Abstract
The general stress response of Bacillus subtilis is controlled by the activity state of the σB transcription factor. Physical stress is communicated to σB via a large-molecular-mass (>106-Da) structure (the stressosome) formed by one or more members of a family of homologous proteins (RsbR, YkoB, YojH, YqhA). The positive regulator (RsbT) of the σB stress induction pathway is incorporated into the complex bound to an inhibitor protein (RsbS). Exposure to stress empowers an RsbT-dependent phosphorylation of RsbR and RsbS, with the subsequent release of RsbT to activate downstream processes. The mechanism by which stress initiates these reactions is unknown. In an attempt to identify changes in stressosome components that could lead to σB activation, a DNA segment encoding these proteins was mutagenized and placed into B. subtilis to create a merodiploid strain for these genes. Eight mutations that allowed heightened σB activity in the presence of their wild-type counterparts were isolated. Two of the mutations are missense changes in rsbR, and six are amino acid changes in rsbS. Additional experiments suggested that both of the rsbR mutations and three of the rsbS mutations likely enhance σB activity by elevating the level of RsbS phosphorylation. All of the mutations were found to be dominant over wild-type alleles only when they are cotranscribed within an rsbR rsbS rsbT operon. The data suggest that changes in RsbR can initiate the downstream events that lead to σB activation and that RsbR, RsbS, and RsbT likely interact with each other concomitantly with their synthesis.
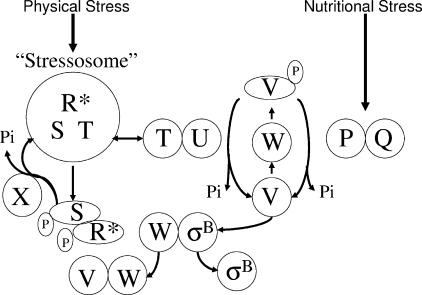
The general stress regulon of Bacillus subtilis consists of over 200 genes whose products confer resistance to multiple forms of stress (18, 27, 28, 33). The general stress regulon is controlled by the activity state of σB, a stress-activated secondary sigma factor (6-8) (Fig. 1). In the absence of stress, σB is held inactive, complexed with the anti-σB protein RsbW (5). σB is released from RsbW when a second protein (RsbV) binds to RsbW in lieu of σB (13, 14). RsbV is unable to catalyze σB release in unstressed B. subtilis due to an inactivating RsbW-dependent phosphorylation (14). The dephosphorylation and reactivation of RsbV-P is catalyzed by either of two stress-responsive phosphatases (RsbP and RsbU) (20, 32, 36). The RsbP phosphatase responds to nutritional stress (glucose or PO4 starvation or azide treatment), while RsbU is activated by physical stress (heat shock, osmotic shock, or ethanol) (1, 20, 32, 38, 40, 41). RsbP is cotranscribed with an additional protein (RsbQ) that is needed for its activity (9, 32). The metabolic inducer of RsbP/RsbQ is unknown, but RsbP/RsbQ activation occurs coincidently with a drop in cellular ATP levels (43, 44).
FIG. 1.
Model of σB control. As depicted in the lower portion of the diagram, σB is normally inactive, complexed with the anti-σB protein RsbW (W). σB is freed from RsbW when a release factor, RsbV (V) binds RsbW in lieu of σB. In the absence of stress, RsbV is unable to trigger σB release due to an RsbW-catalyzed phosphorylation. RsbV is dephosphorylated by one of two stress-responsive phosphatases that uniquely respond to physical or nutritional stress. The nutritional stress phosphatase, RsbP (P), requires a coexpressed protein, RsbQ (Q), for activity. The RsbQ/RsbP trigger is unknown, but its activation coincides with conditions that cause a drop in ATP. The physical stress phosphatase, RsbU (U), is activated by a second protein, RsbT (T), that is ordinarily bound to a negative regulator, RsbS (S), in a large (>106-Da) complex (stressosome) formed from RsbR and a family of paralogous proteins (R*). Following exposure to physical stress, an unknown mechanism allows RsbR and RsbS to become phosphorylated by RsbT. This frees RsbT to activate the RsbU phosphatase. RsbR-P and RsbS-P are dephosphorylated and reactivated by RsbX (X), a phosphatase whose levels increase following σB activation. This model is based on references given in the text.
The physical stress phosphatase (RsbU) also requires an additional protein (RsbT) for activity (39, 41). In the absence of stress, RsbT is unavailable to RsbU, bound to an inhibitory protein (RsbS) in large multiprotein complexes (>106 Da) termed “stressosomes” (11, 24). The stressosome incorporates RsbS and RsbT in a structure formed from one or more members of a family of homologous proteins (RsbR, YkoB, YojH, YqhA) (2, 11, 24). The RsbR proteins are needed for proper interaction between RsbS and RsbT (1, 2). In their absence, RsbS is unable to inhibit the RsbT-dependent activation of RsbU. The specific roles of each of the RsbR family members are unknown, but their functions appear to be at least partially redundant (2, 24). The loss of any one RsbR family member does not significantly affect the activity of σB. Only when multiple members of the RsbR family are lost is the ability of RsbS to inhibit RsbT compromised (24).
Exposure to physical stress triggers RsbT to phosphorylate both RsbR and RsbS (23, 41). Phosphorylation of RsbS is the key event that allows RsbT access to RsbU. Biochemical evidence suggests that the phosphorylation of RsbR, and presumably its paralogs, facilitates the subsequent phosphorylation of RsbS by RsbT (11, 24). This result suggests a sequential series of events in which stress triggers the phosphorylation of RsbR as a prerequisite for the phosphorylation of RsbS (11, 24). Assuming that this progression occurs in vivo, both RsbR and RsbT represent plausible targets for stress-dependent changes that could trigger σB activation. Presumably, stress-induced factors could initiate changes in RsbR to make it more amenable to phosphorylation or alter RsbT in ways that empower its kinase activity.
σB activity levels elevated by the physical stress pathway are restored to prestress levels by RsbX, a phosphatase that dephosphorylates and reactivates both RsbR and RsbS. This allows RsbT to again be sequestered in an inactivating complex (10, 41). RsbX levels increase following σB activation; however, it is not clear whether the increase in RsbX per se is responsible for the reduction in σB activity (15). Artificial manipulation of RsbX levels from an inducible promoter has little effect on σB inducibility (35). Only when RsbX is eliminated does σB activity rise to very high levels (4, 8, 35). The question of whether the inherent activity of RsbX is regulated and, if so, what role this might play in σB induction is unresolved.
The components of the physical stress pathway are encoded by the first four genes of the eight-gene sigB operon (39). The operon appears to be constitutively expressed from a promoter (PA) that is likely recognized by the cell's housekeeping sigma factor (σA), with a σB-dependent promoter (PB) within the operon to upregulate the downstream four genes when σB becomes active (i.e., PA rsbR rsbS rsbT rsbU, PB rsbV rsbW sigB rsbX) (8, 15, 39). In an attempt to identify changes in stressosome components that could lead to σB activation, a DNA segment encoding RsbR, RsbS, RsbT, and RsbU along with the PA promoter element was mutagenized and transferred into B. subtilis in such a way as to create merodiploid strains with two copies of this region. Eight mutations that allowed heightened σB activity in the presence of wild-type copies of the stress pathway genes were isolated. Two of the mutations were missense changes in rsbR, and six were amino acid changes in rsbS. Additional experiments suggest that both rsbR mutations and three of the six rsbS mutations enhance σB activity by allowing a higher background level of RsbS phosphorylation.
MATERIALS AND METHODS
Bacterial strains and plasmids.
All of the B. subtilis strains (Table 1) are derivatives of PY22. BSJ43 is BSA46 (4) transformed with a linearized Escherichia coli plasmid carrying the 1.2-kbp rsbP gene plus the spc cassette from pDG1726 (17) inserted into the unique HindIII site of rsbP. Plasmid pARE7 contains the 2.8-kbp DNA segment (PA rsbR rsbS rsbT rsbU) of pRU13 (31), cloned as a BamHI/SphI fragment into pUK19 (19). The cloned PA rsbR rsbS rsbT rsbU DNA was mutagenized in E. coli by N-methyl-N-nitro-N-nitrosoguanidine, as described by Autret et al. (3). B. subtilis strains BAR6 and its variants (BAR6-5 to BAR6-13) are BSJ43 (rsbP::spc) transformed with untreated or mutagenized (mut) pARE7 and selected for the plasmid-encoded antibiotic resistance. These strains carry integrated plasmids and are merodiploid for PA rsbR rsbS rsbT rsbU.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or features | Source, reference, or construction |
|---|---|---|
| Plasmids | ||
| pUK19 | Apr Kanr | 19 |
| pUR1 | Apr Kanr 520 bp upstream of sigB | 31 |
| pARE7 | Apr KanrPA rsbR rsbS rsbT rsbU | This study |
| pARE30 | AprPA rsbR rsbS(66GR) rsbT rsbU | This study |
| pARE31 | AprPA rsbR(225CY) rsbS rsbT rsbU | This study |
| pARE33 | AprPA rsbR rsbS(22ER) rsbT rsbU | This study |
| pARE34 | AprPA rsbR(136EK) rsbS rsbT rsbU | This study |
| pARE41 | AprPA rsbR rsbS(83GD) rsbT rsbU | This study |
| pARE42 | AprPA rsbR rsbS(23LF) rsbT rsbU | This study |
| pARE43 | AprPA rsbR rsbS(76GR) rsbT rsbU | This study |
| pARE65 | AprPA rsbR rsbS(86PL) rsbT rsbU | This study |
| pARE83 | Apr KanrPA rsbR(225CY) rsbS | This study |
| pARE85 | Apr KanrPA rsbR rsbS(66GR) | This study |
| pARE86 | Apr KanrPA rsbR rsbS(22EK) | This study |
| pARE88 | Apr KanrPA rsbR rsbS(76GR) | This study |
| pARE89 | Apr KanrPA rsbR | This study |
| pARE91 | Apr KanrPA rsbR rsbS(86PL) | This study |
| pARE92 | Apr KanrPA rsbR rsbS(23LF) | This study |
| pARE93 | Apr KanrPA rsbR(136EK) | This study |
| pARE106 | Apr KanrPA rsbR rsbS(83GD) | This study |
| pARE109 | Apr KanrPA rsbR rsbS | This study |
| pARE114 | Apr Kanr pUR1::PA rsbR rsbS(22ER 59SA) rsbT rsbU | This study |
| pARE116 | Apr Kanr pUR1::PA rsbR rsbS(86PL 59SA) rsbT rsbU | This study |
| pARE122 | Apr Kanr pUR1::PA rsbR(136EK) rsbS(59SA) rsbT rsbU | This study |
| pARE123 | Apr Kanr pUR1::PA rsbR(136EK 171TA) rsbS rsbT rsbU | This study |
| pARE124 | Apr Kanr pUR1::PA rsbR(225CY) rsbS(59SA) rsbT rsbU | This study |
| pARE125 | Apr Kanr pUR1::PA rsbR(225CY 171TA) rsbS rsbT rsbU | This study |
| pARE126 | Apr Kanr pUR1::PA rsbR rsbS(66GR 59SA) rsbT rsbU | This study |
| pARE129 | Apr Kanr pUR1::PA rsbR rsbS(83GD 59SA) rsbT rsbU | This study |
| pARE131 | Apr Kanr pUR1::PA rsbR rsbS(76GR 59SA) rsbT rsbU | This study |
| pARE132 | Apr Kanr pUR1::PA rsbR(171TA) rsbS rsbT rsbU | This study |
| pARE133 | Apr Kanr pUR1::PA rsbR rsbS(59SA) rsbT rsbU | This study |
| pARE142 | Apr Kanr pUR1::PA rsbR rsbS(23LF 59SA) rsbT rsbU | This study |
| B. subtilis strains | ||
| PY22 | trpC2 | 4 |
| BSA46 | trpC2 SPβ ctc::lacZ | 4 |
| BSJ43 | trpC2 rsbP::spc | J. Scott |
| BAR6 | trpC2 rsbP::spc aph3′5"/PA rsbR rsbS rsbT rsbU SPβ ctc::lacZ | pARE7-BSJ43 |
| BAR6#5 | trpC2 rsbP::spc aph3′5"/PA rsbR rsbS(83GD) rsbT rsbU SPβ ctc::lacZ | mutpARE7-BSJ43 |
| BAR6#6 | trpC2 rsbP::spc aph3′5"/PA rsbR rsbS(23LF) rsbT rsbU SPβ ctc::lacZ | mutpARE7-BSJ43 |
| BAR6#7 | trpC2 rsbP::spc aph3′5"/PA rsbR rsbS(76GR) rsbT rsbU SPβ ctc::lacZ | mutpARE7-BSJ43 |
| BAR6#8 | trpC2 rsbP::spc aph3′5"/PA rsbR rsbS(86PL) rsbT rsbU SPβ ctc::lacZ | mutpARE7-BSJ43 |
| BAR6#9 | trpC2 rsbP::spc aph3′5"/PA rsbR rsbS(22ER) rsbT rsbU SPβ ctc::lacZ | mutpARE7-BSJ43 |
| BAR6#10 | trpC2 rsbP::spc aph3′5"/PA rsbR(136EK) rsbS rsbT rsbU SPβ ctc::lacZ | mutpARE7-BSJ43 |
| BAR6#11 | trpC2 rsbP::spc aph3′5"/PA rsbR rsbS(66GR) rsbT rsbU SPβ ctc::lacZ | mutpARE7-BSJ43 |
| BAR6#13 | trpC2 rsbP::spc aph3′5"/PA rsbR(225CY) rsbS rsbT rsbU SPβ ctc::lacZ | mutpARE7-BSJ43 |
| BAR11 | trpC2 rsbP::spc rsbS(22ER) SPβ ctc::lacZ | BAR6#9-BSA46 |
| BAR12 | trpC2 rsbP::spc rsbR(136EK) SPβ ctc::lacZ | BAR6#10-BSA46 |
| BAR14 | trpC2 rsbP::spc rsbS(66GR) SPβ ctc::lacZ | BAR6#11-BSA46 |
| BAR15 | trpC2 rsbP::spc rsbR(225CY) SPβ ctc::lacZ | BAR6#13-BSA46 |
| BARM1 | trpC2 rsbP::spc rsbS(83GD) SPβ ctc::lacZ | BAR6#5-BSA46 |
| BARM15 | trpC2 rsbP::spc rsbS(23LF) SPβ ctc::lacZ | BAR6#6-BSA46 |
| BARM24 | trpC2 rsbP::spc rsbS(76GR) SPβ ctc::lacZ | BAR6#7-BSA46 |
| BAR42 | trpC2 rsbP::spc rsbS(86PL) SPβ ctc::lacZ | BAR6#8-BSA46 |
| BAR48 | trpC2 rsbP::spc aph3′5"/PA rsbR(225CY) SPβ ctc::lacZ | pARE83-BSJ43 |
| BAR50 | trpC2 rsbP::spc aph3′5"/PA rsbR rsbS(83GD) SPβ ctc::lacZ | pARE106-BSJ43 |
| BAR54 | trpC2 rsbP::spc aph3′5"/PA rsbR rsbS(66GR) SPβ ctc::lacZ | pARE85-BSJ43 |
| BAR59 | trpC2 rsbP::spc aph3′5"/PA rsbR rsbS(86PL) SPβ ctc::lacZ | pARE91-BSJ43 |
| BAR61 | trpC2 rsbP::spc aph3′5"/PA rsbR SPβ ctc::lacZ | pARE89-BSJ43 |
| BAR62 | trpC2 rsbP::spc aph3′5"/PA rsbR rsbS(23LF) SPβ ctc::lacZ | pARE92-BSJ43 |
| BAR63 | trpC2 rsbP::spc aph3′5"/PA rsbR rsbS(76GR) SPβ ctc::lacZ | pARE88-BSJ43 |
| BAR68 | trpC2 rsbP::spc aph3′5"/PA rsbR(136EK) SPβ ctc::lacZ | pARE93-BSJ43 |
| BAR70 | trpC2 rsbP::spc aph3′5"/PA rsbR rsbS SPβ ctc::lacZ | pARE109-BSJ43 |
| BAR85 | trpC2 rsbP::spc aph3′5"/rsbS(76GR 59SA) SPβ ctc::lacZ | pARE131-BSJ43 |
| BAR87 | trpC2 rsbP::spc aph3′5"/rsbS(22ER 59SA) SPβ ctc::lacZ | pARE114-BSJ43 |
| BAR89 | trpC2 rsbP::spc aph3′5" rsbS(86PL 59SA) SPβ ctc::lacZ | pARE116-BSJ43 |
| BAR90 | trpC2 rsbP::spc aph3′5"/rsbR(136EK) rsbS(59SA) SPβ ctc::lacZ | pARE122-BSJ43 |
| BAR91 | trpC2 rsbP::spc aph3′5"/rsbR(136EK 171TA) SPβ ctc::lacZ | pARE123-BSJ43 |
| BAR92 | trpC2 rsbP::spc aph3′5"/rsbS(83GD 59SA) SPβ ctc::lacZ | pARE129-BSJ43 |
| BAR93 | trpC2 rsbP::spc aph3′5"/rsbS(66GR 59SA) SPβ ctc::lacZ | pARE126-BSJ43 |
| BAR95 | trpC2 rsbP::spc aph3′5"/rsbS(59SA) SPβ ctc::lacZ | pARE133-BSJ43 |
| BAR97 | trpC2 rsbP::spc aph3′5"/rsbR(171TA) SPβ ctc::lacZ | pARE132-BSJ43 |
| BAR99 | trpC2 rsbP::spc aph3′5"/rsbR(225CY 171TA) SPβ ctc::lacZ | pARE125-BSJ43 |
| BAR100 | trpC2 rsbP::spc aph3′5"/rsbR(225CY) rsbS(59SA) SPβ ctc::lacZ | pARE124-BSJ43 |
| BAR104 | trpC2 rsbP::spc aph3′5"/rsbR rsbS(23LF 59SA) SPβ ctc::lacZ | pARE142-BSJ43 |
| E. coli strains | ||
| ARE7 | PA rsbR rsbS rsbT rsbU | pARE7-DH5α |
| ARE31 | PA rsbR(225CY) rsbS rsbT rsbU | pARE31-DH5α |
| ARE34 | PA rsbR(136EK) rsbS rsbT rsbU | pARE34-DH5α |
To obtain B. subtilis strains in which the mutant rsb alleles are present in single copy (i.e., BAR11 to BAR15, BARM1, BARM15, BARM24, and BARM42), each of the two PA rsbR rsbU regions of the mutant strains was separately amplified with oligonucleotides that hybridize to chromosomal sequences outside of those contained in the originally cloned B. subtilis DNA paired with oligonucleotides that hybridize to the vector sequence at either side of the cloned DNA (i.e., the M13 Fwd and Rev sequencing primers). The amplified DNAs were separately transformed into B. subtilis strain BSA46 (ctc::lacZ) (4) in a congression with chromosomal DNA from BSJ43 (rsbP::spc ctc::lacZ). Spcr clones were screened for the mutant phenotype (i.e., blue colony color) on plates containing X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside). Chromosomal DNAs from presumptive mutant strains were analyzed by PCR to verify the presence of the PA rsbR rsbS rsbT rsbU region in single copy. The PA rsbR rsbS rsbT rsbU elements carrying the mutant alleles were cloned into pCR2.1-T0P0 (Invitrogen, Carlsbad, CA) to create plasmids pARE30 to pARE65.
To construct strains in which the mutant rsbR or rsbS genes were expressed as PA rsbR or PA rsbR rsbS transcription units separately from the other genes of the sigB operon (i.e., BAR48, BAR50, BAR54, BAR59, BAR61, BAR62, BAR63, BAR68, and BAR70), DNA segments encoding these alleles were amplified from chromosomal DNA of B. subtilis strains that carry them in single-copy sigB operons. An oligonucleotide primer that hybridized 156 bp upstream of rsbR was used in conjunction with one that hybridized either 42 bp downstream of rsbR or 31 bp downstream of rsbS to amplify PA rsbR or PA rsbR rsbS, respectively. The amplified DNAs were cloned into pUK19 as BamHI/SphI DNA fragments. The resulting plasmids (pARE83 to pARE109) were then transformed into BSJ43 (rsbP::spc SPβ ctc::lacZ). Clones with plasmid integrations were selected on the basis of the plasmid-encoded Kanr. Depending on the site within the PA rsbR or PA rsbR rsbS elements at which recombination with the sigB operon occurred, the mutant rsbR or rsbS alleles would either be expressed separately from the sigB operon or be exchanged for their wild-type counterparts within the sigB operon. When plated on media with X-Gal, two colony types were evident. One had the blue colony color characteristic of the parental mutant strain, while the other had the white phenotype of BSJ43. Chromosomal DNA was extracted from clones of each colony type. The PA rsbR or PA rsbR rsbS regions, both upstream (separate from the sigB operon) and downstream (within the sigB operon) of the integrated vector, were separately amplified with oligonucleotide primers that hybridized either 456 bp upstream of rsbR or 399 bp downstream of rsbS in concert with the M13 Fwd and Rev sequencing primers. This allowed amplification of the rsbR rsbS regions that were either upstream or within the sigB operon, respectively. The identity of the rsbR or rsbS allele in each of these sites was determined by DNA sequencing. In each case, the chromosomal DNA from the blue (mutant phenotype) colonies had a mutant rsbR or rsbS allele as part of their sigB operons, while the white (parental recipient phenotype) colonies contained wild-type sigB operons with the mutant alleles within the DNA element upstream of the integrated plasmid.
DNA segments in which rsbR(171TA) or rsbS(59SA) mutations were added to the previously isolated rsbR and rsbS mutations were constructed by site-directed mutagenesis (Gene Tailor; Invitrogen, Carlsbad, CA). The PA rsbR rsbS rsbT rsbU regions, containing either the wild-type or mutant rsbR or rsbS alleles previously cloned in pUC19 (i.e., pARE30 to pARE65), were cut from these plasmids with BamHI/SphI and cloned downstream of the Kanr gene in pUR1. pUR1 carries 520 bp of chromosomal DNA from the region immediately upstream of the sigB operon. This DNA is inserted next to the plasmid's Kanr gene opposite the side at which the rsbR rsbS rsbT rsbU element is cloned. The resulting plasmids were mutagenized in vitro with oligonucleotides that would specifically add the rsbR(171TA) or rsbS(59SA) mutations. The presence of the desired changes in the mutagenized plasmid DNAs was determined by DNA sequencing. Representative plasmids (pARE114 to pARE142) were linearized with ScaI and transformed into BSJ43 (rsbP::spc SPβ ctc::lacZ). Kanr clones arise by a double recombination between the homologous DNAs on both sides of the kan gene. The proximity of the rsbR and rsbS genes to the kan gene favored incorporation of the mutant alleles into the chromosomes of the transformants. Allelic replacement was verified by amplification of the region from the transformant clones' chromosomal DNA with primers that hybridized 456 bp upstream of rsbR and 31 bp downstream of rsbS, followed by DNA sequencing.
Gel filtration analysis.
Gel filtration was performed as previously described (25). One-liter Escherichia coli cultures expressing the desired rsb alleles were grown to a mid-logarithmic stage (optical density at 540 nm [OD540], 0.5), quickly chilled by the addition of ice, and harvested by centrifugation. Cells were washed, resuspended in 5 ml of a low-salt buffer (10 mM Tris [pH 8.0], 50 μM EDTA, 1.5 mM MgCl2, 1 mM dithiothreitol, 0.03% phenylmethylsulfonyl fluoride), and disrupted with a French pressure cell. Cell debris was removed by centrifugation (5,000 × g for 10 min) at 4°C. Two milliliters of the supernatant was loaded onto a 120-ml Sephacryl S-300 column. Five-milliliter fractions were collected and precipitated with two volumes of ethanol for analysis by 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting (25).
General methods.
B. subtilis was grown with shaking in Luria-Bertani (LB) medium (29). Physical stress was imposed by the addition of ethanol (4% final concentration) to logarithmically growing cultures. β-Galactosidase assays were performed with chloroform-permeabilized cells, as described by Kenny and Moran (22). Western blot assays were undertaken as previously described with mouse monoclonal antibodies against RsbR and RsbS (15). B. subtilis transformation was carried out by the method of Yasbin et al. (42).
RESULTS
Isolation and identification of mutations in the physical stress pathway.
The four principal components (RsbR, RsbS, RsbT, and RsbU) of the σB physical stress activation pathway are encoded by the first four genes of the sigB operon (39). We sought to identify changes in these proteins that lead to σB activation in the absence of stress. Such changes could offer clues into the properties of these proteins and the ways in which exposure to stress might alter them. The stress pathway components include both negative (RsbR, RsbS) and positive (RsbT, RsbU) regulators. To minimize activation of σB due to loss-of-function mutations in the negative regulators, the experiment was conducted with an rsbR rsbS rsbT rsbU merodiploid strain. The use of such a strain was anticipated to favor the isolation of dominant mutations that constitutively activate the σB physical stress pathway.
A DNA fragment containing the coding sequence for rsbR, rsbS, rsbT, and rsbU along with their normal promoter (PA) was mutagenized and transformed into B. subtilis on an integrating plasmid vector. The resulting transformants contain two expressed copies of each of the four rsb genes. The recipient strain also carries a reporter gene (lacZ) under the control of a σB-dependent promoter (Pctc) and a null mutation in the regulatory phosphatase (RsbP) required to activate σB in response to nutritional stress. Transformant clones that form blue colonies on media containing X-Gal are likely to include variant B. subtilis with dominant mutations in components in the rsbR rsbS rsbT rsbU element that allow σB to be active in the absence of stress and the presence of wild-type rsbR rsbS rsbT rsbU alleles. Eight clones with distinct blue colony phenotypes were selected for further study. DNA was extracted from these clones and transformed into a naïve RsbP− Pctc::lacZ strain to verify linkage of the σB activation phenotype (i.e., blue colony color on X-Gal medium) with the antibiotic resistance of the integrated vector. Once the linkage was verified, we created strains in which the rsbR rsbS rsbT rsbU region and the mutant rsb alleles are present in single copy. To accomplish this, each of the two individual rsbR rsbS rsbT rsbU regions of the merodiploid strains was independently amplified from chromosomal DNA with oligonucleotide primers that specifically hybridize to the rsbR rsbS rsbT rsbU region that lies either upstream or downstream of the integrated plasmid vector. The amplified DNAs were separately transformed into B. subtilis by a DNA congression technique. Chromosomal DNA from a B. subtilis parental strain containing an rsbP::spc disruption was added to each of the PCR-amplified DNAs. The mixtures were then transformed into wild-type B. subtilis that carried the Pctc::lacZ reporter system. Transformants selected on the basis of Spcr were screened for the blue colony phenotype associated with heightened σB activity. As anticipated, only one of the two amplified DNAs yielded transformants that included clones with heightened β-galactosidase activity. PCR analysis of chromosomal DNA from representative clones verified the presence of a single copy of the rsbR rsbS rsbT rsbU region in these strains (data not shown).
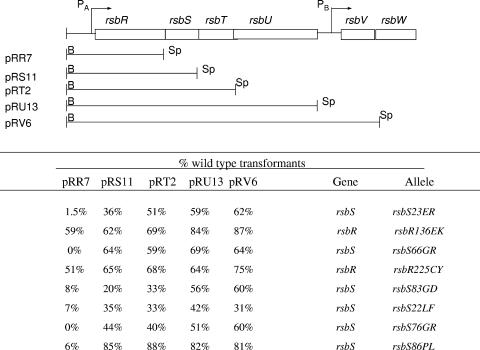
A series of mapping plasmids (31) was then used to localize the position of rsb mutations within the rsbR rsbS rsbT rsbU gene cluster (Fig. 2). Each plasmid in the series contains a Kanr gene adjoining a DNA segment that is homologous with the chromosomal region immediately upstream of the sigB operon. On the other side of the Kanr gene is the sigB operon promoter and increasingly longer segments of the sigB operon. Each plasmid adds a sigB operon gene to the genes that are present on the plasmid that precedes it in the series. Linearization of the plasmids, transformation into the mutant strains, and selection for Kanr results in transformant clones in which the Kanr cassette and increasing lengths of the sigB operon are introduced into the recipient cell's chromosome by homologous recombination. The promoter-distal gene on the smallest plasmid in the series that reversed the blue colony phenotype was inferred to be the likely site of the mutation. A variable number of white colonies (<10%) normally appeared among the transformant clones due to loss of the reporter system during the transformation process. Based on this analysis (Fig. 2), two of the mutations mapped to rsbR and the remaining six mapped to rsbS.
FIG. 2.
Transformation mapping.
To verify the sites of the mutations and identify the changes responsible for the phenotype, the amplified DNA fragments that transformed wild-type B. subtilis to the mutant phenotype were cloned and sequenced. Each sequenced DNA contained a base change in the gene that had been identified in the mapping experiment. In all cases, the base changes substituted an alternative amino acid at the site of the change. These changes are listed in the last column of Fig. 2 and illustrated in Fig. 3. One of the mutations in rsbR results in a charge change (Glu→Lys) at residue 136. The second mutation leads to a replacement of the protein's sole cystine residue (Cys225) with a tyrosine. Three of the rsbS mutations substituted charged amino acids for glycine residues in a glycine-rich region of the protein (i.e., Gly66→Arg, Gly76→Arg, Gly83→Asp). The remaining mutations included two that were clustered near the amino terminus of RsbS, a substitution of Phe for Leu at position 22 and a charge change (Glu→Arg) at the adjoining position 23. The final rsbS mutation replaced a Pro residue at position 86 with Leu. All of the mutations in rsbS, as well as the Glu136→Lys136 change in rsbR, are changes in amino acids that are invariant at these positions in sequenced Bacillus and Listeria species. The Cys changed to Tyr at position 225 of rsbR is less highly conserved but is the only Cys residue in the protein.
FIG. 3.
RsbR and RsbS mutations. The amino acid sequences of RsbR and RsbS are illustrated. Sites of RsbR and RsbS phosphorylation (i.e., T171 and T205 of RsbR and S59 of RsbS) are in boldface type. Amino acid changes in RsbR and RsbS that were mapped and indicated in Fig. 2 are placed above the original residues in the sequence.
Effects of the rsbR and rsbS mutations on σB activity and product abundance.
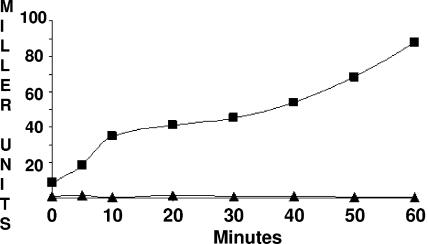
To quantitate the effects of the mutations in rsbR and rsbS on the background activity of the physical stress pathway, strains lacking the nutritional stress pathway (i.e., RsbP−) and carrying either the wild-type or altered rsbR/rsbS alleles were grown in LB and analyzed for σB-dependent β-galactosidase activity. As was seen in previous studies wherein the activities of negative regulators of σB were compromised (4), β-galactosidase specific activity in the mutant cultures increased during growth. This presumably reflects the increase in σB from its autoregulated promoter under conditions where normal negative control is not fully present. A representative plot illustrating this phenomenon in one of the rsbR mutations is presented in Fig. 4. The σB activity levels of each of the mutant strains during mid-log phase (OD = 0.5) and 30 min after entry into stationary phase are illustrated in Table 2. The table, depicting the average values from three independent experiments, includes mid-log-phase data from both the merodiploid and single-copy strains. The effects of the mutations in rsbR or rsbS are not suppressed by the presence of their wild-type counterparts. In general, the σB activities of the mutant rsbR or rsbS strains are elevated to similar degrees regardless of whether or not the mutant genes are expressed as the strain's sole source of RsbR or RsbS.
FIG. 4.
σB activity in a representative mutant. B. subtilis strains BSJ43 (rsbP::spc SPβ ctc::lacZ) (triangles) and BAR12 [rsbP::spc rsbR(136EK) SPβ ctc::lacZ)] (squares) growing logarithmically in LB medium were sampled at the indicated times and analyzed for σB-dependent β-galactosidase levels (Miller units) as described in Materials and Methods.
TABLE 2.
σB activity in mutant rsbR and rsbS strains during growth and physical stress
| Strain | β-Galactosidase (ctc::lacZ) activity (Miller units) under the indicated conditionsa
|
|||
|---|---|---|---|---|
| Merodiploidb | Single copyc
|
4% ethanold | ||
| Growth (OD, 0.5) | Stationary phase | |||
| Wild type (RsbP−) | 1.2 ± 0.3 | 1.0 ± 0.1 | 0.6 ± 0.7 | 37.7 ± 0.1 |
| rsbR(136EK) | 19.2 ± 0.6 | 21.4 ± 0.9 | 34.5 ± 2.6 | 98.4 ± 4.6 |
| rsbR(225CY) | 28.5 ± 2.2 | 42.3 ± 1.8 | 62.4 ± 4.0 | 97.6 ± 2.2 |
| rsbS(22ER) | 42.5 ± 7.2 | 60.7 ± 0.1 | 82.5 ± 3.7 | 119.0 ± 6.9 |
| rsbS(23LF) | 3.6 ± 0.9 | 1.2 ± 0.1 | 6.9 ± 1.3 | 52.8 ± 0.7 |
| rsbS(66GR) | 6.6 ± 1.0 | 13.2 ± 1.1 | 39.9 ± 3.3 | 117.0 ± 9.3 |
| rsbS(76GR) | 3.1 ± 1.1 | 5.3 ± 0.6 | 4.4 ± 0.3 | 75.7 ± 3.7 |
| rsbS(83GD) | 3.1 ± 0.4 | 1.4 ± 0.1 | 10.6 ± 2.4 | 69.5 ± 2.1 |
| rsbS(86PL) | 7.3 ± 2.9 | 11.6 ± 1.6 | 36.4 ± 2.2 | 77.1 ± 8.9 |
Values are averages of three separate determinations ± standard deviations.
Merodiploid strains contain two copies of the PA rsbR rsbS rsbT rsbU element. Both copies are wild type in the first strain listed. The remaining strains contain the indicated allele of rsbR or rsbS in one of the two elements, with its wild-type counterpart in the other. All strains are RsbP−.
Strains are RsbP− and contain single copies of sigB operon genes with the indicated alleles. Stationary-phase values were obtained 30 min after the cessation of growth.
Samples were taken from actively growing cultures at 20 min after ethanol addition.
As expected from the ongoing accumulation of β-galactosidase during growth in these mutant strains (Fig. 4), β-galactosidase levels are significantly lower during a mid-log phase of growth than during stationary phase. During growth, some mutants [e.g., rsbS(23LF) and rsbS(83GD)] exhibited σB activity that was barely above that seen in the wild-type parental strains, while others [e.g., rsbR(136EK), rsbR(225CY), and rsbS(22ER)] displayed σB activities more than 20-fold higher than that of their wild-type parent. By the time the cultures had entered stationary phase, this had increased to levels that were 7- to 100-fold higher than that seen in a stationary-phase culture of the wild-type parental strain.
We next asked whether any of the mutations, in altering the background activity of the physical stress pathway, might also prevent further induction of the pathway by physical stress. To this end, wild-type and mutant B. subtilis cells grown in LB were subjected to ethanol stress (4% ethanol) and analyzed for σB-dependent β-galactosidase activity 20 min after ethanol addition. In each instance, the addition of ethanol resulted in elevated σB activity (Table 2). The increase in σB activity following ethanol treatment in the rsbR mutant strain may be the result of either the residual activity in the mutant RsbR proteins or the compensating activities of the RsbR paralogs (2, 24). In the strains carrying mutant rsbS alleles, the products of these alleles are the strains' only source of RsbS. The stress inducibility of σB activity in these strains suggests that the altered RsbS proteins are still able to at least partially perform their roles as stress-modulated inhibitors of RsbT.
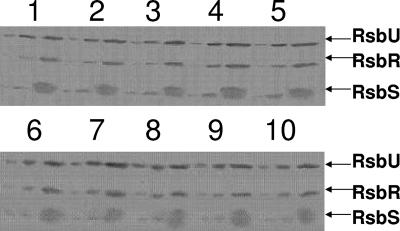
Western blot analyses were next conducted to determine whether the mutations in rsbR or rsbS alter the abundance of their products. Given that loss-of-function mutations in rsbR are essentially without effect on σB activity, it is unlikely that changes in RsbR levels per se could account for the rsbR alleles' mutant phenotype. The loss of RsbS does, however, elevate σB activity. As such, it is formally possible that some of the mutations in rsbS might elevate σB by reducing the levels of RsbS. As a means of standardizing the Western blot reactions, the relative abundance of RsbR and RsbS in each strain's extract was compared to that of the physical stress pathway phosphatase (RsbU) which is also present in the extracts. Figure 5 displays the Western blots. The levels of RsbR and RsbS in each of the mutant strain extracts are indistinguishable from those of their wild-type parent. Thus, the changes in σB activity are not the result of changes in the levels of either RsbR or RsbS but rather changes in their inherent activities.
FIG. 5.
Western blot analysis of RsbR and RsbS abundance in wild-type and mutant B. subtilis. B. subtilis strains BSJ43 (wild-type rsbR and rsbS) (lanes 1 and 6), BAR11 rsbS(22ER) (lane 2), BAR12 rsbR(136EK) (lane 3), BAR14 rsbS(66GR) (lane 4), BAR15 rsbR(225CY) (lane 5), BARM1 rsbS(83GD) (lane 7), BARM15 rsbS(23LT) (lane 8), BARM24 rsbS(79GR) (lane 9), and BAR42 rsbS(86PL) (lane 10) were grown to an OD of 0.5 in LB. Crude extract samples equivalent to 100, 300, and 900 μl of the original cultures were analyzed by Western blotting using monoclonal antibodies specific for RsbU, RsbR, and RsbS. The positions of each of these proteins are indicated.
RsbR and RsbS are negative regulators of the physical stress pathway (2, 20, 24). As such it could be envisioned that mutations that interfere with their ability to act as inhibitors might allow σB to become active even in the presence of a wild-type allele. Although this is at least plausible in the case of the rsbS mutations, where a loss of RsbS leads to a dramatic increase in σB activity, it is unlikely with respect to the rsbR mutations. rsbR deletion mutations have little effect on σB activity. Only when both RsbR and its paralogs are lost is the activity of σB markedly elevated. This suggests that either the changes in RsbR are somehow inhibiting the ability of both the mutant RsbRs and the RsbR paralogs to function in RsbS/RsbT sequestration or the changes in RsbR have created an RsbR variant that has become a positive effector of σB activation. The latter possibility could involve altering RsbR in ways that allow it to facilitate the phosphorylation of RsbS in the absence of stress.
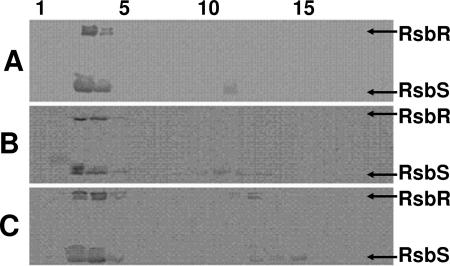
A possible mechanism by which changes in RsbR could impair the ability of both the mutant RsbR and its paralogs to sequester RsbS and RsbT would be if the mutant protein fails to properly engage in stressosome formation and as a consequence disrupts the chimeric stressosome structure that is believed to form from RsbR and its paralogs (12, 24). To test whether the mutant RsbR proteins can form high-molecular-weight associations that are able to sequester RsbS, crude extracts were prepared from E. coli strains carrying DNA segments that express rsbR rsbS rsbT rsbU with either wild-type rsbR or the rsbR(136EK) or rsbR(225CY) alleles. High-molecular-mass RsbR/RsbS structures form when wild-type RsbR and RsbS are expressed in E. coli (25). E. coli extracts from strains expressing the Rsb proteins were fractionated by gel filtration and analyzed by Western blotting for the partitioning of RsbR and RsbS among the fractions. The use of E. coli in this experiment allows visualization of potential RsbR/RsbS complexes in the absence of the RsbR paralogs which might mask any deficiency of RsbR itself in sequestering RsbS. The results of the gel filtration analyses are illustrated in Fig. 6. E. coli extracts containing either the RsbR(136EK) or RsbR(225CY) proteins (Fig. 6B and C, respectively) contained large complexes that included RsbS (fractions 3 and 4). The fractionation properties of these complexes were indistinguishable from those formed by wild-type RsbR (Fig. 6A). Although this result does not rule out the possibility that the 136EK and 225CY mutations cause subtle changes in the interactions among the RsbR subunits or between RsbR and RsbS, the changes in RsbR caused by these mutations do not grossly alter the ability of the mutant proteins to form high-molecular-mass associations and sequester RsbS.
FIG. 6.
Gel filtration chromatography of Rsb proteins in E. coli extracts. Crude extracts were prepared from E. coli strains carrying plasmids pARE7 (PA rsbR rsbS rsbT rsbU) (A), pARE34 [PA rsbR(136EK) rsbS rsbT rsbU] (B), or pARE31 [PA rsbR(225CY) rsbS rsbT rsbU] (C) were fractionated through Sephacryl S-300. Samples from each fraction were analyzed by SDS-PAGE and Western blotting using monoclonal antibodies specific for RsbR and RsbS. Numbers at the top of the figure are fraction numbers, with fraction 1 being the earliest-eluting (high-molecular-mass) fraction. Coomassie-stained gels (not shown) indicate elution of ribosomes between fractions 1 and 5. The positions of RsbR and RsbS on the Western blots are indicated.
Activity of mutant RsbR proteins requires residues that are targets for stress-dependent phosphorylation.
The activation of σB by physical stress is believed to involve an RsbT-dependent phosphorylation of RsbR and then RsbS, with the phosphorylation of RsbR accelerating the subsequent phosphorylation of RsbS (10, 11, 16, 24). In such a model, RsbR initially serves as a negative regulator of the stress pathway by allowing RsbS to sequester RsbT, but once phosphorylated, RsbR acts as a positive element for the reactions that follow. This raises the possibility that one or both of the mutations that we isolated in RsbR are activating σB by changing RsbR so that it either resembles the phosphorylated form of RsbR or is more readily phosphorylated. In either case, the downstream consequence should be σB activation that is dependent on the phosphorylation of RsbS. To determine whether the heightened σB activity seen in the rsbR mutant strains was occurring via RsbS phosphorylation, we created B. subtilis strains in which the mutant rsbR alleles were paired with an rsbS allele, rsbS(59SA), whose product cannot be phosphorylated by RsbT (21) and asked what effect this additional mutation had on the strain's original σB activity. As seen in Table 3, alteration of RsbS so that it can no longer be phosphorylated lowers the mutant strains' σB-dependent reporter gene activity to a level seen in a strain with the rsbS(59SA) allele alone. The data are consistent with the notion that the rsbR mutations do not elevate σB activity by preventing RsbS from binding RsbT but rather by altering RsbR in such a way as to allow RsbS to be more readily phosphorylated by RsbT.
TABLE 3.
σB activity in mutant rsbR and rsbS double mutants
| Relevant allele | β-Galactosidase (ctc::lacZ) activity (Miller units)a |
|---|---|
| Wild type | 1.03 ± 0.42 |
| rsbR(171TA) | 0.52 ± 0.14 |
| rsbS(59SA) | 0.62 ± 0.39 |
| rsbR(136EK) | 20.1 ± 0.67 |
| rsbR(136EK 171TA) | 0.80 ± 0.27 |
| rsbR(136EK) rsbS(59SA) | 0.85 ± 0.51 |
| rsbR(225CY) | 39.7 ± 2.42 |
| rsbR(225CY 171TA) | 0.39 ± 0.22 |
| rsbR(225CY) rsbS(59SA) | 0.41 ± 0.20 |
| rsbS(22ER) | 63.2 ± 3.46 |
| rsbS(22ER 59SA) | 50.3 ± 4.10 |
| rsbS(23LF) | 1.20 ± 0.225 |
| rsbS(23LF 59SA) | 0.49 ± 0.69 |
| rsbS(66GR) | 11.7 ± 0.75 |
| rsbS(66GR 59SA) | 4.51 ± 0.27 |
| rsbS(76GR) | 4.39 ± 1.76 |
| rsbS(76GR 59SA) | 1.70 ± 0.28 |
| rsbS(83GD) | 1.28 ± 0.12 |
| rsbS(83GD 59SA) | 0.47 ± 0.10 |
| rsbS(86PL) | 13.8 ± 0.08 |
| rsbS(86PL 59SA) | 0.42 ± 0.27 |
Values are averages of three determinations ± standard deviations for cultures harvested during mid-logarithmic-phase growth.
In vitro phosphorylation of the threonine residues at position 171 or 205 of RsbR enhances the kinase activity of RsbT for RsbS (10, 11). Thr 171 is the site in RsbR that is preferentially phosphorylated by RsbT and the likely site in RsbR at which stress-dependent phosphorylation occurs to stimulate the phosphorylation of RsbS (10). If the mutant RsbR proteins facilitate the phosphorylation of RsbS via an RsbR-P intermediate, prevention of RsbR phosphorylation may block the activation. To test this idea, rsbR alleles were created in which a Thr-to-Ala substitution at position 171 was added to the 136EK and 225CY mutations. When σB activity was assayed (Table 3), the β-galactosidase levels in the strains carrying both mutations in rsbR had fallen to the level observed in a strain with the rsbR(171TA) mutation alone. Thus, the rsbR(171TA) mutation is dominant over both the rsbR 136EK and 225CY changes. Although it is possible that the Thr→Ala substitution may have altered properties of RsbR beyond merely preventing its phosphorylation (10, 16), it is clear that altering this residue abolishes the ability of the mutant rsbR alleles to activate σB.
We next turned to the mutations in rsbS. Given the role of RsbS as a direct inhibitor of RsbT, there are at least two possible ways that the mutations in rsbS could enhance σB activity. The changes could either reduce the ability of the mutant RsbS to bind RsbT and prevent the activation of RsbT or make the RsbS products more prone to phosphorylation and RsbT release. Although mutations of the former kind (i.e., those that inactivate RsbS) were not initially anticipated due to the presence of a potentially complementing wild-type rsbS allele, there is increasing evidence that RsbS predominantly regulates the RsbT protein with which it is cotranscribed (see below and reference 45). Thus, mutations that impair RsbS function could be included in our collection. In an attempt to distinguish changes in RsbS that impair its ability to bind and hold RsbT inactive from those which facilitate RsbS phosphorylation by RsbT, we added the rsbS(59SA) mutation to the altered rsbS alleles. Mutations that impair the ability of RsbS to inhibit RsbT should still allow σB to be active in the presence of the serine-to-alanine substitution, while those that promote σB activity via RsbS phosphorylation should be no more active than strains with the rsbS(59SA) mutation alone. When B. subtilis strains carrying both mutations in rsbS were assayed for σB-dependent activity (Table 3), three of the six rsbS alleles retained most [rsbS(22ER)] or part [rsbS(66GR) and rsbS(76GR)] of their original heightened σB activity. Presumably, these strains are compromised in the ability to inhibit RsbT and so their phosphorylation is not critical. The σB activity in the remaining three mutant strains fell to the level seen in a strain with the rsbS(59SA) mutation alone. This is particularly evident in the case of the rsbS(86PL 59SA)-expressing strain, where introduction of the rsbS(59SA) mutation caused a 30-fold drop in σB activity. These results suggest that the rsbS mutations include variants with impaired RsbT inhibition and others that are more readily phosphorylated.
cis/trans analyses of rsbR and rsbS alleles.
The observation that some of the rsbS mutations allow elevated σB activity when coupled with a mutation, rsbS(59SA), that normally prevents RsbT release/σB activation suggests that these rsbS variants encode RsbS proteins that are defective in binding and inhibiting of RsbT. It is curious that these mutant RsbS proteins are not complemented by a wild-type rsbS allele. A possible explanation for the failure of the wild-type rsbS allele to substitute for the impaired RsbS proteins is offered by our recent observation that RsbT activity is principally controlled by the product of the rsbS gene with which it is cotranscribed (45). Thus, the wild-type rsbS allele might fail to effectively compensate for the mutant rsbS allele when it is expressed in trans to the rsbT gene whose product must be controlled. This characteristic of RsbS/RsbT raises the possibility it might also apply to the rsbR mutations. In such a case, the failure of the mutant rsbR genes to be complemented by their wild-type counterpart might reflect a similar effect of their coexpression with rsbS and rsbT. To examine the possibility that the rsbR and rsbS mutants display their σB activation phenotypes only when cotranscribed with a subset of their potential binding partners, we created B. subtilis strains in which the mutant rsbR alleles or the mutant rsbS alleles plus wild-type rsbR were inserted with their promoters into the B. subtilis chromosome immediately upstream of the wild-type sigB operon. In the case of the rsbS allele, the operons also included a wild-type rsbR gene. This places the mutant rsbR alleles on transcription units separate from rsbS, rsbT, and rsbU and the rsbS alleles separate from rsbT and rsbU. As seen in Table 4, removal of the mutant alleles from the other genes of the sigB operon lowered σB activity to the level seen in a wild-type strain. Thus, the heightened σB activity caused by each of the mutations is dependent on cotranscription of the mutant alleles with other stressosome components. In the case of rsbS, this is consistent with the notion that RsbS and RsbT function as a unit upon synthesis; however, given that the RsbR paralogs, transcribed from diverse sites, can substitute for RsbR in properly regulating RsbS/RsbT interactions, this was not expected of rsbR. The finding that the rsbR alleles must also be joined to the sigB operon for heightened σB activity suggests that RsbR, RsbS, and RsbT rapidly interact following synthesis and that RsbS and RsbT may join RsbR prior to association within the stressosome.
TABLE 4.
σB activity in merodiploid rsbR and rsbS strains during growth
| rsbR/rsbS allelea | β-Galactosidase (ctc::lacZ) activity (Miller units) for indicated duplicated elementb
|
|
|---|---|---|
| PA rsbR rsbS rsbT rsbU | PA rsbR/PA rsbR rsbS | |
| Wild type | 1.2 ± 0.3 | 0.64 ± 0.45/0.76 ± 0.38c |
| rsbR(136EK) | 19.2 ± 0.6 | 1.2 ± 0.02 |
| rsbR(225CY) | 28.5 ± 2.2 | 1.0 ± 0.46 |
| rsbS(22ER) | 42.5 ± 7.2 | 0.67 ± 0.07 |
| rsbS(23LF) | 3.6 ± 0.9 | 0.57 ± 0.45 |
| rsbS(66GR) | 6.6 ± 1.0 | 0.38 ± 0.25 |
| rsbS(76GR) | 3.1 ± 1.1 | 0.40 ± 0.20 |
| rsbS(83GD) | 3.1 ± 0.4 | 0.53 ± 0.33 |
| rsbS(86PL) | 7.3 ± 2.9 | 0.62 ± 0.21 |
Each strain contains a wild-type sigB operon plus the indicated rsbR or rsbS allele in a transcription unit containing rsbR rsbS rsbT rsbU or rsbR (for rsbR mutations), rsbR rsbS rsbT rsbU or rsbR rsbS or rsbR rsbS (for rsbS mutations).
All strains are RsbP−. The indicated allele is present in the diploid element outside of the sigB operon.
Values represent two copies of wild-type rsbR/rsbR rsbS.
DISCUSSION
The B. subtilis stressosome is a large protein assemblage that couples the occurrence of physical stress to the activation of σB (11, 12, 24). The principal structural components of the stressosome are a family of homologous proteins (the RsbR family) that are needed for proper interactions between the other regulatory components. Central to σB induction is the release of RsbT, a positive regulatory protein trapped within the stressosome, from an association with its primary negative regulator (RsbS). Release of RsbT follows its phosphorylation of RsbR and then RsbS (10, 12, 24). The mechanism by which stress empowers RsbT to initiate these phosphorylations is unknown.
In the present work we describe missense changes in RsbR and RsbS that allow σB to be active in the absence of applied stress. Previous mutations in rsbS which enhanced σB activity during growth consisted either of deletions of the rsbS gene itself or a serine-to-aspartate change at the amino acid (Ser 59) that is believed to be phosphorylated by RsbT (39, 41). The latter mutation is believed to mimic RsbS phosphorylation. Both mutations eliminate or reduce the ability of RsbS to sequester RsbT in an inhibitory complex. Several of the RsbS mutations that were isolated in the present study are also likely to compromise the ability of RsbS to bind RsbT. These are the RsbS variants that allow residual enhanced σB activity when paired with a mutation, rsbS(59SA), that normally blocks the release of RsbT from RsbS. Among the variants are an RsbS protein with a glutamate-to-arginine substitution at position 22 and two others with glycine-to-arginine changes at positions 66 or 76. A third glycine substitution (Gly 83→Glu 83) in the same general region also elevates σB activity; however, this mutation failed to elevate σB activity when paired with the rsbS(59SA) change. Although such a result would be consistent with a change in rsbS that elevated σB activity via altered RsbS phosphorylation, this particular mutant allele, as well as another, rsbS(23LF), causes relatively modest increases in σB activity. The addition of the rsbS(59SA) mutation reduces σB activity as much as 40% even in rsbS variants where substantial σB activity persists [e.g., rsbS(76GR)]. It thus appears possible that in the case of the two rsbS alleles with relatively low increases in σB activity, the smaller reduction in their products' ability to bind and hold RsbT may be masked by the secondary effects of the rsbS(59SA) mutation. This makes their dependence on RsbS phosphorylation ambiguous. Ambiguity is not evident, however, in the instance of the rsbS(86PL) mutation. Addition of the Ser→Ala substitution to this allele leads to a 30-fold drop in σB activity. The ability of this variant to be phosphorylated is clearly important for activation of σB. The RsbS(59SA) protein, believed to bind RsbT in a complex from which it is not released, still requires an interaction with the RsbR proteins to effect this inhibition. The RsbS(59SA) variant is unable to block σB activation in the absence of the RsbR proteins (24). Thus, the loss of elevated σB activity caused by the RsbS(86PL) mutation upon the addition of the rsbS(59SA) mutation argues that this variant RsbS still interacts with the RsbR proteins and that the heightened σB activity is not due to a failure to be sequestered within the stressosome. It seems more likely that this mutation heightens the phosphorylation state of RsbS. Presumably the Pro→Leu change at residue 86 creates an RsbS variant that is more accessible to phosphorylation by RsbT, resistant to dephosphorylation by RsbX, or both.
The σB-activating mutations in rsbR are particularly interesting. Null rsbR mutations have little effect on σB activity in strains that still express the RsbR paralogs (1, 24). Both the rsbR136EK and rsbRT225CY mutations elevate σB activity in the presence of both wild-type rsbR and its paralogs. The ability of the RsbR variants to form high-molecular-weight associations that sequester RsbS lessens the possibility that their phenotype is a consequence of stressosome disruption. Instead, the observation that the rsbR alleles allow σB to be active only if both their products and RsbS carry the amino acid residues that are phosphorylated during activation argues that these rsbR mutations increase the likelihood that RsbR will be phosphorylated, with heightened phosphorylation of RsbS as a consequence. As with the rsbS allele [rsbS(86PL)], which also requires its phosphorylation site for σB activity, it is unresolved whether the mutations in rsbR alter their products' phosphorylation rate by RsbT or dephosphorylation by RsbX.
The finding that modifications of RsbR can alter σB activity lends credence to the notion that RsbR and its paralogs may be potential targets for stress induction. It is unlikely, however, even if the RsbR proteins are the stressosome components that receive stress signals, that the RsbR proteins themselves undergo stress-induced changes to initiate the response. The known Rsb proteins, expressed and functional in E. coli, fail to activate σB in response to applied stress (31). It appears more likely that if the RsbR proteins are a target for stress activation, unknown Bacillus-specific factors interact with them to modify their rate of phosphorylation or dephosphorylation.
An additional interesting observation coming from the present work is the finding that mutations in either rsbR or rsbS are dominant only when coexpressed with other rsb genes. We had previously noted that rsbT is unable to complement an rsbT deletion unless it is cotranscribed with other rsb genes (45). It was also determined that an rsbS variant, rsbS(59SD), that normally allows heightened σB activity during growth must be cotranscribed with rsbT to exert this effect (45). These results were interpreted as evidence that RsbS and RsbT interact concomitantly with their synthesis to form a stable association that is critical to RsbT activity. In such a model, RsbS principally controls the activity of the RsbT protein with which it is cosynthesized. If this is true, it is not surprising that our mutant rsbS alleles must also be cotranscribed with rsbT for them to display their phenotypes. The observation that our mutant rsbR genes fail to activate σB unless cotranscribed with rsbS and rsbT suggests that all three of these stress pathway components interact soon after their synthesis. This implies that RsbS and RsbT may bind to RsbR prior to RsbR becoming part of the stressosome and that RsbR, RsbS, and RsbT could, in fact, enter the stressosome as a preformed complex. The significance of this to stressosome assembly is not known.
Acknowledgments
This work was supported by U.S. National Institutes of Health grant GM-48220.
Footnotes
Published ahead of print on 8 December 2006.
REFERENCES
- 1.Akbar, S., C. M. Kang, T. A. Gaidenko, and C. W. Price. 1997. Modulator protein RsbR regulates environmental signaling in the general stress pathway of Bacillus subtilis. Mol. Microbiol. 24:567-578. [DOI] [PubMed] [Google Scholar]
- 2.Akbar, S., T. A. Gaidenko, C. M. Kang, M. O'Reilly, K. M. Devine, and C. W. Price. 2001. New family of regulators in the environmental signaling pathway which activates the general stress transcription factor σB of Bacillus subtilis. J. Bacteriol. 183:1329-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Autret, S., R. Nair, and J. Errington. 2001. Genetic analysis of the chromosome segregation protein Spo0J of Bacillus subtilis: evidence for separate domains involved in DNA binding and interactions with Soj protein. Mol. Microbiol. 41:743-755. [DOI] [PubMed] [Google Scholar]
- 4.Benson, A. K., and W. G. Haldenwang. 1992. Characterization of a regulatory network that controls σB expression in Bacillus subtilis. J. Bacteriol. 174:749-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benson, A. K., and W. G. Haldenwang. 1993. Bacillus subtilis σB is regulated by a binding protein (RsbW) that blocks its association with core RNA polymerase. Proc. Natl. Acad. Sci. USA 90:2330-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benson, A. K., and W. G. Haldenwang. 1993. The σB-dependent promoter of the Bacillus subtilis sigB operon is induced by heat shock. J. Bacteriol. 175:1929-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boylan, S. A., A. R. Redfield, M. S. Brody, and C. W. Price. 1993. Stress-induced activation of the σB transcription factor of Bacillus subtilis. J. Bacteriol. 175:7931-7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boylan, S. A., A. Rutherford, S. M. Thomas, and C. W. Price. 1992. Activation of Bacillus subtilis transcription factor σB by a regulatory pathway responsive to stationary phase signals. J. Bacteriol. 174:3695-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brody, M. S., K. Vijay, and C. W. Price. 2001. Catalytic function of an α/β hydrolase is responsible for energy stress activation of the σB transcription factor in Bacillus subtilis. J. Bacteriol. 183:6422-6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, C.-C., M. D. Yudkin, and O. Delumeau. 2004. Phosphorylation and RsbX-dependent dephosphorylation of RsbR in the RsbR-RsbS complex of Bacillus subtilis. J. Bacteriol. 186:6830-6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, C.-C., R. J. Lewis, R. Harris, M. D. Yudkin, and O. Delumeau. 2003. A supermolecular complex in the environmental stress signalling pathway of Bacillus subtilis. Mol. Microbiol. 49:1657-1669. [DOI] [PubMed] [Google Scholar]
- 12.Delumeau, O., C.-C. Chen, J. W. Murray, M. D. Yudkin, and R. J. Lewis. 2006. High-molecular-weight complexes of RsbR and paralogues in the environmental signaling pathway of Bacillus subtilis. J. Bacteriol. 188:7885-7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delumeau, O., R. J. Lewis, and M. D. Yudkin. 2002. Protein-protein interactions that regulate the energy stress activation of σB in Bacillus subtilis. J. Bacteriol. 184:5583-5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dufour, A., and W. G. Haldenwang. 1994. Interactions between a Bacillus subtilis anti-σ factor (RsbW) and its antagonist (RsbV). J. Bacteriol. 176:1813-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dufour, A., U. Voelker, A. Voelker, and W. G. Haldenwang. 1996. Relative levels and fractionation properties of Bacillus subtilis σB and its regulators during balanced growth and stress. J. Bacteriol. 178:3701-3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaidenko, T. A., X. Yang, Y. M. Lee, and C. W. Price. 1999. Threonine phosphorylation of modulator protein RsbR governs its ability to regulate a serine kinase in the stress signaling pathway of Bacillus subtilis. J. Mol. Biol. 288:29-39. [DOI] [PubMed] [Google Scholar]
- 17.Guerout-Fleury, A.-M., K. Shazand, and P. Stragier. 1995. Antibiotic-resistance cassettes for Bacillus subtilis. Gene 167:335-336. [DOI] [PubMed] [Google Scholar]
- 18.Hecker, M., W. Schumann, and U. Voelker. 1996. Heat-shock and general stress response in Bacillus subtilis. Mol. Microbiol. 19:417-428. [DOI] [PubMed] [Google Scholar]
- 19.Ju, J., T. Luo, and W. G. Haldenwang. 1998. Forespore expression and processing of the SigE transcription factor in wild-type and mutant B. subtilis. J. Bacteriol. 180:1673-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang, C. M., M. S. Brody, S. Akbar, X. Yang, and C. W. Price. 1996. Homologous pairs of regulatory proteins control activity of Bacillus subtilis transcription factor σB in response to environmental stress. J. Bacteriol. 178:3846-3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang, C. M., K. Vijay, and C. W. Price. 1998. Serine kinase activity of a Bacillus subtilis switch protein is required to transducer environmental stress signals but not to activate its target PP2C phosphatase. Mol. Microbiol. 30:189-196. [DOI] [PubMed] [Google Scholar]
- 22.Kenney, T. J., and C. P. Moran, Jr. 1987. Organization and regulation of an operon that encodes a sporulation-essential sigma factor of Bacillus subtilis. J. Bacteriol. 169:3329-3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim, T.-J., T. A. Gaidenko, and C. W. Price. 2004. In vivo phosphorylation of partner switching regulators correlates with stress transmission in the environmental signaling pathway of Bacillus subtilis. J. Bacteriol. 186:6124-6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim, T.-J., T. A. Gaidenko, and C. W. Price. 2004. A multi-component protein complex mediates environmental stress signaling in Bacillus subtilis. J. Mol. Biol. 341:135-150. [DOI] [PubMed] [Google Scholar]
- 25.Kuo, S., S. Zhang, R. L. Woodbury, and W. G. Haldenwang. 2004. Associations between Bacillus subtilis σB regulators in cell extracts. Microbiology 150:4125-4136. [DOI] [PubMed] [Google Scholar]
- 26.Reference deleted.
- 27.Petersohn, A., J. Bernhardt, U. Gerth, D. Hoper, T. Koburger, U. Voelker, and M. Hecker. 1999. Identification of σB-dependent genes in Bacillus subtilis using a promoter consensus-directed search and oligonucleotide hybridization. J. Bacteriol. 181:5718-5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price, C. W., P. Fawcett, H. Ceremonie, N. Su, C. K. Murphy, and P. Youngman. 2001. Genome-wide analysis of the general stress response in Bacillus subtilis. Mol. Microbiol. 41:757-774. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 30.Reference deleted.
- 31.Smirnova, N., J. Scott, U. Voelker, and W. G. Haldenwang. 1998. Isolation and characterization of Bacillus subtilis sigB operon mutations that suppress the loss of the negative regulator RsbX. J. Bacteriol. 180:3671-3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vijay, K., M. S. Brody, E. Fredlund, and C. W. Price. 2000. A PP2C phosphatase containing a PAS domain is required to convey signals of energy stress to the σB transcription factor of Bacillus subtilis. Mol. Microbiol. 35:180-188. [DOI] [PubMed] [Google Scholar]
- 33.Voelker, U., S. Engelmann, B. Maul, S. Riethdorf, A. Voelker, R. Schmid, H. Mach, and M. Hecker. 1994. Analysis of the induction of general stress proteins of Bacillus subtilis. Microbiology 140:741-752. [DOI] [PubMed] [Google Scholar]
- 34.Reference deleted.
- 35.Voelker, U., T. Luo, N. Smirnova, and W. G. Haldenwang. 1997. Stress activation of Bacillus subtilis σB can occur in the absence of the σB negative regulator RsbX. J. Bacteriol. 179:1980-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voelker, U., A. Voelker, and W. G. Haldenwang. 1996. Reactivation of the Bacillus subtilis anti-σB antagonist, RsbV, by stress or starvation-induced phosphatase activities. J. Bacteriol. 178:5456-5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reference deleted.
- 38.Voelker, U., A. Voelker, B. Maul, M. Hecker, A. Dufour, and W. G. Haldenwang. 1995. Separate mechanisms activate σB of Bacillus subtilis in response to environmental and metabolic stresses. J. Bacteriol. 177:3771-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wise, A. A., and C. W. Price. 1995. Four additional genes in the sigB operon of Bacillus subtilis that control activity of the general stress factor σB in response to environmental signals. J. Bacteriol. 177:123-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woodbury, R. L., T. Luo, L. Grant, and W. G. Haldenwang. 2004. Mutational analysis of RsbT, an activator of the Bacillus subtilis stress response transcription factor, σB. J. Bacteriol. 186:2789-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang, X., C. M. Kang, M. S. Brody, and C. W. Price. 1996. Opposing pairs of serine protein kinases and phosphatases transmit signals of environmental stress to activate a bacterial transcription factor. Genes Dev. 10:2265-2275. [DOI] [PubMed] [Google Scholar]
- 42.Yasbin, R. E., G. A. Wilson, and F. E. Young. 1973. Transformation and transfection of lysogenic strains of Bacillus subtilis 168. J. Bacteriol. 113:540-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang, S., and W. G. Haldenwang. 2003. RelA is a component of the nutritional stress activation pathway of Bacillus subtilis transcription factor σB. J. Bacteriol. 185:5714-5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang, S., and W. G. Haldenwang. 2005. Contributions of ATP, GTP, and redox state to nutritional stress activation of the Bacillus subtilis σB transcription factor. J. Bacteriol. 187:7554-7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang, S., A. Reeves, R. L. Woodbury, and W. G. Haldenwang. 2005. Coexpression patterns of σB regulators in Bacillus subtilis affect σB inducibility. J. Bacteriol. 187:8520-8525. [DOI] [PMC free article] [PubMed] [Google Scholar]