription is required for the temporal expression of multiple phenotypic phases that occurs in response to a shift from Bvg−-phase conditions to Bvg+-phase conditions. Autoregulation was also shown to contribute to steady-state regulation; it influences the sensitivity of the system in response to subtle differences in signal intensity. In addition, considered in relation to BvgA and BvgS activities demonstrated in vitro, our results provide insight into how BvgA and BvgS function mechanistically.
Like all organisms, bacteria must be able to sense their environment and control their behavior appropriately to survive. They must also be able to change their behavior in a timely manner when they move from one environment to another or when the environment around them changes. Behavioral changes in bacteria usually require changes in gene expression, and therefore the transition from one behavior to another is neither instantaneous nor abrupt (i.e., without transient expression of alternate or intermediate behaviors). The sequence of events that occurs during adaptation from one set of environmental conditions to another can be considered temporal regulation. Once adaptation to the new environment occurs, the gene expression profile will, in general, remain constant as long as conditions remain constant. The ability to maintain a specific and appropriate gene expression profile depending on specific environmental conditions can be considered environmental or steady-state regulation. Although temporal and steady-state regulation probably occurs continuously and simultaneously in nature, there are circumstances in which temporal regulation may play a particularly important role. For example, the initial entry of a bacterium into a host or into a specific environment within a host may trigger a temporally defined program of gene expression that facilitates the ability of the bacterium to survive in that new environment and/or that temporarily prevents the bacterium from committing fully to the new environment so that it can readapt quickly if it is expelled back into the former environment. The inability to alter host environments in a controlled way precludes studying these processes in vivo. Laboratory media and growth conditions, however, can be controlled easily and precisely, allowing temporal and steady-state regulation to be separated and studied independently.
The most common mechanism used by bacteria to sense and respond to environmental conditions is the two-component regulatory system (TCS) (see reference 42 for a review). A subset of the TCS family, phosphorelays, uses a four-step His-Asp-His-Asp phosphorylation-phosphotransfer mechanism to convert environmental signals into behavioral changes, and a prototypical member of the subfamily of phosphorelays that contain polydomain sensors is BvgAS (see reference 10 for a review). BvgS, the sensor, and BvgA, the response regulator, control the expression of over 250 genes, including those encoding all known protein virulence factors, in Bordetella spp. (14). The Bordetella genus includes Bordetella pertussis, the causative agent of human whooping cough, and Bordetella bronchiseptica, which causes respiratory infections in a broad range of mammals (9, 11).
A variety of genetic and biochemical analyses have shown that Bvg-regulated genes fall into (at least) four classes. Class 1 genes include those encoding toxins, such as cyaA-E, which encode adenylyl cyclase, and ptxA-E, which encode pertussis toxin. Class 1 gene promoters contain low-affinity BvgA binding sites far upstream of the transcription start site, and relatively high levels of BvgA-phosphate (BvgA∼P) are required to activate their transcription (6, 21, 27, 41, 52). Class 2 genes include those encoding adhesins, such as fhaB, which encodes filamentous hemagglutinin (17, 24, 34). Class 2 gene promoters contain high-affinity BvgA binding sites close to the transcription start site, and low levels of BvgA∼P are sufficient to activate their transcription (5, 7, 8). The only class 3 gene characterized so far is bipA, whose product bears amino acid sequence similarity to intimins of enteropathogenic and enterohemorrhagic Escherichia coli and invasins of Yersinia species (43). The bipA promoter contains high-affinity BvgA binding sites just upstream of the transcription start site and low-affinity BvgA binding sites downstream of the transcription start site (15, 50). bipA transcription is activated by low levels of BvgA∼P and repressed by high levels of BvgA∼P (15, 50). Class 4 genes are repressed by BvgAS. Although no class 4 gene promoter has been characterized with regard to BvgA binding, the frlAB promoter contains discernible BvgA binding sites overlapping the transcription start site (1), suggesting that relatively low levels of BvgA∼P are sufficient to repress frlAB transcription. frlAB encodes orthologs of E. coli FlhDC and controls the expression of flagellum, motility, and chemotaxis genes in B. bronchiseptica (2). Together, these in vitro studies suggest that by controlling the intracellular concentration of BvgA∼P, BvgAS can control three distinct gene expression profiles: when BvgA∼P levels are at or near zero, class 4 gene expression will be maximal and class 1, 2, and 3 gene expression minimal; when BvgA∼P levels are relatively low, class 2 and 3 gene expression will be maximal and class 1 and 4 gene expression minimal; and when BvgA∼P levels are high, class 1 and 2 gene expression will be maximal, class 4 gene expression will be minimal, and class 3 gene expression will be low. Although intracellular BvgA∼P levels have not been measured, these three gene expression profiles correspond to three phenotypic phases that have been characterized in vivo and are known as the Bvg−, Bvgi, and Bvg+ phases, respectively (see reference 10 for a review).
The signals sensed by BvgAS in nature are unknown. In the laboratory, however, BvgAS activity varies in response to temperature and the concentration of MgSO4 or nicotinic acid (NA) present in the growth medium (23, 31, 32, 37). These “modulators” have therefore been used to study Bvg-mediated gene regulation, and several analyses confirmed that BvgAS controls expression of the Bvg−, Bvgi, and Bvg+ phases in both a steady-state and temporal manner. For example, it has been well established that Bordetella grown in Stainer-Scholte (SS) broth or on Bordet-Gengou (BG) agar at 37°C displays a gene expression profile indicative of the Bvg+ phase, suggesting that the BvgAS phosphorelay is fully active under these conditions (14, 30, 38). Growth at 25°C or at 37°C in medium containing high concentrations of MgSO4 or NA causes expression of the Bvg− phase, suggesting that the BvgAS phosphorelay is inactive under these conditions (14, 30, 38). Growth at intermediate temperatures or in medium containing low levels of MgSO4 or NA results in expression of the Bvgi phase, suggesting that the BvgAS phosphorelay is semiactive under these conditions (14, 15, 43). Temporal regulation has been observed by growing B. pertussis under Bvg−-phase conditions and then switching it abruptly to Bvg+-phase conditions and monitoring gene expression over time. This kind of experiment has shown that the Bvgi phase is expressed for about 4 hours postshift before the Bvg+ phase is expressed and maintained (14, 25, 36). BvgAS's ability to control three distinct phenotypic phases that are each characterized by a specific and distinct gene expression profile makes it extremely useful for investigating the mechanisms underlying steady-state and temporal regulation.
The expression of genes encoding many TCSs is positively autoregulated. Examples include phoPQ in Salmonella, cpxAR in E. coli, misRS in Neisseria meningitidis, and bvgAS (16, 37, 39, 44). What role autoregulation plays in the ability of these systems to control gene expression precisely and coordinately is unknown. We investigated the role of autoregulation in the ability of BvgAS to control gene expression in both a temporal and steady-state manner by constructing B. bronchiseptica mutants in which bvgAS was expressed constitutively. Our results show that positive autoregulation is essential for normal temporal regulation and is important, but less crucial, for steady-state regulation.
MATERIALS AND METHODS
Bacterial strains.
The bacterial strains used in this study are listed in Table 1. Bordetella strains were cultured on BG agar (BD Biosciences, San Jose, CA) supplemented with 7.5% defibrinated sheep blood for 48 h at 37°C. For β-galactosidase assays and total RNA isolations, bacteria were grown in SS broth (40) at 37°C, with shaking. E. coli strains were cultured on LB agar or in LB broth. When appropriate, culture media were supplemented with gentamicin (Gm; 20 μg ml−1), streptomycin (Sm; 25 μg ml−1), or ampicillin (Ap; 100 μg ml−1).
TABLE 1.
Strains and plasmids used in this analysis
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| DH5α | E. coli strain used for molecular cloning | BRL, Gaithersburg, MD |
| SM10λpir | E. coli strain used for conjugation with Bordetella | 33 |
| RB50 | Wild-type B. bronchiseptica strain | 12 |
| RBL | RB50 with the bvgAS promoter replaced with the recA promoter | This study |
| RBH | RB50 with the bvgAS promoter replaced with the aacC1 promoter | This study |
| RB50::pEG111 | RB50 containing a chromosomal fhaB::lacZ fusion | 26 |
| RB50::pEG112 | RB50 containing a chromosomal frl::lacZ fusion | 30 |
| RB50::pTEN34 | RB50 containing a chromosomal bipA::lacZ fusion | 18 |
| RB50::pCW27 | RB50 containing ptxA promoter-lacZYA fusion | This study |
| RBL::pEG111 | RBL containing a chromosomal fhaB::lacZ fusion | This study |
| RBL::pEG112 | RBL containing a chromosomal frl::lacZ fusion | This study |
| RBL::pTEN34 | RBL containing a chromosomal bipA::lacZ fusion | This study |
| RBL::pCW27 | RBL containing ptxA promoter-lacZYA fusion | This study |
| RBH::pEG111 | RBH containing a chromosomal fhaB::lacZ fusion | This study |
| RBH::pEG112 | RBH containing a chromosomal frl::lacZ fusion | This study |
| RBH::pTEN34 | RBH containing a chromosomal bipA::lacZ fusion | This study |
| RBH::pCW27 | RBH containing ptxA promoter-lacZYA fusion | This study |
| TohamaI | Clinical B. pertussis isolate | 28 |
| Plasmids | ||
| pUC19 | Cloning vector; Apr | 51 |
| pSS3110 | Promoterless lacZYA fusion vector, integrates at an untranscribed region of the chromosome; Apr Gmr | 49 |
| pEG7S | Allelic exchange vector; Apr Gmr | 26 |
| pEG111 | Chromosomal fhaB-lacZ fusion plasmid; Apr Gmr | 26 |
| pEG112 | Chromosomal frl-lacZ fusion plasmid; Apr Gmr | 30 |
| pTEN34 | Chromosomal bipA-lacZ fusion plasmid; Apr Gmr | 18 |
| pCW27 | ptxA promoter-lacZYA fusion in pSS3110 | This study |
| pCW46 | pUC19 backbone with the recA promoter fused to bvgA homology region | This study |
| pCW54 | pEG7S backbone plasmid for replacing the bvgAS promoter with the recA promoter | This study |
| pCW57 | pUC19 backbone with the aacC1 promoter fused to bvgA homology region | This study |
| pCW60 | pEG7S backbone plasmid for replacing the bvgAS promoter with the aacC1 promoter | This study |
Molecular cloning and DNA sequence analysis.
Standard cloning techniques were used for all DNA manipulations (35). Restriction enzymes, T4 DNA ligase, Taq polymerase, and high-fidelity Phusion polymerase were purchased from Promega Corp. (Madison, WI), New England Biolabs (Beverly, MA), and MJ Research (Waltham, MA) and were used according to the manufacturers' instructions. All plasmids used in this study are listed in Table 1. Plasmid pCW27 was constructed by PCR amplifying the −203-to-+45 region of the ptxA promoter from B. pertussis strain Tohama 1, using primers adding a 5′ EcoRI site (CWptxPF [5′-CCGAATTCGCATACGTGTTGGCAA-3′]) and a 3′ SalI site (CWptxPR [5′-CCGTCGACAGTGCAACGCACC-3′]) (restriction sites are shown in bold), and ligating the digested PCR product into the EcoRI and SalI sites of pSS3110.
Construction of bacterial strains.
The bvgAS promoter was replaced with two nonautoregulated promoters by using an allelic exchange system employing the sacB gene as previously described (1, 30). For replacement of the bvgAS promoter with the recA promoter, we amplified a DNA fragment containing 483 bp upstream of the recA translational start site, using primers that added a 5′ EcoRI site (CWrecAPF [5′-CCGAATTCCGGCGGCGGTGAGGATCAGCACCGGCA-3′]) and a 3′ EarI site (CWrecAPREar [5′-GCGCTCTTCGCATGTAAAGTCCTGTATTGAAGCGGCGCC-3′]), and cloned this fragment along with a 295-bp fragment from the bvgA locus beginning at the translational start site, obtained with primers that added a 5′ EarI site (CWbvgAPEarI [5′-CTACTCTTCGATGCGTTGCAGGATTTTTTTCTCGCC-3′]) and a 3′ HindIII site (CWbvgARHind [5′-GGAAGCTTAGCACCAGAACGCGTAGCGGCAACCCC-3′]), into the EcoRI and HindIII sites of pUC19 to create pCW46. The resulting 785-bp EcoRI/HindIII fragment was then cloned, along with a 269-bp BamHI/EcoRI fragment from the fhaB promoter (immediately upstream of bvgAS), including 15 bp downstream of the ATG, into BamHI/HindIII-cut pEG7S to create pCW54. The 269-bp BamHI/EcoRI fragment from the fhaB promoter was generated by PCR amplification using a primer that adds a 5′ BamHI site (CWfhaBFBam [5′-GGGGATCCACAGGTTCGTGTTCATATTCC-3′]) and a primer that binds downstream of a natural EcoRI site (CWfhaBR [5′-GGGAATTCACAGGTTCGTGTTCATATTCC-3′]). Plasmid pCW54 was used for allelic exchange with RB50 to create strain RBL. For replacement of the bvgAS promoter with the aacC1 promoter, we amplified a DNA fragment containing 470 bp upstream of the translational start site of the aacC1 gene in plasmid pSS3110, using primers that added a 5′ EcoRI site (CWaacC1FEcoRI [5′-GAATTCGCCGTTTCTGTAATGAAGGAG-3′]) and a 3′ EarI site (CWaacC1REar [5′-CCGCTCTTCGCATCGTTGCTGCTCCATAACATC-3′]), and ligated this fragment along with a 295-bp fragment from the bvgA locus beginning at the translational start site, obtained with primers that added a 5′ EarI site and a 3′ HindIII site (CWbvgAPEarI and CWbvgARHind), into the EcoRI and HindIII sites of pUC19 to create pCW57. We then ligated the 269-bp BamHI/HindIII fragment from pCW54 with the EcoRI/HindIII insert from pCW57 with BamHI/HindIII-cut pEG7S to create the allelic exchange vector pCW60. Plasmid pCW60 was used for allelic exchange with RB50 to create strain RBH.
To construct B. bronchiseptica strains containing chromosomal lacZ reporter plasmids, strains RB50, RBL, and RBH were mated with E. coli SM10λpir containing pEG110, pEG112, pTEN34, or pCW27, and exconjugants were selected on BG agar containing streptomycin and gentamicin. Plasmids pEG110, pEG112, and pTEN34 contain internal fragments of fhaB, frl, and bipA, respectively, upstream of and in the same orientation as a promoterless lacZ gene. Integration of these plasmids into the B. bronchiseptica chromosome results in the formation of fhaB-lacZ, frl-lacZ, and bipA-lacZ transcriptional fusions, respectively. pCW27 contains the ptxA promoter from B. pertussis upstream of and in the same orientation as a promoterless lacZ gene. This plasmid also contains sequences homologous to a nonessential region of the chromosome, and integration of this plasmid at this location results in the formation of a strain containing a ptxA-lacZ fusion. PCR was used to verify the integration of all plasmids at the proper chromosomal location.
Bacterial conjugations.
Matings between B. bronchiseptica strains and E. coli strain SM10λpir were achieved by mixing stationary-phase cultures of the strains on BG agar plus 7.5% sheep blood at a 10:1 (B. bronchiseptica to E. coli) ratio. The mating mixture was incubated at 37°C for 5 h and then plated onto BG agar plus 7.5% sheep blood containing Gm and Sm to select for cointegrates.
Total RNA isolation and cDNA synthesis.
Total RNAs were isolated from RB50, RBL, and RBH grown for 16 to 18 h, to an optical density at 600 nm (OD600) of about 1.0 (mid-log phase for B. bronchiseptica grown in SS broth), under Bvg+-phase conditions (0 mM MgSO4), Bvgi-phase conditions (2 mM MgSO4 for RB50 and RBL, 6 mM MgSO4 for RBH), or Bvg−-phase conditions (12 mM MgSO4), following the protocol of an RNAqueous-4PCR kit from Ambion Inc. (Austin, TX). For the reverse transcription step, 10 ng of total RNA was transcribed using oligo(dT) and random priming following the protocol supplied with Super Script II reverse transcriptase (Invitrogen Inc., Carlsbad, CA).
mRNA quantification.
Relative levels of bvgA and recA transcripts were determined using quantitative real-time PCR using the following primers: RTrecAF (5′-GCCAGGGCAAGGACAATGT-3′), RTrecAR (5′-CTTCGCTGGCGGGAAG-3′), RTbvgAF (5′-TCCGGGTCCTGATGGAAAA-3′), and RTbvgAR (5′-CTTTTCCTCGCGACGATTATTG-3′). Quantitative real-time PCRs were performed in SYBR green super mix (Bio-Rad Laboratories, Hercules, CA), using a Bio-Rad iCycler PCR machine and software (Bio-Rad Laboratories, Hercules, CA). All samples were run in triplicate, and bvgA transcription was normalized to recA transcription for each sample.
β-Galactosidase assays.
For steady-state β-galactosidase activity assays, a single colony of each RB50, RBL, and RBH lacZ fusion strain was inoculated into 3 ml of SS broth containing 0, 2, 4, 6, 8, or 12 mM MgSO4 and grown for 16 to 18 h, to an OD600 of between 1.0 and 2.0. These cells were in mid-log phase because the final density of RB50 grown in SS broth is ∼4.7 (13). Cells were permeabilized by the addition of sodium dodecyl sulfate and CHCl3, and β-galactosidase activity was determined as described previously (30), except that measurements were taken using a Victor3 1420 microplate reader (Perkin-Elmer Life Sciences, Boston, MA). For Bvg+-phase-to-Bvg−-phase time courses, RB50, RBL, and RBH lacZ fusion strains were grown overnight in SS broth containing 25 mM MgSO4, as described above, an aliquot of each strain was taken at time zero, and the remaining cells were harvested, washed twice with SS broth without MgSO4, and resuspended to an OD600 of 0.3 in 2 ml fresh medium. A 1-ml aliquot was taken at 1.5, 3, 4.5, 6, 7.5, and 9 h postshift for β-galactosidase measurement, and 1 ml fresh medium was added to the remaining cells. The doubling time for B. bronchiseptica growing in SS broth is ∼90 min. Removal of 1 ml of culture and replacement of 1 ml of fresh medium every 90 min therefore maintained the culture at a relatively constant density in mid-log phase. For the reverse time courses, RB50, RBL, and RBH strains were grown overnight in SS broth without MgSO4, as described above, an aliquot of each strain was taken at time zero, and the remaining cells were harvested, resuspended in SS broth containing 25 mM MgSO4, and adjusted to an OD600 of 0.3 in 2 ml fresh medium. A 1-ml aliquot was taken at 1.5, 3, 4.5, 6, and 7.5 h postshift, and 1 ml fresh medium was added to keep cells in mid-log phase as described above. β-Galactosidase assays were performed on each sample as described above. For steady-state and β-galactosidase assays, relative transcription was calculated by setting the maximum β-galactosidase activity value for each gene transcribed in RB50 to 100%. For temporal (Bvg−-to-Bvg+ time courses and Bvg+-to-Bvg− time courses) β-galactosidase assays, relative transcription was calculated by setting the maximum β-galactosidase activity value for each gene transcribed in RB50 during the Bvg−-to-Bvg+ time course to 100%.
RESULTS
Steady-state regulation of gene expression in wild-type B. bronchiseptica.
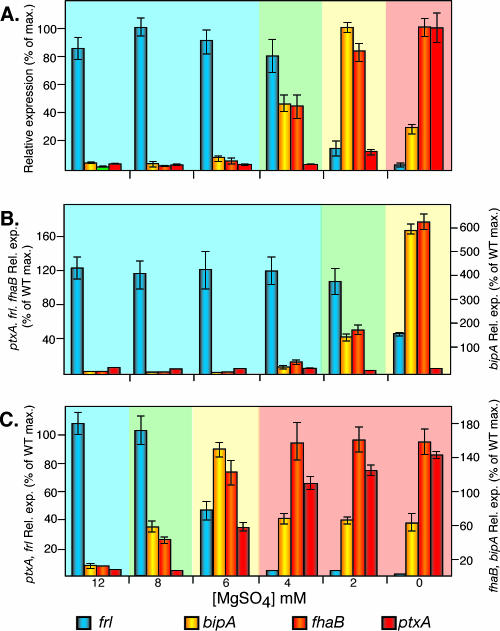
We used B. bronchiseptica RB50 derivatives containing lacZ fusions to representative class 1, 2, 3, and 4 genes to determine Bvg-regulated gene expression patterns in bacteria grown under different environmental conditions, i.e., to characterize steady-state regulation in wild-type B. bronchiseptica. Since the best-characterized class 1 gene promoter, that of ptx, is not expressed in B. bronchiseptica due to nucleotide differences at the primary BvgA binding site (3), we fused the B. pertussis ptx promoter to lacZ in plasmid pSS3110, which integrates into a nonessential region of the chromosome (49), and used the resulting cointegrate strain to measure ptx (class 1) gene expression in RB50. Bacteria grown in SS broth containing 6 to 12 mM MgSO4 displayed a gene expression pattern characteristic of the Bvg− phase: the class 4 gene frl was expressed at maximal levels, while expression of the class 1, 2, and 3 genes was barely detectable (Fig. 1A). Bacteria grown in SS broth without added MgSO4 displayed a gene expression pattern characteristic of the Bvg+ phase: ptx and fha (class 1 and 2 genes, respectively) were expressed at high levels, and frl and the class 3 gene bipA were expressed at low levels (Fig. 1A). Bacteria grown in medium containing 2 mM MgSO4 displayed a gene expression pattern characteristic of the Bvgi phase: expression of fha and bipA was maximal, and expression of frl and ptx was minimal (Fig. 1A). Bacteria grown in medium containing 4 mM MgSO4 expressed high levels of frl, moderate levels of fha and bipA, and low levels of ptx (Fig. 1A). We refer to this phase as Bvg−/i since it displays a gene expression pattern intermediate between those of the Bvg− and Bvgi phases.
FIG. 1.
Steady-state gene expression in RB50 (A), RBL (B), and RBH (C). lacZ fusion strains were grown overnight in SS broth containing 0 mM, 2 mM, 4 mM, 6 mM, 8 mM, or 12 mM MgSO4. Relative expression of frlAB (blue bars), bipA (yellow bars), fhaB (orange bars), and ptxA (red bars) was calculated by setting the maximum β-galactosidase activity of each gene in RB50 (wild type [WT]) to 100%. The light blue background indicates Bvg−-phase expression, the yellow background indicates the Bvgi phase, the light green background indicates the Bvg−/i phase, and the light red background indicates the Bvg+ phase.
Temporal regulation in wild-type B. bronchiseptica in response to a shift from Bvg−-phase conditions to Bvg+-phase conditions.
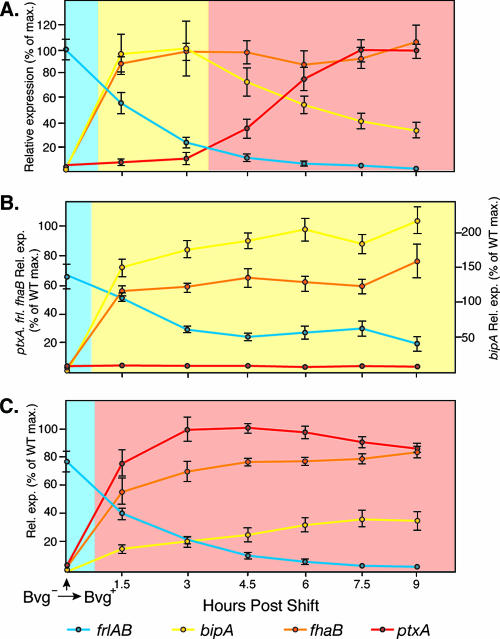
To characterize Bvg-mediated gene regulation in response to a shift from Bvg−-phase conditions to Bvg+-phase conditions, we grew bacteria overnight in SS broth containing 25 mM MgSO4, washed the cells twice in SS broth without added MgSO4, resuspended the cells in SS broth without MgSO4 to an OD600 of 0.3, incubated the cultures at 37°C with shaking, removed samples every 1.5 h, and measured β-galactosidase activity. The gene expression pattern in the overnight cultures was characteristic of the Bvg− phase, as expected (Fig. 2A). By 1.5 h postshift, β-galactosidase activity was maximal in the strains containing bipA-lacZ and fha-lacZ fusions, minimal in the strain containing the ptx-lacZ fusion, and half-maximal in the strain containing the frl-lacZ fusion (Fig. 2A). Because of the stability of the β-galactosidase enzyme, it is likely that frl transcription was actually minimal at this time point and that the β-galactosidase activity detected was due to enzyme that was produced preshift that had not yet degraded. At 3 and 4.5 h postshift, β-galactosidase activity remained high in the fha-lacZ- and bipA-lacZ-containing strains and low in the frl-lacZ- and ptx-lacZ-containing strains (Fig. 2A). At 6 h postshift and beyond, fha-lacZ and ptx-lacZ expression was high, frl-lacZ expression was nearly undetectable, and bipA-lacZ expression was low (Fig. 2A). These results indicate that when wild-type B. bronchiseptica is shifted abruptly from Bvg−-phase conditions to Bvg+-phase conditions, the Bvgi phase is expressed for approximately 3 hours before the transition to the Bvg+ phase is complete. Expression of the Bvg+ phase was maintained for the remainder of the time course. These results are consistent with observations for B. pertussis (14, 25, 36).
FIG. 2.
Temporal gene expression in cells shifted from Bvg−-phase conditions to Bvg+-phase conditions in RB50 (A), RBL (B), and RBH (C). lacZ fusion strains were grown overnight in SS broth containing 25 mM MgSO4 and then washed and resuspended in SS broth without added MgSO4 at time zero, and samples were taken every 1.5 h postshift. Relative expression of frlAB (blue lines), bipA (yellow lines), fhaB (orange lines), and ptxA (red lines) was calculated by setting the maximum β-galactosidase activity of each gene in RB50 (wild type [WT]) to 100%. The light blue background indicates Bvg−-phase expression, the yellow background indicates the Bvgi phase, and the light red background indicates the Bvg+ phase.
Temporal regulation in wild-type B. bronchiseptica in response to a shift from Bvg+-phase conditions to Bvg−-phase conditions.
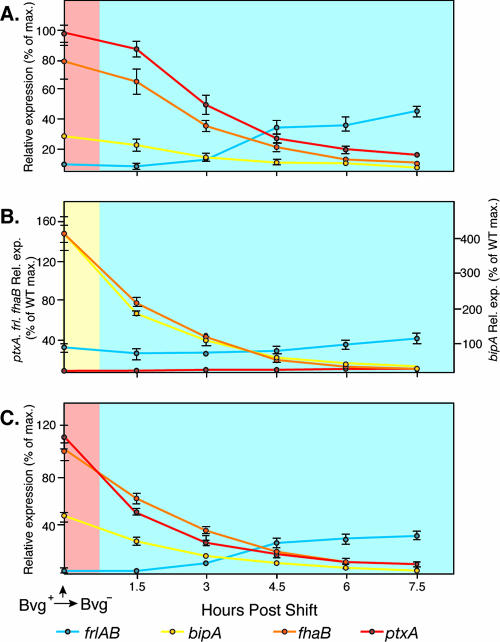
To characterize Bvg-mediated gene regulation in response to a shift from Bvg+-phase conditions to Bvg−-phase conditions, we grew bacteria overnight in SS broth without added MgSO4, harvested the cells, resuspended them in SS broth containing 25 mM MgSO4 to an OD600 of 0.3, incubated the cultures at 37°C with shaking, removed samples every 1.5 h, and measured β-galactosidase activity. fha-lacZ, ptx-lacZ, and bipA-lacZ levels were approximately 80% of their preshift levels at 1.5 h postshift, approximately 40% of preshift levels by 3 h postshift, and ≤20% of preshift levels at 4.5 h postshift (Fig. 3A). bipA-lacZ expression was not higher than the preshift level at any time point. frl-lacZ expression increased gradually over the course of the experiment (Fig. 3A). Therefore, when wild-type B. bronchiseptica is shifted from Bvg+-phase conditions to Bvg−-phase conditions, the temporal pattern of gene expression that occurs is not the reverse of that which occurs when cells are shifted from Bvg−-phase conditions to Bvg+-phase conditions. Instead, the transition from the Bvg+ phase to the Bvg− phase occurs directly, without even transient expression of the Bvgi phase.
FIG. 3.
Temporal gene expression in cells shifted from Bvg+-phase conditions to Bvg−-phase conditions in RB50 (A), RBL (B), and RBH (C). lacZ fusion strains were grown overnight in SS broth without added MgSO4 and then resuspended in SS broth containing 25 mM MgSO4 at time zero, and samples were taken every 1.5 h postshift. Relative expression of frlAB (blue lines), bipA (yellow lines), fhaB (orange lines), and ptxA (red lines) was calculated by setting the maximum β-galactosidase activity of each gene in RB50 (wild type [WT]) shifted from Bvg−-phase conditions to Bvg+-phase conditions (i.e., the values from Fig. 2A) to 100%. The light blue background indicates Bvg−-phase expression, the yellow background indicates the Bvgi phase, and the light red background indicates the Bvg+ phase.
Construction of bvgAS autoregulation mutants.
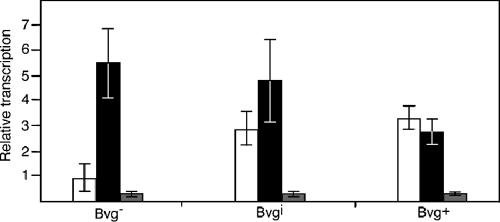
Because bvgAS is autoregulated, we could not measure its expression using bvg-lacZ fusions and therefore used quantitative reverse transcription-PCR (RT-PCR). Consistent with previous reports, bvgAS transcripts were of very low abundance in bacteria grown under Bvg−-phase conditions, of moderate abundance in bacteria grown under Bvgi-phase conditions, and of high abundance in bacteria grown under Bvg+-phase conditions (Fig. 4). To investigate the role of autoregulation in the ability of BvgAS to control multiple patterns of gene expression in a steady-state and temporal manner, we constructed strains in which bvgAS was not autoregulated. We sought to construct one strain in which bvgAS was expressed constitutively at a high level, similar to the level at which wild-type bvgAS is expressed in the Bvg+ phase, and one strain in which bvgAS was expressed constitutively at a low level, similar to that at which wild-type bvgAS is expressed under Bvg−-phase conditions. We tested the expression of several promoters for constitutive expression in RB50, and although we were unable to find promoters that met either condition exactly, the recA and aacC1 promoters proved useful and adequate for our analyses. We constructed strain RBL by replacing the native bvgAS promoter with the B. bronchiseptica recA promoter. Quantitative RT-PCR indicated that in this strain, bvgAS expression under all growth conditions was about half that of bvgAS in wild-type bacteria grown under Bvg−-phase conditions (Fig. 4). We constructed strain RBH by replacing the native bvgAS promoter with that of the aacC1 gene, which encodes gentamicin acetyltransferase. Quantitative RT-PCR indicated that in this strain, bvgAS expression was slightly higher in cells grown under Bvg−- and Bvgi-phase conditions and the same in cells grown under Bvg+-phase conditions as that of bvgAS in wild-type bacteria grown under Bvg+-phase conditions (Fig. 4). Although bvgAS expression in these strains is not exactly the same as that in wild-type B. bronchiseptica grown under either Bvg+- or Bvg−-phase conditions, it is not autoregulated, and therefore the role of autoregulation in steady-state and temporal gene regulation can be determined using these strains.
FIG. 4.
Relative bvgA mRNA levels in RB50 (white bars), RBH (black bars), and RBL (gray bars). Quantitative PCR was used to determine relative levels of the bvgA transcript in RB50, RBL, and RBH grown under Bvg−-, Bvgi-, and Bvg+-phase conditions.
Steady-state gene expression profiles of bvgAS autoregulation mutants.
We measured frl-lacZ, bipA-lacZ, fha-lacZ, and ptx-lacZ expression in RBL and RBH after growing the bacteria in SS broth containing various concentrations of MgSO4, as described above for RB50. The gene expression patterns displayed by RBL indicated that this strain expressed the Bvg− phase when grown in medium containing 4 to 12 mM MgSO4, the Bvg−/i phase when grown in medium containing 2 mM MgSO4, and the Bvgi phase when grown in medium without added MgSO4 (Fig. 1B). bipA-lacZ levels in this strain grown without added MgSO4 were approximately sixfold greater than the level in RB50 grown in 2 mM MgSO4 (Fig. 1B). (The reason for this high level of bipA expression is addressed in Discussion.) The low level of bvgAS expressed in RBL is therefore sufficient to induce a Bvgi-phase pattern of gene expression but not a Bvg+-phase pattern of gene expression. The gene expression patterns displayed by RBH indicated that this strain was able to control all of the various Bvg-regulated gene expression patterns, but with a slightly different sensitivity to MgSO4; the Bvg−/i phase was expressed in cells grown in medium containing 8 mM MgSO4, and the Bvgi phase was expressed in cells grown in medium containing 6 mM MgSO4 (Fig. 1C). Together, these data indicate that autoregulation is not required for BvgAS to control multiple gene expression patterns if it is expressed at a high level. However, positive autoregulation appears to influence the sensitivity of the system, since more MgSO4 was required to modulate the activity of BvgAS in RBH than in RB50.
Temporal gene expression profiles of bvgAS autoregulation mutants in response to a shift from Bvg−-phase conditions to Bvg+-phase conditions.
When shifted from Bvg−-phase conditions to Bvg+-phase conditions, RBL and RBH switched within 1.5 h to gene expression patterns indicative of the Bvgi and Bvg+ phases, respectively, and these gene expression profiles were maintained for the duration of the experiment (Fig. 2B and C). Notably, the Bvgi phase was not expressed even briefly in RBH (Fig. 2C). Positive autoregulation is therefore essential for BvgAS to control multiple gene expression patterns in a temporal manner in response to a shift from Bvg−-phase conditions to Bvg+-phase conditions.
Temporal gene expression profiles of bvgAS autoregulation mutants in response to a shift from Bvg+-phase conditions to Bvg−-phase conditions.
When shifted from Bvg+-phase conditions to Bvg−-phase conditions, the expression of Bvg-activated genes in RBL and RBH was significantly decreased by the first time point (1.5 h) (except that of ptx in RBL, which was never activated in this strain even under Bvg+-phase conditions) (Fig. 3B and C). frl-lacZ expression increased gradually in all strains over the course of the experiment. These gene expression patterns are similar to those measured for RB50 and therefore indicate that positive autoregulation does not play a significant role in the ability of BvgAS to control gene expression temporally in response to a shift from Bvg+-phase conditions to Bvg−-phase conditions.
DISCUSSION
A variety of pathogens regulate virulence gene expression in response to the surrounding environment through the use of two-component regulatory systems (13, 32, 34). Although many of these systems are positively autoregulated, how autoregulation affects their functionality is not understood. Using Bordetella BvgAS as a model, we have shown that autoregulation of bvgAS transcription is critical for temporal expression of multiple phenotypic phases in response to a shift from Bvg−-phase conditions to Bvg+-phase conditions. We also showed that bvgAS autoregulation contributes to steady-state regulation, as it influences the sensitivity of the system in response to subtle differences in signal intensity. As discussed below, our results also provide insight into how BvgAS functions mechanistically.
A model for how BvgA and BvgS respond to a shift from Bvg−-phase conditions to Bvg+-phase conditions, based primarily on experiments done with B. pertussis, is as follows. Both protein quantification and gene expression data indicate that BvgA and BvgS levels are very low in Bvg−-phase cells (14, 25, 37, 38). The fact that mutations in bvgA or bvgS that prevent phosphorylation of BvgA in vitro result in constitutive Bvg−-phase expression in vivo suggests that the amount of BvgA that is phosphorylated under Bvg−-phase conditions is at or near zero (12, 45, 47). When cells are shifted to Bvg+-phase conditions, BvgS autophosphorylates, and the phosphoryl group is transferred to BvgA. The amount of BvgA∼P that is present immediately postshift (which must be very low) is sufficient to activate transcription of class 2 and 3 genes as well as bvgAS itself but not to activate transcription of class 1 genes or to repress bipA, and therefore the Bvgi phase is expressed. After 3 to 4 h of positive autoregulation, BvgS and BvgA (and, most importantly, BvgA∼P) reach levels sufficient to activate the expression of class 1 genes and to repress the expression of bipA. Cells then express the Bvg+ phase as long as they are exposed to Bvg+-phase conditions.
Consistent with the results of studies done with B. pertussis (14, 25, 36), our studies showed that when wild-type B. bronchiseptica was shifted from Bvg−-phase conditions to Bvg+-phase conditions, the Bvgi phase was expressed for approximately 3 hours before the Bvg+ phase was expressed. However, when bvgAS was expressed constitutively at a low level, the bacteria switched from the Bvg− phase to the Bvgi phase and never expressed the Bvg+ phase, and when bvgAS was expressed constitutively at a high level, the bacteria switched from the Bvg− phase to the Bvg+ phase without ever expressing the Bvgi phase. Positive autoregulation is therefore essential for the bacteria to express multiple phenotypic phases in a temporal manner in response to a shift from Bvg−-phase conditions to Bvg+-phase conditions. Consistent with the model, therefore, positive autoregulation appears to be responsible for the relatively slow accumulation of BvgA (and hence BvgA∼P) postshift, which is responsible for the transient (2 to 3 h) expression of the Bvgi phase. These data also provide insight into BvgS activation kinetics: the lack of Bvgi-phase expression in RBH postshift indicates that the kinase activity of BvgS can be activated rapidly upon exposure to Bvg+-phase conditions.
When wild-type B. bronchiseptica was shifted from Bvg+-phase conditions to Bvg−-phase conditions, the cells switched directly to the Bvg− phase without transient Bvgi-phase expression. This result concurs with previous studies with B. pertussis in which mRNA transcripts for fhaB, cyaA, and ptxA decreased rapidly postshift (Bvg+-phase conditions to Bvg−-phase conditions) (38). Scarlato et al. hypothesized that BvgA was rapidly inactivated under noninducing conditions and that BvgA levels remained high, at least temporarily, to prime the cells for detection of a new signal (37). This study was done prior to the demonstration that BvgS and BvgA communicate via phosphorylation and prior to the identification of the Bvgi-phase-specific gene bipA (43, 45, 47). Our experiments showed that in B. bronchiseptica, class 1 and 2 gene expression decreased significantly by the first time point following a shift from Bvg+-phase conditions to Bvg−-phase conditions and that bipA expression never increased, demonstrating conclusively that the Bvgi phase was not expressed even transiently postshift. Like wild-type B. bronchiseptica, both RBL and RBH switched directly to the Bvg− phase postshift, indicating that autoregulation is not required for temporal regulation in response to a shift to Bvg−-phase conditions. The fact that RBH switched directly to the Bvg− phase indicates that, consistent with the hypothesis put forth by Scarlato et al., BvgA is inactivated rapidly postshift. Because in vitro studies indicate that BvgA∼P is stable in the absence of BvgS (48), these data suggest that the phosphatase activity of BvgS is activated immediately upon exposure to Bvg−-phase conditions.
While the results of our temporal regulation experiments support the hypothesis that BvgS converts rapidly to a kinase or a phosphatase upon exposure to Bvg+- or Bvg−-phase conditions, respectively, the results of our steady-state regulation experiments support the hypothesis that the kinase and phosphatase activities of BvgS can be adjusted to intermediate levels, i.e., that BvgS functions like a rheostat rather than a switch. Although this hypothesis was proposed nearly a decade ago (13), the mechanistic basis for how BvgS activity is fine-tuned is still unknown. Moreover, the possibility that expression of the Bvgi phase results from autoregulation rather than intermediate BvgS activity could not previously be ruled out. The fact that RBH was capable of expressing all of the same phenotypic phases as RB50, however, indicates that BvgA∼P levels can be adjusted precisely and maintained indefinitely at specific levels in response to specific environmental cues, even when BvgA is maintained at a high level. Since there is considerable evidence that phosphorylation and dephosphorylation of BvgA are controlled only by BvgS (41, 45-48), these data provide strong evidence that the kinase and/or phosphatase activity of BvgS can be adjusted to and maintained at intermediate levels. Our data also suggest that it is the absolute amount of BvgA∼P, rather than the ratio of BvgA∼P to BvgA, that is important in controlling gene expression, because this ratio must be well below one in RBH cells grown under Bvgi-phase conditions but one or nearly one in RBL cells grown under Bvg+-phase conditions and in RB50 cells immediately after a shift from Bvg−-phase conditions to Bvg+-phase conditions, yet in all of these cases, the Bvgi phase is expressed.
The fact that the sensitivity of RBH to environmental cues differs from that of RB50 demonstrates that although it is relatively subtle, autoregulation does contribute to the ability of BvgAS to control gene expression in a steady-state manner. This result suggests that BvgS activity is controlled primarily in response to environmental signals and that if a feedback mechanism that adjusts BvgS activity in response to the absolute amount of BvgA∼P exists, it plays only a minor role. If such a feedback mechanism played a major role, then RB50 and RBH would control the same pattern of gene expression in response to the same environmental conditions. Instead, it appears that BvgS activity is controlled predominantly in response to environmental cues and that BvgA∼P levels reflect BvgS activity and the amount of BvgA and BvgS present in the cell. Thus, the data suggest that BvgS activities in RB50 and RBH grown in the presence of 6 M MgSO4, for example, are the same, but the higher absolute amount of BvgA and BvgS in RBH than in RB50 under these conditions results in higher levels of BvgA∼P in RBH and therefore in expression of the Bvgi phase instead of the Bvg− phase.
In the course of our experiments, we noted that bipA expression in RBL grown under Bvg+-phase conditions was much higher than bipA expression in RB50 under any condition. This extremely high level of bipA expression is most likely due to binding of BvgA∼P to the high-affinity BvgA binding sites present upstream of the bipA transcription start site and the lack of BvgA∼P binding to the low-affinity BvgA binding sites present downstream of the bipA transcription start site (50). The concentration of BvgA∼P produced in RBL under Bvg+-phase conditions must therefore be greater than the binding constants of the high-affinity sites and less than the binding constants of the low-affinity sites. The failure of RB50 to express bipA at this high level indicates that the concentration of BvgA∼P present in RBL cells grown under Bvg+-phase conditions is never stably produced in RB50. This is most likely due to the fact that bvgAS transcription is activated by binding of BvgA∼P to sites that are of similar high affinity to those present upstream of the bipA promoter. Together, these data indicate that although the concentration of BvgA∼P in the cell can be adjusted over a broad range, autoregulation prevents the stable expression of some (low) concentrations. Thus, while BvgAS may function like a rheostat, autoregulation apparently makes it an imperfect one.
Our experiments have provided insight into the role of autoregulation in the ability of BvgAS to control multiple gene expression profiles in response to changes in environmental conditions and have also provided clues regarding how BvgA and BvgS function mechanistically. Important questions begging to be addressed, however, relate to the role of autoregulation in survival of the organism in its natural environments. For both B. pertussis and B. bronchiseptica, there is considerable evidence that the Bvg+ phase is necessary and sufficient for the bacteria to cause respiratory infection and that a failure to repress Bvg−-phase phenotypes is detrimental to the development of infection (1, 12, 29). Autoregulation therefore must not play an important role during respiratory infection (other than to maintain high levels of BvgA and BvgS), and the fact that Bvg+-phase-locked strains are indistinguishable from wild-type B. pertussis and B. bronchiseptica in various animal models supports this conclusion (12, 29). It has been hypothesized that transition to the Bvgi phase is important for aerosol transmission by both B. pertussis and B. bronchiseptica and that the Bvg− phase is required for B. bronchiseptica to survive for extended periods of time outside the mammalian host, an ability that B. pertussis apparently lacks. We hypothesize that bvgAS autoregulation plays an important role in transmission between mammalian hosts, either directly or via an environmental reservoir (in the case of B. bronchiseptica). Testing this hypothesis, however, will require the use of models that encompass the entire Bordetella infectious cycle, which are not yet available.
Although this hypothesis has not been tested, it seems likely that autoregulation of two-component systems is important generally. In Salmonella, for example, the PhoPQ TCS, which is positively autoregulated, controls the expression of many virulence genes, including those required for survival within macrophages (see reference 20 for a review). It is possible that autoregulation of phoPQ and subsequent (at least partial) autoregulation of ssrA and ssrB control a temporal pattern of virulence gene expression that is important for survival of Salmonella cells after they enter macrophage phagosomes (see reference 4 for a review). In Bacillus subtilis, the Spo0A response regulator controls the expression of genes required for sporulation. Spo0A synthesis is controlled by a positive feedback loop in which Spo0A∼P stimulates expression of the gene encoding the RNA polymerase sigma factor σH, which leads, in turn, to increased spo0A transcription (22). This feedback loop results in a gradual increase in Spo0A after cells are shifted to nutrient-limiting conditions (19). Fujita and Losick hypothesized that the gradual increase in Spo0A∼P concentration allowed early-threshold genes to be expressed before late-threshold genes, and by ectopically expressing spo0A from an inducible promoter, they showed that a gradual increase in Spo0A was indeed required for cells to sporulate properly after they were shifted to starvation conditions (19). The role of autoregulation in other regulatory systems remains to be determined.
Acknowledgments
We thank Robin Hulbert and Jessica Sexton for comments on the manuscript and Christopher Osovitz for technical assistance with quantitative PCR experiments.
This work was supported by grants from the National Institutes of Health (AI43876) and the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service (2003-35204-13555). C.L.W. was supported in part by a UC Regents Fellowship.
Footnotes
Published ahead of print on 8 December 2006.
REFERENCES
- 1.Akerley, B. J., P. A. Cotter, and J. F. Miller. 1995. Ectopic expression of the flagellar regulon alters development of the Bordetella-host interaction. Cell 80:611-620. [DOI] [PubMed] [Google Scholar]
- 2.Akerley, B. J., and J. F. Miller. 1993. Flagellin gene transcription in Bordetella bronchiseptica is regulated by the BvgAS virulence control system. J. Bacteriol. 175:3468-3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arico, B., and R. Rappuoli. 1987. Bordetella parapertussis and Bordetella bronchiseptica contain transcriptionally silent pertussis toxin genes. J. Bacteriol. 169:2847-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beier, D., and R. Gross. 2006. Regulation of bacterial virulence by two-component systems. Curr. Opin. Microbiol. 9:143-152. [DOI] [PubMed] [Google Scholar]
- 5.Boucher, P. E., A. E. Maris, M. S. Yang, and S. Stibitz. 2003. The response regulator BvgA and RNA polymerase alpha subunit C-terminal domain bind simultaneously to different faces of the same segment of promoter DNA. Mol. Cell 11:163-173. [DOI] [PubMed] [Google Scholar]
- 6.Boucher, P. E., F. D. Menozzi, and C. Locht. 1994. The modular architecture of bacterial response regulators. Insights into the activation mechanism of the BvgA transactivator of Bordetella pertussis. J. Mol. Biol. 241:363-377. [DOI] [PubMed] [Google Scholar]
- 7.Boucher, P. E., K. Murakami, A. Ishihama, and S. Stibitz. 1997. Nature of DNA binding and RNA polymerase interaction of the Bordetella pertussis BvgA transcriptional activator at the fha promoter. J. Bacteriol. 179:1755-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boucher, P. E., M. S. Yang, D. M. Schmidt, and S. Stibitz. 2001. Genetic and biochemical analyses of BvgA interaction with the secondary binding region of the fha promoter of Bordetella pertussis. J. Bacteriol. 183:536-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherry, J. D. 1999. Epidemiological, clinical, and laboratory aspects of pertussis in adults. Clin. Infect. Dis. 28(Suppl. 2):S112-S117. [DOI] [PubMed] [Google Scholar]
- 10.Cotter, P. A., and A. M. Jones. 2003. Phosphorelay control of virulence gene expression in Bordetella. Trends Microbiol. 11:367-373. [DOI] [PubMed] [Google Scholar]
- 11.Cotter, P. A., and J. F. Miller. 2001. Bordetella, p. 619-674. In E. A. Groisman (ed.), Principles of bacterial pathogenesis. Academic Press, San Diego, CA.
- 12.Cotter, P. A., and J. F. Miller. 1994. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect. Immun. 62:3381-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cotter, P. A., and J. F. Miller. 1997. A mutation in the Bordetella bronchiseptica bvgS gene results in reduced virulence and increased resistance to starvation, and identifies a new class of Bvg-regulated antigens. Mol. Microbiol. 24:671-685. [DOI] [PubMed] [Google Scholar]
- 14.Cummings, C. A., H. J. Bootsma, D. A. Relman, and J. F. Miller. 2006. Species- and strain-specific control of a complex, flexible regulon by Bordetella BvgAS. J. Bacteriol. 188:1775-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deora, R., H. J. Bootsma, J. F. Miller, and P. A. Cotter. 2001. Diversity in the Bordetella virulence regulon: transcriptional control of a Bvg-intermediate phase gene. Mol. Microbiol. 40:669-683. [DOI] [PubMed] [Google Scholar]
- 16.De Wulf, P., O. Kwon, and E. C. Lin. 1999. The CpxRA signal transduction system of Escherichia coli: growth-related autoactivation and control of unanticipated target operons. J. Bacteriol. 181:6772-6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Domenighini, M., D. Relman, C. Capiau, S. Falkow, A. Prugnola, V. Scarlato, and R. Rappuoli. 1990. Genetic characterization of Bordetella pertussis filamentous haemagglutinin: a protein processed from an unusually large precursor. Mol. Microbiol. 4:787-800. [DOI] [PubMed] [Google Scholar]
- 18.Fuchslocher, B., L. L. Millar, and P. A. Cotter. 2003. Comparison of bipA alleles within and across Bordetella species. Infect. Immun. 71:3043-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujita, M., and R. Losick. 2005. Evidence that entry into sporulation in Bacillus subtilis is governed by a gradual increase in the level and activity of the master regulator Spo0A. Genes Dev. 19:2236-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groisman, E. A. 2001. The pleiotropic two-component regulatory system PhoP-PhoQ. J. Bacteriol. 183:1835-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gross, R., and R. Rappuoli. 1988. Positive regulation of pertussis toxin expression. Proc. Natl. Acad. Sci. USA 85:3913-3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoch, J. A. 1991. Spo0 genes, the phosphorelay, and the initiation of sporulation, p. 747-755. In A. L. Sonenshein et al. (ed.), Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. American Society for Microbiology, Washington, DC.
- 23.Idigbe, E. O., R. Parton, and A. C. Wardlaw. 1981. Rapidity of antigenic modulation of Bordetella pertussis in modified Hornibrook medium. J. Med. Microbiol. 14:409-418. [DOI] [PubMed] [Google Scholar]
- 24.Jacob-Dubuisson, F., B. Kehoe, E. Willery, N. Reveneau, C. Locht, and D. A. Relman. 2000. Molecular characterization of Bordetella bronchiseptica filamentous haemagglutinin and its secretion machinery. Microbiology 146:1211-1221. [DOI] [PubMed] [Google Scholar]
- 25.Jones, A. M., P. E. Boucher, C. L. Williams, S. Stibitz, and P. A. Cotter. 2005. Role of BvgA phosphorylation and DNA binding affinity in control of Bvg-mediated phenotypic phase transition in Bordetella pertussis. Mol. Microbiol. 58:700-713. [DOI] [PubMed] [Google Scholar]
- 26.Julio, S. M., and P. A. Cotter. 2005. Characterization of the filamentous hemagglutinin-like protein FhaS in Bordetella bronchiseptica. Infect. Immun. 73:4960-4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karimova, G., J. Bellalou, and A. Ullmann. 1996. Phosphorylation-dependent binding of BvgA to the upstream region of the cyaA gene of Bordetella pertussis. Mol. Microbiol. 20:489-496. [DOI] [PubMed] [Google Scholar]
- 28.Kasuga, T., Y. Nakase, K. Ukishima, and K. Takatsu. 1954. Studies on Haemophilus pertussis. V. Relation between the phase of bacilli and the progress of the whooping-cough. Kitasato Arch. Exp. Med. 27:57-62. [PubMed] [Google Scholar]
- 29.Martinez de Tejada, G., P. A. Cotter, U. Heininger, A. Camilli, B. J. Akerley, J. J. Mekalanos, and J. F. Miller. 1998. Neither the Bvg− phase nor the vrg6 locus of Bordetella pertussis is required for respiratory infection in mice. Infect. Immun. 66:2762-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez de Tejada, G., J. F. Miller, and P. A. Cotter. 1996. Comparative analysis of the virulence control systems of Bordetella pertussis and Bordetella bronchiseptica. Mol. Microbiol. 22:895-908. [DOI] [PubMed] [Google Scholar]
- 31.McPheat, W. L., A. C. Wardlaw, and P. Novotny. 1983. Modulation of Bordetella pertussis by nicotinic acid. Infect. Immun. 41:516-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller, J. F., C. R. Roy, and S. Falkow. 1989. Analysis of Bordetella pertussis virulence gene regulation by use of transcriptional fusions in Escherichia coli. J. Bacteriol. 171:6345-6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Relman, D. A., M. Domenighini, E. Tuomanen, R. Rappuoli, and S. Falkow. 1989. Filamentous hemagglutinin of Bordetella pertussis: nucleotide sequence and crucial role in adherence. Proc. Natl. Acad. Sci. USA 86:2637-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook, J., E. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 36.Scarlato, V., B. Arico, A. Prugnola, and R. Rappuoli. 1991. Sequential activation and environmental regulation of virulence genes in Bordetella pertussis. EMBO J. 10:3971-3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scarlato, V., A. Prugnola, B. Arico, and R. Rappuoli. 1990. Positive transcriptional feedback at the bvg locus controls expression of virulence factors in Bordetella pertussis. Proc. Natl. Acad. Sci. USA 87:6753-6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scarlato, V., and R. Rappuoli. 1991. Differential response of the bvg virulence regulon of Bordetella pertussis to MgSO4 modulation. J. Bacteriol. 173:7401-7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soncini, F. C., E. G. Vescovi, and E. A. Groisman. 1995. Transcriptional autoregulation of the Salmonella typhimurium phoPQ operon. J. Bacteriol. 177:4364-4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stainer, D. W., and M. J. Scholte. 1970. A simple chemically defined medium for the production of phase I Bordetella pertussis. J. Gen. Microbiol. 63:211-220. [DOI] [PubMed] [Google Scholar]
- 41.Steffen, P., S. Goyard, and A. Ullmann. 1996. Phosphorylated BvgA is sufficient for transcriptional activation of virulence-regulated genes in Bordetella pertussis. EMBO J. 15:102-109. [PMC free article] [PubMed] [Google Scholar]
- 42.Stock, A. M., V. L. Robinson, and P. N. Goudreau. 2000. Two-component signal transduction. Annu. Rev. Biochem. 69:183-215. [DOI] [PubMed] [Google Scholar]
- 43.Stockbauer, K. E., B. Fuchslocher, J. F. Miller, and P. A. Cotter. 2001. Identification and characterization of BipA, a Bordetella Bvg-intermediate phase protein. Mol. Microbiol. 39:65-78. [DOI] [PubMed] [Google Scholar]
- 44.Tzeng, Y. L., X. Zhou, S. Bao, S. Zhao, C. Noble, and D. S. Stephens. 2006. Autoregulation of the MisR/MisS two-component signal transduction system in Neisseria meningitidis. J. Bacteriol. 188:5055-5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uhl, M. A., and J. F. Miller. 1994. Autophosphorylation and phosphotransfer in the Bordetella pertussis BvgAS signal transduction cascade. Proc. Natl. Acad. Sci. USA 91:1163-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uhl, M. A., and J. F. Miller. 1995. BvgAS is sufficient for activation of the Bordetella pertussis ptx locus in Escherichia coli. J. Bacteriol. 177:6477-6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uhl, M. A., and J. F. Miller. 1996. Central role of the BvgS receiver as a phosphorylated intermediate in a complex two-component phosphorelay. J. Biol. Chem. 271:33176-33180. [DOI] [PubMed] [Google Scholar]
- 48.Uhl, M. A., and J. F. Miller. 1996. Integration of multiple domains in a two-component sensor protein: the Bordetella pertussis BvgAS phosphorelay. EMBO J. 15:1028-1036. [PMC free article] [PubMed] [Google Scholar]
- 49.Veal-Carr, W. L., and S. Stibitz. 2005. Demonstration of differential virulence gene promoter activation in vivo in Bordetella pertussis using RIVET. Mol. Microbiol. 55:788-798. [DOI] [PubMed] [Google Scholar]
- 50.Williams, C. L., P. E. Boucher, S. Stibitz, and P. A. Cotter. 2005. BvgA functions as both an activator and a repressor to control Bvg phase expression of bipA in Bordetella pertussis. Mol. Microbiol. 56:175-188. [DOI] [PubMed] [Google Scholar]
- 51.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 52.Zu, T., R. Manetti, R. Rappuoli, and V. Scarlato. 1996. Differential binding of BvgA to two classes of virulence genes of Bordetella pertussis directs promoter selectivity by RNA polymerase. Mol. Microbiol. 21:557-565. [DOI] [PubMed] [Google Scholar]