Abstract
UvrD is a helicase that is widely conserved in gram-negative bacteria. A uvrD homologue was identified in Mycobacterium tuberculosis on the basis of the homology of its encoded protein with Escherichia coli UvrD, with which it shares 39% amino acid identity, distributed throughout the protein. The gene was cloned, and a histidine-tagged form of the protein was expressed and purified to homogeneity. The purified protein had in vitro ATPase activity that was dependent upon the presence of DNA. Oligonucleotides as short as four nucleotides were sufficient to promote the ATPase activity. The DNA helicase activity of the enzyme was only fueled by ATP and dATP. UvrD preferentially unwound 3′-single-stranded tailed duplex substrates over 5′-single-stranded ones, indicating that the protein had a duplex-unwinding activity with 3′-to-5′ polarity. A 3′ single-stranded DNA tail of 18 nucleotides was required for effective unwinding. By using a series of synthetic oligonucleotide substrates, we demonstrated that M. tuberculosis UvrD has an unwinding preference towards nicked DNA duplexes and stalled replication forks, representing the likely sites of action in vivo. The potential role of M. tuberculosis UvrD in maintenance of bacterial genomic integrity makes it a promising target for drug design against M. tuberculosis.
Tuberculosis (TB) continues to be one of the greatest sources of mortality and morbidity worldwide, with approximately 8 million new infections and 2 million deaths per year (64). The success of Mycobacterium tuberculosis, the etiological agent of TB, as a pathogen is largely attributable to its ability to persist in host tissues (25), where drugs that are rapidly bactericidal in vitro require prolonged administration to achieve comparable effects. Latency is a frequent outcome of M. tuberculosis infection, creating a long-standing reservoir of future disease and contagion. The identification of pathways used by the microbe to resist elimination by the host immune response may suggest new targets for the prevention or treatment of tuberculosis.
The establishment of a persistent infection in the macrophages of the host demands that microbes evade and subvert various host immune mechanisms that are meant to eliminate the pathogens. Activated macrophages produce reactive oxygen and nitrogen species (22, 34), which damage DNA, among other targets. Thus, pathways involved in maintenance of genomic integrity appear to be important for M. tuberculosis pathogenesis and persistence in the host. Recent reports have revealed that the uvrB gene has a role in M. tuberculosis pathogenesis in mice (13, 14). In Escherichia coli, the uvrB gene product takes part in the prokaryotic nucleotide excision repair (NER) pathway, which removes bulky adducts on DNA by a dual incision bracketing the lesion carried out by the sequential and partially overlapping functions of UvrA, UvrB, UvrC, and UvrD (58). Inactivation of the uvrD gene has been found to reduce M. tuberculosis persistence in a mouse model of tuberculosis infection (61a). This suggests an important role for the uvrD gene product in the ability of M. tuberculosis to survive prolonged exposure to DNA-damaging conditions.
The M. tuberculosis UvrD protein shares 39% amino acid sequence identity with E. coli UvrD and 46% and 43% amino acid sequence identity, respectively, with the Bacillus stearothermophilus and Staphylococcus aureus PcrA proteins. Furthermore, these proteins share >95% identity in the seven conserved motifs (Fig. 1) found in helicases belonging to superfamily I (9). Gram-negative bacteria carry both UvrD and another closely related helicase, termed Rep (Fig. 1), whereas gram-positive bacteria have only a single equivalent protein, known as PcrA. While single uvrD and rep mutants of E. coli are viable, double mutants are not, suggesting that their activities might overlap (52). Notably, the single homologue, PcrA, is essential in Bacillus subtilis (51). Furthermore, genetic studies have shown that PcrA appears to incorporate at least some functions of both the Rep and UvrD proteins of E. coli, as expression of the pcrA gene product in E. coli restored the viability of the uvrD rep double mutant (51).
FIG. 1.
Amino acid sequence alignment of M. tuberculosis UvrD, E. coli UvrD, B. stearothermophilus PcrA, S. aureus PcrA, and E. coli Rep. Helicase motifs are boxed and labeled as motifs Ia to VI.
In E. coli, UvrD plays an important role in DNA repair, being involved in both the NER pathway (8) and the mismatch repair pathway (65), as well as being implicated in replication (23, 46) and recombination (26, 44). Consistent with these multiple roles, UvrD-deficient cells are more susceptible to DNA-damaging agents, exhibit elevated mutation rates, and are hyperrecombinogenic (63). In NER, UvrD is required to unwind the excised oligonucleotide containing the damaged base, as well as to displace UvrC, permitting resynthesis by DNA polymerase I (8). During mismatch repair, UvrD is loaded by MutL at the nicked GATC sequence and unwinds the DNA to just beyond the mismatch, providing a suitable substrate for appropriate single-strand exonucleases (1, 41). UvrD also plays a critical role in replication. It promotes the replication of small drug resistance plasmids by a rolling circle mechanism (7), and it maintains the viability of cells lacking DNA polymerase I (46) and Rep (52). The E. coli UvrD protein unwinds both DNA duplexes and DNA-RNA hybrids in an ATP-dependent manner, with 3′-to-5′ polarity. The average number of base pairs unwound per successful unwinding cycle is 4 or 5 bp (2, 38, 40). In addition to defects in DNA repair, the uvrD null mutation causes an increase in the frequency of homologous recombination. UvrD participates in homologous recombination initiated by RecFOR in recBC sbcBC mutants (43, 63), yet, conversely, it acts as an antirecombinase in vitro and in vivo (5, 47, 53). The uvrD mutant hyperrecombination phenotype likely results from the capacity of the purified UvrD protein to destroy joint molecules (recombination intermediates) made by RecA and to dislodge RecA from single-stranded DNA (ssDNA) (47, 62). An increasing number of reports have suggested a role for UvrD at blocked replication forks. UvrD removes RecA from inactivated forks in dnaE(Ts) and dnaN(Ts) mutants (20, 21). After removal of RecA from blocked forks in Rec+ cells, UvrD may translocate further and unwind the ends of the newly synthesized DNA strands, promoting fork reversal (21).
In the gram-positive bacteria Bacillus subtilis, B. stearothermophilus, and S. aureus, PcrA is required for both DNA repair and rolling circle replication of plasmids of the pT181 family (10, 28, 51). PcrA suppresses the UV sensitivity of a uvrD mutant of E. coli, but not its defect in mismatch repair (51). However, PcrA does not complement a single rep mutant, and when expressed at high levels, it confers a Rep-negative phenotype on a wild-type strain (51). The PcrA helicase of B. stearothermophilus has been purified, and its crystal structure has been determined (6, 60). B. stearothermophilus PcrA shows a strong preference for double-stranded substrates containing a 3′ single-stranded tail over substrates containing a 5′ single-stranded tail (19). Interestingly, Bacillus anthracis, Bacillus cereus, and S. aureus PcrAs were equally active as 5′-3′ helicases and 3′-5′ helicases (4, 48).
In this paper, we describe the expression, purification, and characterization of the UvrD helicase from M. tuberculosis. We found that M. tuberculosis UvrD had an ATPase activity that was strictly DNA dependent and exhibited helicase activity with 3′-to-5′ polarity of unwinding. This UvrD protein acted as a monomer and showed structure-specific substrate preferences. M. tuberculosis UvrD unwound in vitro duplex DNAs containing a nick or fork structures extremely efficiently, consistent with a role for M. tuberculosis UvrD in the NER pathway as well as in clearing stalled replication forks.
MATERIALS AND METHODS
Materials, DNA oligonucleotides, and nucleotides.
Polyacrylamide gel electrophoresis (PAGE)-purified oligonucleotides were purchased from Eurogentec and Sigma Genesis. Nucleotides were purchased from Sigma. Radiolabeled nucleotides were purchased from Amersham GE Healthcare (6,000 mCi/mmol).
Cloning, expression, and purification of M. tuberculosis UvrD.
The UvrD (Rv0949) sequence data for the M. tuberculosis genome were obtained from the website of the Institut Pasteur (www.pasteur.fr/Bio/TubercuList/). The genomic DNA of M. tuberculosis was isolated by the procedure described by Davis et al. (15) and was used as a template for the amplification of the uvrD gene (2.3 kb). The forward primer (5′-GAGAATTCCATGGGTGTGCACGCGACCGACGC-3′) included an NcoI site, while the reverse primer (5′-GGATTACTCGAGGAGCTTGGTGACAGGGGCG-3′) included an XhoI site. In order to use the ATG of the NcoI site as the starting codon, the second amino acid codon was changed from a Ser to a Gly codon. Moreover, usage of this cloning strategy necessitated the addition of Leu and Glu residues at the C-terminal end of the protein before the histidine (His6) tag. The DNA sequence was amplified by PCR, using 5 U of KOD Hot Start DNA polymerase (Novagen), a 200 μM concentration of each deoxynucleoside triphosphate (dNTP), 50 ng of the genomic DNA, and 1 μM of each primer. The PCR product was cloned into the pET28b expression vector to incorporate a C-terminal His6 tag. The sequence of the pET28b-UvrD construct was verified, and the plasmid was transformed into E. coli BL21(DE3) Star cells (Invitrogen) for overexpression. Conventional expression protocols yielded insoluble protein. In order to obtain soluble protein, a heat shock protocol was adopted. The rationale behind this protocol is that heating the culture to 42°C causes the expression of E. coli heat shock chaperones that can help the folding of the overexpressed target protein. E. coli BL21(DE3) Star cells harboring the pET28b-UvrD expression plasmid were grown at 37°C until the optical density at 600 nm reached 0.6. At this point, the culture was heated at 42°C for 20 min and then allowed to cool to room temperature for a further 20 min. Following the heat shock, the expression of M. tuberculosis UvrD was induced using 1 mM isopropyl-β-d-thiogalactopyranoside at 37°C for 4 h.
The cells were resuspended in lysis buffer (50 mM Tris-HCl, pH 8.5, 500 mM NaCl, 0.5 mg/ml lysozyme, 1 U/ml DNase I, complete EDTA-free protease inhibitor cocktail [Roche]) and lysed by sonication. The clarified lysate was applied to a Ni2+-nitrilotriacetic acid (QIAGEN) column and equilibrated with buffer A (50 mM Tris-HCl, pH 8.5, 500 mM NaCl), and the protein was eluted with a linear gradient of 0 to 250 mM imidazole. Relevant fractions were analyzed by sodium dodecyl sulfate-PAGE (SDS-PAGE) to verify the purity of the protein and then pooled before dialysis against buffer B (50 mM Tris-HCl, pH 8.5, 400 mM NaCl, 2 mM EDTA, and 5 mM dithiothreitol [DTT]). The dialyzed M. tuberculosis UvrD protein was further purified using a heparin FF column (Amersham GE Healthcare) preequilibrated with buffer C, which is identical to buffer B but at half ionic strength. Since the protein was more soluble at high salt concentrations, it was diluted immediately prior to being loaded onto the column to a conductivity equivalent to that of buffer C by adding an equivalent volume of buffer D (50 mM Tris-HCl, pH 8.5, 2 mM EDTA, 5 mM DTT, and 2 mM ATP). The addition of ATP was essential for the purification of M. tuberculosis UvrD from a 70-kDa contaminant. The protein was eluted with a linear gradient of 200 to 800 mM NaCl. The protein eluted at a conductivity equivalent to that of buffer D plus 400 mM NaCl. The peak fractions were pooled and applied to a 50-ml Q-Sepharose anion-exchange column. The sample was again diluted prior to being loaded to a conductivity equal to that of buffer C. The column was washed with buffer C, and the protein was eluted with a gradient of 200 to 800 mM NaCl. Traces of contaminants were removed by gel filtration using a Superdex S200 column (GE Healthcare) equilibrated with buffer E (50 mM Tris-HCl, pH 8.5, 400 mM NaCl, 2 mM EDTA, and 5 mM DTT). Prior to storage of the protein in aliquots at −80°C, the protein was dialyzed in buffer E containing 25% (vol/vol) glycerol. The purity of the sample was monitored by SDS-PAGE.
Protein and DNA concentration determination.
The extinction coefficient of M. tuberculosis UvrD (ɛ280 nm = 63,260 M−1 cm−1) was calculated using the program ProtParam (www.expasy.org). The protein concentration was determined from the absorbance at 280 nm. The concentration was comparable to the value from the Bradford assay (Bio-Rad), using bovine serum albumin as a standard. The DNA oligonucleotide concentrations were determined from the absorbance at 260 nm, using calculated extinction coefficients.
ATPase assays.
The hydrolysis of ATP by M. tuberculosis UvrD was tested using a coupled assay (49). This assay measures the loss of NADH absorbance at 340 nm (ɛM = 6,250 cm−1 M−1) as ADP is converted to ATP through the actions of pyruvate kinase and lactate dehydrogenase in the presence of phosphoenolpyruvate. The reaction mixtures (200 μl) contained 4.7 U lactate dehydrogenase (Sigma-Aldrich), 5.3 U pyruvate kinase (Sigma-Aldrich), 0.2 mM NADH, and 2 mM phosphoenolpyruvate (Sigma-Aldrich) in addition to 50 mM Tris-HCl, pH 7.5, 50 mM NaCl, 5 mM MgCl2, 10% glycerol, DNA (1 to 15 μM), ATP (5 μM to 2 mM), and 100 nM enzyme (unless otherwise stated). The reactions were initiated by the addition of ATP and monitored at 340 nm for 15 min. The sequences of the DNA oligonucleotide substrates are reported in Table 1, with the exception of the poly(dT)n sequences. The data were plotted by nonlinear regression using the program KaleidaGraph (Synergy Software, Reading, PA). The steady-state kinetic parameters for ATP hydrolysis were determined by plotting the observed initial rates of hydrolysis at different substrate concentrations versus the substrate concentrations and fitting the data to a typical Michaelis-Menten equation, as follows: v = (Vmax × [S])/(Km + [S]), where v is the rate of hydrolysis at each nucleotide concentration, Vmax is the maximal velocity, [S] is the substrate concentration considered, and Km is the concentration of substrate at which v = 1/2Vmax. To determine the constant KDNA, defined as the concentration of DNA that supports a half-maximum rate of catalysis, reactions were performed in the presence of 1 mM ATP with various concentrations of DNA, and the data were fitted to the equation v = (Vmax × [NA])/(KDNA + [NA]), where v is the velocity of ATP hydrolysis at each DNA concentration, Vmax is the maximal velocity, [NA] is the substrate concentration considered, and KDNA is the concentration of DNA required to stimulate a half-maximal rate of ATP hydrolysis. The data are reported as the averages for at least three independent experimental data sets.
TABLE 1.
Sequences of the oligonucleotides used in this study
| Name | Sequence (5′ to 3′) |
|---|---|
| 1 | GTACCCGTGGATCCTCTAGAGT |
| 2 | ACTCTAGAGGATCCACGGGTAC |
| 3 | ACTCTAGAGGATCCACGGGTACGTTATTGCATGAAAGCCCGGCTG |
| 4 | GTTATTGCATGAAAGCCCGGCTGACTCTAGAGGATCCACGGGTAC |
| 5 | CAGCCGGGCTTTCATGCAATAAG |
| 6 | GTTATTGCATGAAAGCCCGGCTGACTCTAGAGGATCCCCGGGTACGTT ATTGCATGAAAGCCCGGCTG |
| 7 | ACTCTAGAGGATCCCCGGGTACTTTT |
| 8 | ACTCTAGAGGATCCCCGGGTACTTTTTTTTTTTT |
| 9 | ACTCTAGAGGATCCCCGGGTACTTTTTTTTTTTTTTTTTT |
| 10 | ACTCTAGAGGATCCCCGGGTACTTTTTTTTTTTTTTTTTTTT |
| 11 | CAGCCGGGCTTTCATGCAATAAGAGCGTAACCGTACTAGGCAGGC |
| 12 | GCCTGCCTAGTACGGTTACGCTC |
| 13 | CAGCCGGGCTTTCATGCAATAAG |
Helicase assay.
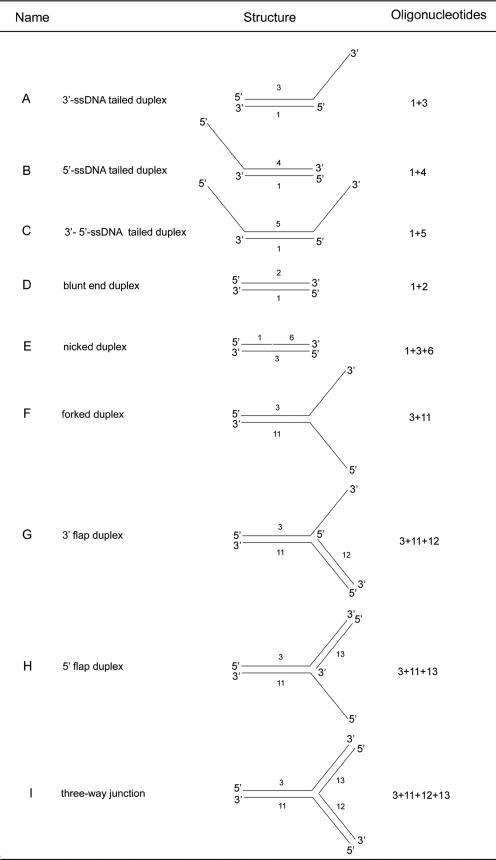
We used a variety of oligonucleotides to study the helicase activity of UvrD (Table 1). The DNA substrates used in this study are listed in Table 2. The DNA duplexes were prepared by annealing a 5′-end-radiolabeled ssDNA oligonucleotide (oligonucleotide 1) (Table 1) to a series of fully or partially complementary ssDNA oligonucleotides to generate either a 23-nucleotide (23-nt) 3′-ssDNA tailed duplex (substrate A), a 23-nt 5′-ssDNA tailed duplex (substrate B), a 23-nt 3′- and 5′-ssDNA tailed substrate (substrate C), a blunt-ended duplex (substrate D), a nicked substrate (substrate E), a forked duplex (substrate F), a 3′- or 5′-flap duplex (substrates G and H), or a three-way junction (substrate I). The radiolabeled oligonucleotide was prepared by incubating the DNA with 50 μCi [γ-32P]ATP and T4 polynucleotide kinase (New England Biolabs) according to the manufacturer's instructions. The nonincorporated nucleotides were removed by centrifugation through a G25 gel filtration spin column (Amersham GE Healthcare). Labeled and unlabeled oligonucleotides were mixed at a 1:1.5 molar ratio. The annealing mixture was heated at 95°C for 2 min and then slowly cooled to room temperature (25°C) over a period of 1 h. The helicase assays (20 μl) were performed by mixing 1 nM radiolabeled DNA duplex, 1 mM ATP, and 200 nM UvrD, unless otherwise stated, in reaction buffer containing 20 mM Tris-HCl, pH 7.5, 50 mM NaCl, 5 mM MgCl2, and 10% glycerol. Reactions were initiated by the addition of ATP, and the mixtures were incubated at 37°C for the indicated length of time. Time course analyses of the helicase reactions were carried out by scaling up the reaction volume to 250 μl and withdrawing 20-μl aliquots at the indicated times. Reactions were terminated by the addition of 6 μl of stop solution (0.1% [wt/vol] bromophenol blue, 0.1% [wt/vol] xylene cyanol, 8% [vol/vol] glycerol, 0.4% [wt/vol] SDS, 50 mM EDTA). To minimize reannealing of the unwound oligonucleotides, a 10-fold molar excess (10 nM) of unlabeled DNA trap, corresponding to the 22-mer (oligonucleotide 1), was added at the same time as the stop solution. As a positive control, DNA substrates were heat denatured at 95°C for 15 min in the absence of helicase. As a negative control, the substrates were incubated in the reaction mix in the absence of helicase. Samples were resolved in a 12% (vol/vol) polyacrylamide gel in 1× Tris-borate-EDTA. The gel was dried under vacuum and exposed to a PhosphorImager screen. Radioactively labeled bands were visualized using a Storm860 PhosphorImager (Molecular Dynamics) and quantified with ImageQuant software (Molecular Dynamics).
TABLE 2.
Structures of the various substrates used in this study
Analytical ultracentrifugation.
Sedimentation equilibrium experiments were performed at 20°C in a Beckman XL-A analytical ultracentrifuge, using an An-60 Ti rotor and 1.2-cm six-channel charcoal-Epon centerpieces (Beckman Coulter). Before centrifugation, the protein was purified by size-exclusion chromatography and extensive dialysis against the blank buffer, containing 20 mM Tris-HCl, pH 8.5, 400 mM NaCl, 1 mM EDTA, and 5 mM DTT. Data were collected at protein concentrations of 1, 2, and 5 mg/ml and rotor speeds of 9,000, 11,000, and 13,000 rpm. After centrifugation for 20 h, cells were scanned radially at 2-hour intervals (λ = 280 nm) until no further change in the absorbance profile was observed. The data were fitted to a least-squares nonlinear regression, using XL-A/XL-I data analysis software, version 4.0, by Beckman, based on the program Origin (MicroCal Software Inc., Northampton, MA). The monomeric molecular masses and partial specific volumes were calculated by using the program SEDINTERP (30).
RESULTS
The finding that inactivation of the M. tuberculosis uvrD gene is associated with attenuation of persistence in a mouse model of TB suggested a role for this protein in bacterial survival during chronic infection. This prompted us to characterize the enzymatic activity of UvrD in vitro.
Cloning, expression, and purification of M. tuberculosis UvrD.
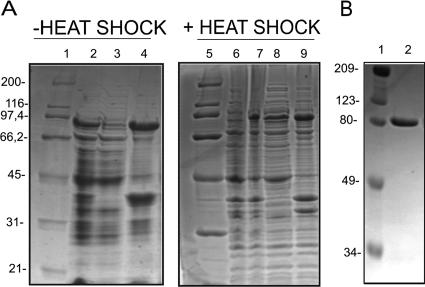
To obtain sufficient quantities of protein for in vitro studies, the uvrD gene, which comprises an open reading frame encoding 771 amino acids, was amplified by PCR and fused in frame to codons for six His residues, carried by pET28b, at its C-terminal end for expression in E. coli. Conventional expression protocols led to insoluble recombinant protein. Heating the culture at 42°C before induction was essential to obtain soluble protein (Fig. 2A). The M. tuberculosis UvrD His6-tagged protein was purified by nickel-affinity chromatography, followed by heparin-affinity chromatography, anion-exchange chromatography, and gel filtration chromatography. SDS-PAGE analysis showed that the full-length protein was at least 95% pure (Fig. 2B). The 85,962-Da (±10 Da) molecular mass determined by mass spectrometry was in agreement with the calculated molecular mass of 85,953 Da.
FIG. 2.
Expression and purification of M. tuberculosis UvrD. (A) Expression of M. tuberculosis UvrD without heat shock (lanes 1 to 4) and with heat shock (lanes 5 to 9). Lanes 1 and 5, unstained broad-range protein standards (Bio-Rad); lanes 2 and 7, induced cell extract; lanes 3 and 8, soluble fraction; lanes 4 and 9, insoluble fraction; lane 6, uninduced cell extract. (B) Lane 1, prestained broad-range markers (Bio-Rad); lane 2, purified M. tuberculosis UvrD.
ATP hydrolysis by M. tuberculosis UvrD.
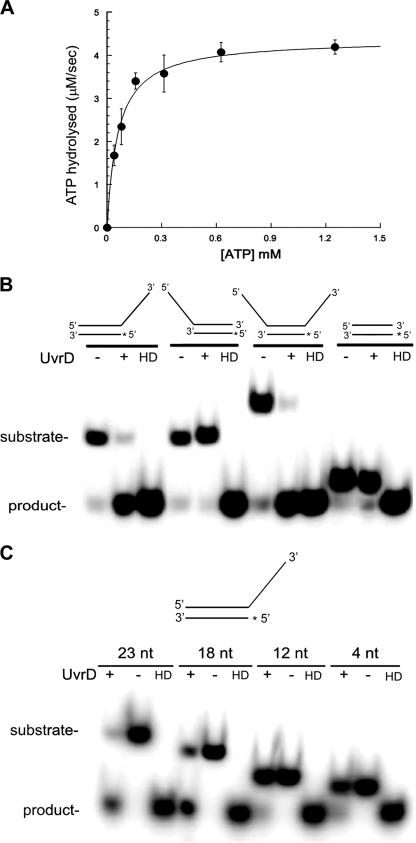
We first tested the purified UvrD protein for ATP hydrolysis activity. Using a continuous coupled ATPase assay, we determined that M. tuberculosis UvrD has a strong ATPase activity (Km = 60.2 μM; kcat = 43 s−1) that is strictly dependent on the presence of DNA and Mg2+ (Fig. 3A). In the absence of DNA or in the presence of EDTA, any ATPase activity was lower than the detection limit of the assay (data not shown). Optimal assay conditions are reported in Table 3. We also examined the effects of different DNA substrates on the ATPase activity of UvrD. ssDNA, double-stranded DNA, and 5′- and 3′-tailed DNA duplexes induced ATPase activity with the same rate (data not shown). We investigated the effect of the length of single-stranded poly(dT)n on the ATPase rate. Homopolymeric oligonucleotides as short as four nucleotides were sufficient to stimulate ATPase activity (Table 4) to a rate similar to those observed for the longer sequences. However, the enzyme displayed higher affinities for the longer sequences, as reflected by the KD values (Table 4).
FIG. 3.
ATPase and helicase activities of M. tuberculosis UvrD. (A) UvrD ATPase activity was assayed on 10 μM poly(T)18 with 100 nM UvrD in the presence of 2 mM ATP. (B) Polarity of unwinding of M. tuberculosis UvrD. Reaction mixtures containing 1 mM ATP, 5 mM MgCl2, 1 nM 32P-labeled helicase substrates (as depicted at the top), and 200 nM UvrD were incubated for 20 min at 37°C. The reaction mixtures were analyzed by native PAGE. HD, heat-denatured substrates. The asterisks indicate the 32P label. (C) Effect of the length of the 3′-ssDNA tail on UvrD helicase activity. Reaction mixtures containing 1 mM ATP, 5 mM MgCl2, 1 nM 32P-labeled helicase substrates, and 200 nM UvrD were incubated for 20 min at 37°C. The reaction mixtures were analyzed by native PAGE. HD, heat-denatured substrates. The asterisk indicates the 32P label.
TABLE 3.
Optimal assay conditions for ATPase and helicase assays
| Parameter | Range | Optimal value |
|---|---|---|
| pH | 6.0-9.0 | ≤7.5 |
| MgCl2 concn (mM) | 0.1-10 | 5 |
| NaCl concn (mM) | 10-250 | 50 |
| Temp (°C) | 20-50 | 37 |
TABLE 4.
KD and kcat values for different lengths of poly(dT)
| Oligonucleotide | KD (μM) | kcat (s−1) |
|---|---|---|
| poly(dT)4 | 3.06 ± 0.65 | 23.3 ± 0.2 |
| poly(dT)6 | 1.99 ± 0.38 | 16.4 ± 0.1 |
| poly(dT)10 | 0.37 ± 0.03 | 32.7 ± 0.4 |
| poly(dT)13 | 0.16 ± 0.03 | 27.9 ± 0.1 |
| poly(dT)18 | 0.059 ± 0.003 | 29.4 ± 0.3 |
| poly(dT)23 | 0.045 ± 0.004 | 24.7 ± 0.5 |
| poly(dT)40 | 0.021 ± 0.002 | 28.6 ± 0.6 |
Polarity of unwinding of M. tuberculosis UvrD.
Helicases of the SF1 family bind to the single-stranded tails of their partial duplex substrates with a specific orientation with respect to the polarity of the sugar-phosphate backbone. This property determines the directionality (or polarity) of the duplex-unwinding reaction and allows for classification into 3′-to-5′ helicases and 5′-to-3′ helicases. A standard in vitro assay was used, with a set of four duplex substrates carrying different single-stranded regions, in order to investigate the directionality of unwinding of M. tuberculosis UvrD. The substrate was incubated with UvrD in the presence of ATP and magnesium. The addition of a trap in the reaction stop buffer was necessary to avoid reannealing of the unwound complementary strands. The separation of the partial duplex substrate into single-stranded products was examined by nondenaturing gel electrophoresis. The four substrates each had a common 22-nt double-stranded region, but they had various overhangs. The first substrate had a 23-nt 3′-ssDNA tail, the second substrate had a 23-nt 5′-ssDNA tail, the third substrate possessed both the 3′- and 5′-ssDNA tails, and the fourth substrate consisted simply of the 22-nt blunt-ended duplex. UvrD was able to efficiently unwind the substrate with a single 3′-ssDNA tail and the substrate with both tails; in contrast, very inefficient unwinding was observed with both the blunt-ended and the 5′-ssDNA-tailed substrates (Fig. 3B). These data suggest that the DNA duplex-unwinding activity strictly depends on the presence of 3′ single-stranded tails, indicating that UvrD is a helicase with 3′-to-5′ polarity. The trace activities observed with 5′-tailed and blunt-ended substrates likely represent nonspecific background activities, not genuine translocation of the enzyme in the opposite, 5′-3′ direction. It is likely that the enzyme gains entrance at the blunt end via thermal fraying at the ends and then translocates 3′ to 5′.
Since the helicase activity of M. tuberculosis UvrD required the presence of a 3′-single-stranded region, we investigated the effect of the length of the ssDNA region on the unwinding activity of the enzyme. We performed additional assays using another set of four substrates, in which the same 22-nt double-stranded region was joined to 3′ oligo(dT) tails of 4, 12, 18, and 23 nt. The overhanging DNA sequence was chosen to be a poly(dT)n to avoid effects of possible secondary structures. The results showed that duplex unwinding decreased marginally as the length of the tail was shortened from 23 to 18 nt but that duplexes with a tail of 12 nt or shorter were not unwound (Fig. 3C).
Nucleotide and metal ion specificities.
As more information on helicases has become available, the diversity of these enzymes has become apparent. Helicases have been shown to have specific nucleotide preferences and diverse abilities to couple the hydrolysis of nucleotides to DNA unwinding (6). We measured the strand displacement activity of M. tuberculosis UvrD with different NTPs and dNTPs (Fig. 4A). No activity was observed in the absence of nucleotides, consistent with previous results showing that helicase-catalyzed unwinding of nucleic acids is an energy-dependent process (31-33). Only ATP and dATP supported the displacement of the annealed oligonucleotides, but with different efficiencies (Fig. 4A). While 80% of the duplex was separated in the presence of 1 mM ATP in a 15-min reaction, only 58% was unwound in the presence of 1 mM dATP (Fig. 4B).
FIG. 4.
Helicase activity of UvrD is fueled by different NTPs and metal ions. (A) Dependence of helicase activity on different NTPs. The 3′-ssDNA-tailed duplex (1 nM) was incubated in the presence of NTPs or dNTPs (1 mM [each]), 5 mM MgCl2, and 200 nM UvrD for 10 min at 37°C. The control reaction lacked NTPs (−). HD, heat-denatured substrate. Reactions were analyzed by native PAGE, and the PhosphorImager scan of the dried gel is shown. (B) Quantification of UvrD helicase activity in the presence of different NTPs and dNTPs. The data are reported as the averages for at least three independent experiments, and the error bars represent the standard deviations of the averages. (C) Dependence of helicase activity of UvrD on metal ion cofactors. The 3′-ssDNA-tailed duplex (1 nM) was incubated in the presence of 1 mM ATP, 200 nM UvrD, and 1 mM of each metal ion for 20 min at 37°C. The control reaction lacked metal ions (−). HD, heat-denatured substrate. Reactions were analyzed by native PAGE, and the PhosphorImager scan of the dried gel is shown. (D) Quantification of UvrD helicase activity in the presence of different metal ions. The data are reported as the averages for at least three independent experiments, and the error bars represent the standard deviations of the averages.
Although it has been established that the ATPase and helicase activities of this family of enzymes are dependent on the presence of Mg2+ ions, little is known about their metal cofactor specificity. Metal cofactors are essential for the correct positioning of nucleotides in the active sites of a broad range of enzymes. We investigated the possibility that different metal ions could substitute for Mg2+ in M. tuberculosis UvrD. We firstly examined the effects of several divalent ions on the M. tuberculosis ATPase activity. In this set of experiments, MgCl2 (1 mM) was replaced with 1 mM Ni2+, Ca2+, Cu2+, Mn2+, Zn2+, Co2+, or Cd2+. ATPase activity was observed in the presence of Mn2+, Co2+, and Ni2+. Steady-state kinetic analyses of ATPase rates (Table 5) showed that Co2+ could support a catalytic efficiency similar to that with Mg2+, while Mn2+ and Ni2+ could support ATPase activity, but with two- and threefold rate decreases, respectively. We also investigated the possibility that M. tuberculosis UvrD could couple ATP hydrolysis to DNA unwinding in the presence of these metal ions. UvrD could efficiently separate duplex DNA in the presence of Mn2+ and, to a lesser extent, in the presence of Co2+ and Ni2+ (Fig. 4C and D). Interestingly, unwinding activity was also detected in the presence of Cu2+ (Fig. 4C and D). It is likely that the lack of ATPase activity in the presence of Cu2+ is a reflection of an inhibitory effect of Cu2+ on one of the enzymes in the coupled ATPase assay. All of the negative control experiments, performed by incubating the DNA substrate in the presence of the metal ions but in the absence of UvrD, showed no DNA unwinding (data not shown).
TABLE 5.
Steady-state kinetic parameters for ATP hydrolysis in the presence of different metal ions (1 mM)
| Metal | Km (μM) | Vmax (μM s−1) | kcat (s−1) | kcat/Km (s−1/μM) |
|---|---|---|---|---|
| Mg2+ | 80 ± 12 | 2.98 ± 0.13 | 29.8 | 0.372 |
| Mn2+ | 86 ± 7.5 | 1.98 ± 0.04 | 19.8 | 0.230 |
| Ni2+ | 128 ± 30 | 1.31 ± 0.11 | 13.1 | 0.102 |
| Co2+ | 55.3 ± 7.3 | 2.22 ± 0.07 | 22.2 | 0.401 |
M. tuberculosis UvrD unwinds structure-specific DNA substrates.
We next wanted to investigate if M. tuberculosis UvrD was able to unwind DNA substrates with specific structures. The structures of the substrates used resembled those expected to be present in various DNA metabolic pathways, such as replication, repair, recombination, and transcription. These substrates (Table 2) were generated by annealing oligonucleotides (Table 1) based on a common core sequence represented by oligonucleotide 1. By using these substrates, we aimed to eliminate, as far as possible, any variation in unwinding efficiency due to sequence differences between substrates. We tested a nicked DNA duplex, a forked structure, both 3′-flap and 5′-flap duplexes, and a three-way junction. The nicked DNA resembles reaction intermediates generated during the UvrABC-mediated NER pathway, whereas the other substrates resemble structures proposed for stalled replication forks, which are thought to be subject to recombinational repair. The 3′-flap substrate can be envisioned as a replication fork where the synthesis of the leading strand has been blocked while the synthesis of the lagging strand has continued. The 5′-flap substrate resembles the structure proposed for a stalled replication fork where synthesis of the lagging strand has been blocked. The three-way junction substrate resembles a stalled replication fork where both the leading and the lagging arms have been converted to duplex DNA.
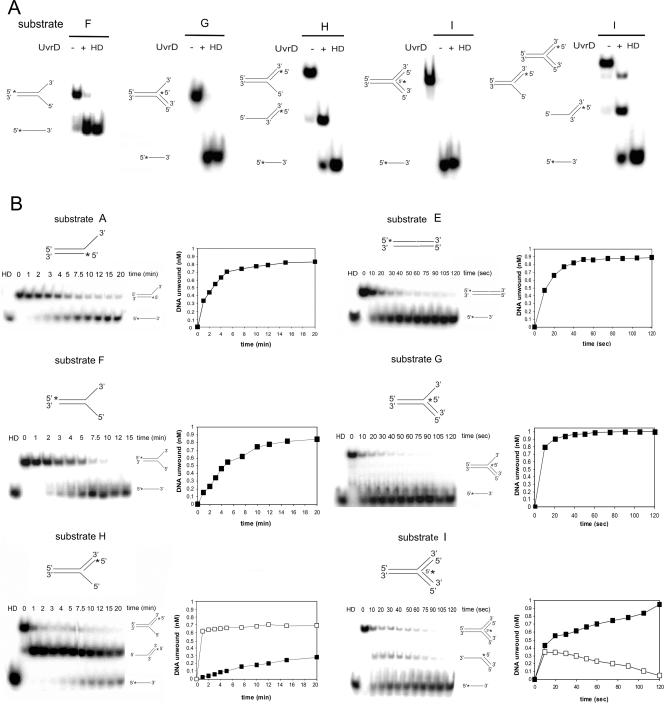
Initially, we tested whether unwinding of these substrates was catalyzed by UvrD at a detectable level. We found that the enzyme is capable of efficient unwinding of all of the structures tested, with the exception of the 5′ flap, where the extent of unwinding did not exceed 20% after 20 min (Fig. 5A). Having identified DNA structures that were relatively good substrates for unwinding by UvrD, we then conducted a more detailed and comparative analysis of unwinding of those structures. Time course studies were carried out to determine the efficiency of the reaction (Fig. 5B). Interestingly, M. tuberculosis UvrD unwound a DNA duplex containing a nick with surprising efficiency. Time course data showed that UvrD was able to unwind a nicked substrate with a sevenfold increase in the unwinding rate compared to that for the partial duplex with a 3′-ssDNA tail (Fig. 5B).
FIG. 5.
Unwinding of substrates with different structures by UvrD. (A) Substrates (1 nM) were incubated in the presence of 1 mM ATP, 5 mM MgCl2, and 200 nM UvrD at 37°C for 10 min. Reactions were analyzed by native PAGE, and PhosphorImager scans of the dried gels are shown. HD, heat denatured. The 32P label is indicated with asterisks. (B) Time course analysis of the unwinding of different substrates (A to I, as reported in Table 2). Each substrate (1 nM) was incubated in the presence of 1 mM ATP, 5 mM MgCl2, and 200 nM UvrD at 37°C for the indicated times. The data for the unwinding reactions were quantified and plotted; note that the units for the scales of the x axes are minutes for the left panels but seconds for the right panels. The amounts of labeled DNA unwound in time are represented as closed squares, while the intermediate structures are represented as open squares. The data reported are the averages for at least three independent experiments, and the gels are representative images.
With respect to the fork structures, UvrD was only active on certain strands at the fork. It could unwind the duplex ahead of the fork (duplex 3-11, in substrate F) as well as the “lagging” strand (duplex 11-12, in substrates G and I). Unwinding of the duplex ahead of the fork in substrate F is likely to result from UvrD translocating on the “leading” strand template, while unwinding of the 11-12 duplex in substrates G and I is likely to occur from UvrD entering at the junction and translocating on the lagging strand. The failure to efficiently unwind the 5′-flap structure (substrate H) is consistent with the previous finding that UvrD cannot unwind a blunt-ended DNA substrate or translocate 5′ to 3′. As shown in Fig. 5, UvrD catalyzed unwinding of the fork structure (substrate F) with the same efficiency displayed for the 3′-tailed duplex but unwound the 11-12 duplex in the 3′-flap (substrate G) and three-way junction (substrate I) structures substantially more efficiently. Time course data on the early stages of the unwinding reaction with the 5′-flap structure (substrate H) revealed the presence of a reaction intermediate consisting of a partial duplex. This suggests that UvrD binds at the junction and translocates 3′ to 5′, unwinding the duplex region reasonably efficiently, thus resulting in the formation of a 5′-tailed duplex, which is a poor substrate for further unwinding. Similarly, unwinding of the three-way junction (substrate I) also led to the formation of reaction intermediates. The intermediates visualized depended on the position of the label in the substrate. Thus, when oligonucleotide 13 was labeled, unwinding to release the oligonucleotide was inefficient, and a long-lived 5′-tailed intermediate was produced (Fig. 5A, rightmost panel). In contrast, when oligonucleotide 12 was labeled, the oligonucleotide was rapidly released, and a 3′-tailed intermediate that was only detected by kinetic analysis (Fig. 5B) was transiently formed.
UvrD unwinds DNA as a monomer.
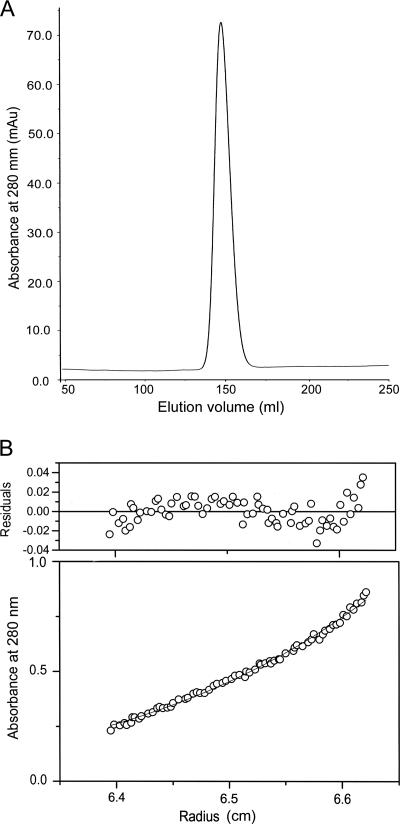
Although the increasing number of available crystal structures of helicases belonging to the SF1 family seems to point towards the common belief that SF1 helicases act as monomers, the oligomeric state of UvrD-like helicases has remained somewhat controversial. Several models for DNA unwinding catalyzed by a helicase have been proposed, some of which invoke a monomeric state, while others require an oligomeric state as the functional entity. The M. tuberculosis UvrD protein elutes from a Superdex S200 gel filtration column as a single peak (Fig. 6A), suggesting the presence of only one major species in solution. To confirm that M. tuberculosis UvrD was monomeric, not dimeric or multimeric, sedimentation equilibrium analytical ultracentrifugation was carried out. These experiments were performed using three protein concentrations and three rotor speeds. Global analysis of the data indicated that UvrD in solution behaves as an ∼85,000-kDa monomeric protein. No self-associations were observed at protein concentrations of up to 4 mg/ml (Fig. 6B). Importantly, the data show no evidence for a monomer-dimer equilibrium under the experimental conditions employed. Fitting the data to a reversible monomer-dimer model did not significantly improve the fitting of the data.
FIG. 6.
UvrD is a monomer in solution. (A) Elution profile of M. tuberculosis UvrD from Superdex S200 gel filtration column. (B) Sedimentation equilibrium distribution of UvrD at a loading concentration of 1 mg/ml, acquired at a rotor speed of 9,000 rpm and at 20°C.
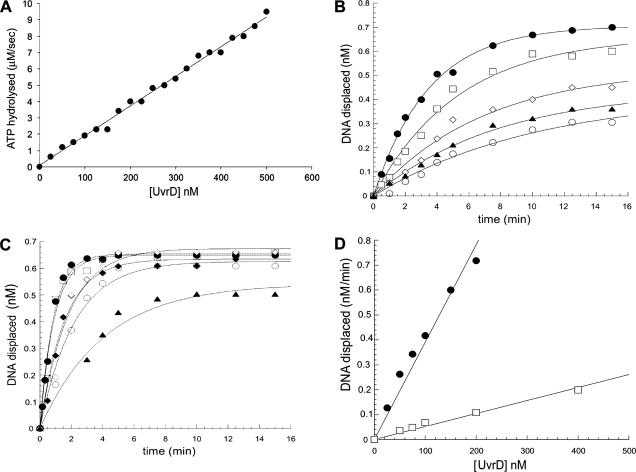
To shed more light on the oligomeric state of M. tuberculosis UvrD in the active form, we analyzed its ATPase and helicase activities as a function of protein concentration. Analysis of the dependence of an enzyme activity on protein concentration has been a commonly used method to determine the presence of cooperativity between catalytic units. If M. tuberculosis UvrD oligomers are more active than the monomers, then the activities of the protein should increase sigmoidally with increasing protein concentrations, as increasing protein concentrations should shift the equilibrium toward the formation of oligomers. A plot of the initial rates of ATP hydrolysis versus protein concentration (Fig. 7A) showed a nonsigmoidal increase in ATPase rate with the protein concentration in the range tested (20 nM to 500 nM), indicating the absence of any significant cooperativity between protein molecules. A similar nonsigmoidal correlation was obtained by plotting the rates of unwinding at different protein concentrations (25 nM to 400 nM) (Fig. 7D), using either a nicked substrate (Fig. 7B) or a 3′-ssDNA-tailed substrate (Fig. 7C). Again, these data suggest the absence of a shift towards a more active oligomeric form at higher protein concentrations and, therefore, a lack of cooperativity between UvrD molecules. The lack of cooperativity observed here for both the ATPase and helicase activities therefore lends support to the view that UvrD monomers are the functional unit.
FIG. 7.
Dependence of UvrD ATPase and helicase activities on protein concentration. (A) ATPase activity dependence on protein concentration. Poly(dT)18 (10 μM) was incubated with 1 mM ATP, 5 mM MgCl2, and increasing concentrations of UvrD in an NADH-coupled assay. The data for each enzyme concentration were determined as averages for three individual experiments. (B) The 3′-ssDNA-tailed substrate (1 nM) was incubated with UvrD at concentrations of 50 nM (open circles), 75 nM (closed triangles), 100 nM (open diamonds), 200 nM (open squares), and 400 nM (closed circles) and with 1 mM ATP at 37°C for the indicated times. Data were fitted to a single exponential equation. (C) The nicked substrate (1 nM) was incubated with UvrD at concentrations of 25 nM (closed triangles), 50 nM (open circles), 75 nM (closed diamonds), 100 nM (open diamonds), 150 nM (open squares), and 200 nM (closed circles) for the indicated times. Data were fitted to a single exponential equation. The curves in panels B and C were used to determine the initial rates. (D) Plot of the initial rates calculated from the data in panels B and C versus protein concentration. The fit of the data shows a linear dependence of the helicase activity on the protein concentration.
DISCUSSION
Genetic and biochemical data have demonstrated that the E. coli uvrD gene product is required for plasmid replication as well as for DNA repair by both the methyl-directed mismatch repair (24, 57) and UvrABC-mediated nucleotide excision repair pathways (8, 27, 29). However, the genes mediating methyl-directed mismatch repair are not conserved in M. tuberculosis (45, 61), and so far there is no direct evidence for the involvement of UvrD in nucleotide excision repair in M. tuberculosis, although sequence homology suggests that this is likely to be the case. Thus, the precise cellular function of this enzyme in M. tuberculosis has yet to be established. Preliminary studies (61a) have shown that an M. tuberculosis strain in which the uvrD gene has been inactivated is defective in persistence in a mouse model of tuberculosis, suggesting a role for this enzyme in the establishment or maintenance of persistence in the host. Thus, M. tuberculosis UvrD could serve as a target for drug development against the bacterium in the persistent state.
We have expressed and purified the UvrD protein from M. tuberculosis and characterized its ATPase and helicase activities. We demonstrated that the ATPase activity is strongly dependent on the presence of DNA. Single-stranded poly(dT) as short as four nucleotides was sufficient to stimulate ATP hydrolysis to a rate similar to that induced by a 23-mer or longer poly(dT) oligonucleotide. It was suggested recently that the stimulatory effect on the ATPase activity upon binding to single-stranded nucleic acid most likely reflects a conformational change that stabilizes the bound ATP molecule in a conformation that is required for rapid hydrolysis (59). This conformational change has long been proposed to occur in most NTPases, and recently it was indeed verified by structural data (59). M. tuberculosis UvrD could couple DNA unwinding to the hydrolysis of ATP and dATP only (Fig. 4A). ATP is likely to be the substrate used in vivo, as it is commonly present in cells at a much higher concentration than dATP. Nucleotide preferences differ considerably among helicases, as a biochemical reflection of the structures of their active sites. The gene 4 helicase from bacteriophage T7 preferentially hydrolyzed dTTP (41); the E. coli Rep and UvrD proteins preferred ATP and dATP (39). On the other hand, B. stearothermophilus PcrA displayed a wider nucleotide specificity and was able to hydrolyze all nucleotides and the ethenonucleotide analogues (6, 59). The determined kinetic parameters for ATP hydrolysis, Km and kcat (Km = 60.2 μM; kcat = 43 s−1), were similar to the equivalent parameters for E. coli UvrD (53 μM and 95 s−1, respectively) (16) but less comparable to the parameters determined for B. stearothermophilus PcrA (225 μM and 25 s−1, respectively) (59). Like E. coli UvrD and most helicases, M. tuberculosis UvrD required Mg2+ for optimal activity. Metal replacement studies showed, however, that Mn2+, Cu2+, Ni2+, and Co2+ were also effective as metal cofactors (Fig. 4B). To our knowledge, similar studies for other prokaryotic helicases have not been reported. For the eukaryotic helicase WRN, Mn2+ and Ni2+ could replace Mg2+ as a cofactor; however, Ni2+ was less effective than either Mg2+ or Mn2+ (11).
We also demonstrated that M. tuberculosis UvrD has a 3′-to-5′ polarity of unwinding. Like E. coli UvrD (38, 55), M. tuberculosis UvrD showed preference for a 3′-ssDNA-tailed duplex template compared to a 5′-ssDNA-tailed duplex. This finding implies that the helicase binds to the 3′-single-stranded region of a partial duplex DNA and unwinds this duplex in a 3′-to-5′ direction with respect to the DNA strand used for entry. In this respect, it should be kept in mind that there are many DNA SF1 helicases with proven 3′-to-5′ directionality, although a number of helicases in this family have been reported to have a 5′-to-3′ polarity or a bipolar mode of unwinding. Thermoanaerobacter tengcongensis UvrD showed a 5′-to-3′ polarity of unwinding (3). The PcrA helicase from Bacillus anthracis showed robust 3′-to-5′ as well as 5′-to-3′ helicase activities with substrates containing a duplex region and a 3′- or 5′-single-stranded poly(dT) tail (48). Also, the E. coli RecBCD complex has been reported to contain bipolar enzyme activity, where the RecB and RecD components of the complex unwind DNA in the 3′-to-5′ and 5′-to-3′ directions, respectively (17, 18). Recently, Constantinesco et al. have shown that the HerA DNA helicase from thermophilic archaea is able to utilize either 3′- or 5′-ssDNA extensions for loading and subsequent DNA duplex unwinding (12). For simple duplex DNA with a 3′-ssDNA tail, an overhang of 18 nucleotides was required for productive unwinding. This finding is consistent with the idea that productive unwinding requires simultaneous binding of UvrD molecules on DNA. UvrD molecules may bind next to each other on the tail and move forward like wagon trains on the single-stranded DNA. If the leading molecules dissociate from the DNA, then the following molecules carry on the unwinding, leading to an overall increase in processivity.
The substrate requirement for the helicase activity of UvrD was characterized using a variety of DNA duplexes with structures that were designed to represent the intermediates in various DNA transaction pathways, such as replication, transcription, and repair. Our first conclusion was that M. tuberculosis UvrD was able to unwind DNA substrates containing a nick. This was not surprising, since the ability of E. coli UvrD to unwind nicked DNA substrates has already been reported by Runyon and Lohman (55). However, while high concentrations of the E. coli protein were required to unwind nicked substrates, M. tuberculosis UvrD catalyzed unwinding of nicked DNA very efficiently. Interestingly, M. tuberculosis UvrD was approximately sevenfold more active on a nicked substrate than on 3′-tailed substrates (Fig. 5). With the exception of the 5′-flap structure, M. tuberculosis UvrD was also able to unwind forked structures. Indeed, the behavior of M. tuberculosis UvrD with respect to forked structures was surprisingly similar to that of E. coli RecG helicase, which has been shown to be involved in the recovery of stalled replication forks (54). Although UvrD and RecG are not homologues by sequence, they may have overlapping functions. Another recently purified member of the RecQ family, RecQ5 from Drosophila melanogaster, has shown very similar substrate specificity (50). Similar to RecQ5, M. tuberculosis UvrD could unwind the fork structure and the 3′-flap structure as well as a three-way junction, with a strong preference for the “lagging” strand over the fork. The “lagging” strands in both the 3′-flap structure and the three-way junction were unwound with approximately eightfold greater efficiency than that for the fork structure. Analysis of the reaction intermediates showed that UvrD unwound a three-way junction outward from the fork rather than towards the fork. Such results suggest that UvrD is a structure-specific helicase which is active at stalled replication forks and is probably targeted to the “lagging” strand.
Although data suggesting that E. coli UvrD is active as a dimer have been reported (2, 35-37, 56), evidence in favor of a monomeric active form of E. coli UvrD in vitro and in vivo has been presented (42), and this issue is still a matter of debate. The data presented here support a monomeric form of M. tuberculosis UvrD as the active species. Since our initial solution studies showed that UvrD was a monomer (Fig. 6), we measured the ATPase activity and helicase activity as a function of the protein concentration (Fig. 7). The nonsigmoidal dependence of ATPase activity on the enzyme concentration indicates that there is no cooperativity between the protein molecules. Since it is arguable that M. tuberculosis UvrD could hydrolyze ATP as a monomer but require dimerization to function as a helicase, we also measured the helicase activity of the protein at a range of protein concentrations. Two substrates, the 3′-ssDNA-tailed DNA and the nicked DNA, were used for these experiments. In both cases, no sigmoidal increase of helicase activity was observed with increasing protein concentrations. The lack of cooperativity observed for both ATPase and helicase activities suggests that M. tuberculosis UvrD functions as a monomer.
Considering the characteristics of the UvrD helicase activity reported here, we suggest that UvrD may play several roles in the DNA metabolism of M. tuberculosis. The specificity of M. tuberculosis UvrD towards the nicked duplex suggests that this substrate may be a close analogue of its physiological substrate and supports the notion that UvrD may play a function in the UvrABC-mediated NER pathway in M. tuberculosis. In addition, the efficiency of unwinding of the “lagging” strand in fork structures suggests that UvrD may support the reversal of stalled replication forks as well as potentially having a role in recombination (20, 21). In the absence of a mismatch repair pathway in M. tuberculosis, UvrD is not constrained by the requirement that it should interact with MutL, as it is in E. coli. It is possible that in this situation, M. tuberculosis UvrD has evolved in such a way that it has heightened efficiency on substrates of particular importance to this organism. Indeed, the alignment in Fig. 1 reveals short sequences that are unique to the M. tuberculosis UvrD protein which may influence its function. Thus, it could be that damage to DNA that is subject to repair by the NER pathway and/or stalling of replication is a relatively frequent event in M. tuberculosis.
Acknowledgments
This work was supported by the Medical Research Council Technology UK and the Medical Research Council UK.
We thank Arsen Petrovic for performing analytical ultracentrifugation experiments and Martin Webb and Christian Kurtis for critical readings of the manuscript.
Footnotes
Published ahead of print on 8 December 2006.
REFERENCES
- 1.Ahn, B. 2000. A physical interaction of UvrD with nucleotide excision repair protein UvrB. Mol. Cell 10:592-597. [DOI] [PubMed] [Google Scholar]
- 2.Ali, J. A., and T. M. Lohman. 1997. Kinetic measurement of the step size of DNA unwinding by Escherichia coli UvrD helicase. Science 275:377-380. [DOI] [PubMed] [Google Scholar]
- 3.An, L., W. Tang, T. A. Ranalli, H. J. Kim, J. Wytiaz, and H. Kong. 2005. Characterization of a thermostable UvrD helicase and its participation in helicase-dependent amplification. J. Biol. Chem. 280:28952-28958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anand, S. P., P. Mitra, A. Naqvi, and S. A. Khan. 2004. Bacillus anthracis and Bacillus cereus PcrA helicases can support DNA unwinding and in vitro rolling-circle replication of plasmid pT181 of Staphylococcus aureus. J. Bacteriol. 186:2195-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bierne, H., M. Seigneur, S. D. Ehrlich, and B. Michel. 1997. uvrD mutations enhance tandem repeat deletion in the Escherichia coli chromosome via SOS induction of the RecF recombination pathway. Mol. Microbiol. 26:557-567. [DOI] [PubMed] [Google Scholar]
- 6.Bird, L. E., J. A. Brannigan, H. S. Subramanya, and D. B. Wigley. 1998. Characterization of Bacillus stearothermophilus PcrA helicase: evidence against an active rolling mechanism. Nucleic Acids Res. 26:2686-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruand, C., and S. D. Ehrlich. 2000. UvrD-dependent replication of rolling-circle plasmids in Escherichia coli. Mol. Microbiol. 35:204-210. [DOI] [PubMed] [Google Scholar]
- 8.Caron, P. R., S. R. Kushner, and L. Grossman. 1985. Involvement of helicase II (uvrD gene product) and DNA polymerase I in excision mediated by the UvrABC protein complex. Proc. Natl. Acad. Sci. USA 82:4925-4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caruthers, J. M., and D. B. McKay. 2002. Helicase structure and mechanism. Curr. Opin. Struct. Biol. 12:123-133. [DOI] [PubMed] [Google Scholar]
- 10.Chang, T. L., A. Naqvi, S. P. Anand, M. G. Kramer, R. Munshi, and S. A. Khan. 2002. Biochemical characterization of the Staphylococcus aureus PcrA helicase and its role in plasmid rolling circle replication. J. Biol. Chem. 277:45880-45886. [DOI] [PubMed] [Google Scholar]
- 11.Choudhary, S., J. A. Sommers, and R. M. Brosh, Jr. 2004. Biochemical and kinetic characterization of the DNA helicase and exonuclease activities of Werner syndrome protein. J. Biol. Chem. 279:34603-34613. [DOI] [PubMed] [Google Scholar]
- 12.Constantinesco, F., P. Forterre, E. V. Koonin, L. Aravind, and C. Elie. 2004. A bipolar DNA helicase gene, herA, clusters with rad50, mre11 and nurA genes in thermophilic archaea. Nucleic Acids Res. 32:1439-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darwin, K. H., S. Ehrt, J. C. Gutierrez-Ramos, N. Weich, and C. F. Nathan. 2003. The proteasome of Mycobacterium tuberculosis is required for resistance to nitric oxide. Science 302:1963-1966. [DOI] [PubMed] [Google Scholar]
- 14.Darwin, K. H., and C. F. Nathan. 2005. Role for nucleotide excision repair in virulence of Mycobacterium tuberculosis. Infect. Immun. 73:4581-4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis, E. O., S. G. Sedgwick, and M. J. Colston. 1991. Novel structure of the recA locus of Mycobacterium tuberculosis implies processing of the gene product. J. Bacteriol. 173:5653-5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dessinges, M. N., T. Lionnet, X. G. Xi, D. Bensimon, and V. Croquette. 2004. Single-molecule assay reveals strand switching and enhanced processivity of UvrD. Proc. Natl. Acad. Sci. USA 101:6439-6444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dillingham, M. S., M. Spies, and S. C. Kowalczykowski. 2003. RecBCD enzyme is a bipolar DNA helicase. Nature 423:893-897. [DOI] [PubMed] [Google Scholar]
- 18.Dillingham, M. S., M. R. Webb, and S. C. Kowalczykowski. 2005. Bipolar DNA translocation contributes to highly processive DNA unwinding by RecBCD enzyme. J. Biol. Chem. 280:37069-37077. [DOI] [PubMed] [Google Scholar]
- 19.Dillingham, M. S., D. B. Wigley, and M. R. Webb. 2000. Demonstration of unidirectional single-stranded DNA translocation by PcrA helicase: measurement of step size and translocation speed. Biochemistry 39:205-212. [DOI] [PubMed] [Google Scholar]
- 20.Flores, M. J., V. Bidnenko, and B. Michel. 2004. The DNA repair helicase UvrD is essential for replication fork reversal in replication mutants. EMBO Rep. 5:983-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flores, M. J., N. Sanchez, and B. Michel. 2005. A fork-clearing role for UvrD. Mol. Microbiol. 57:1664-1675. [DOI] [PubMed] [Google Scholar]
- 22.Flynn, J. L., and J. Chan. 2005. What's good for the host is good for the bug. Trends Microbiol. 13:98-102. [DOI] [PubMed] [Google Scholar]
- 23.George, J. W., R. M. Brosh, Jr., and S. W. Matson. 1994. A dominant negative allele of the Escherichia coli uvrD gene encoding DNA helicase II. A biochemical and genetic characterization. J. Mol. Biol. 235:424-435. [DOI] [PubMed] [Google Scholar]
- 24.Hall, M. C., J. R. Jordan, and S. W. Matson. 1998. Evidence for a physical interaction between the Escherichia coli methyl-directed mismatch repair proteins MutL and UvrD. EMBO J. 17:1535-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernandez-Pando, R., M. Jeyanathan, G. Mengistu, D. Aguilar, H. Orozco, M. Harboe, G. A. Rook, and G. Bjune. 2000. Persistence of DNA from Mycobacterium tuberculosis in superficially normal lung tissue during latent infection. Lancet 356:2133-2138. [DOI] [PubMed] [Google Scholar]
- 26.Horii, Z., and A. J. Clark. 1973. Genetic analysis of the RecF pathway to genetic recombination in Escherichia coli K12: isolation and characterization of mutants. J. Mol. Biol. 80:327-344. [DOI] [PubMed] [Google Scholar]
- 27.Husain, I., B. Van Houten, D. C. Thomas, M. Abdel-Monem, and A. Sancar. 1985. Effect of DNA polymerase I and DNA helicase II on the turnover rate of UvrABC excision nuclease. Proc. Natl. Acad. Sci. USA 82:6774-6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iordanescu, S., and R. Basheer. 1991. The Staphylococcus aureus mutation pcrA3 leads to the accumulation of pT181 replication initiation complexes. J. Mol. Biol. 221:1183-1189. [DOI] [PubMed] [Google Scholar]
- 29.Kumura, K., M. Sekiguchi, A. L. Steinum, and E. Seeberg. 1985. Stimulation of the UvrABC enzyme-catalyzed repair reactions by the UvrD protein (DNA helicase II). Nucleic Acids Res. 13:1483-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laue, T. M., B. D. Shah, T. M. Ridgeway, and S. L. Pelletier. 1992. Computer-aided interpretation of analytical sedimentation data for proteins, p. 90-125. In S. E. Harding, A. J. Rowe, and J. C. Horton (ed.), Analytical ultracentrifugation in biochemistry and polymer science. Royal Society of Chemistry, Cambridge, United Kingdom.
- 31.Lohman, T. M. 1992. Escherichia coli DNA helicases: mechanisms of DNA unwinding. Mol. Microbiol. 6:5-14. [DOI] [PubMed] [Google Scholar]
- 32.Lohman, T. M. 1993. Helicase-catalyzed DNA unwinding. J. Biol. Chem. 268:2269-2272. [PubMed] [Google Scholar]
- 33.Lohman, T. M., and K. P. Bjornson. 1996. Mechanisms of helicase-catalyzed DNA unwinding. Annu. Rev. Biochem. 65:169-214. [DOI] [PubMed] [Google Scholar]
- 34.MacMicking, J., Q. W. Xie, and C. Nathan. 1997. Nitric oxide and macrophage function. Annu. Rev. Immunol. 15:323-350. [DOI] [PubMed] [Google Scholar]
- 35.Maluf, N. K., J. A. Ali, and T. M. Lohman. 2003. Kinetic mechanism for formation of the active, dimeric UvrD helicase-DNA complex. J. Biol. Chem. 278:31930-31940. [DOI] [PubMed] [Google Scholar]
- 36.Maluf, N. K., C. J. Fischer, and T. M. Lohman. 2003. A dimer of Escherichia coli UvrD is the active form of the helicase in vitro. J. Mol. Biol. 325:913-935. [DOI] [PubMed] [Google Scholar]
- 37.Maluf, N. K., and T. M. Lohman. 2003. Self-association equilibria of Escherichia coli UvrD helicase studied by analytical ultracentrifugation. J. Mol. Biol. 325:889-912. [DOI] [PubMed] [Google Scholar]
- 38.Matson, S. W. 1986. Escherichia coli helicase II (uvrD gene product) translocates unidirectionally in a 3′ to 5′ direction. J. Biol. Chem. 261:10169-10175. [PubMed] [Google Scholar]
- 39.Matson, S. W., and J. W. George. 1987. DNA helicase II of Escherichia coli. Characterization of the single-stranded DNA-dependent NTPase and helicase activities. J. Biol. Chem. 262:2066-2076. [PubMed] [Google Scholar]
- 40.Matson, S. W., and K. A. Kaiser-Rogers. 1990. DNA helicases. Annu. Rev. Biochem. 59:289-329. [DOI] [PubMed] [Google Scholar]
- 41.Mechanic, L. E., B. A. Frankel, and S. W. Matson. 2000. Escherichia coli MutL loads DNA helicase II onto DNA. J. Biol. Chem. 275:38337-38346. [DOI] [PubMed] [Google Scholar]
- 42.Mechanic, L. E., M. C. Hall, and S. W. Matson. 1999. Escherichia coli DNA helicase II is active as a monomer. J. Biol. Chem. 274:12488-12498. [DOI] [PubMed] [Google Scholar]
- 43.Mendonca, V. M., K. Kaiser-Rogers, and S. W. Matson. 1993. Double helicase II (uvrD)-helicase IV (helD) deletion mutants are defective in the recombination pathways of Escherichia coli. J. Bacteriol. 175:4641-4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mendonca, V. M., and S. W. Matson. 1995. Genetic analysis of delta helD and delta uvrD mutations in combination with other genes in the RecF recombination pathway in Escherichia coli: suppression of a ruvB mutation by a uvrD deletion. Genetics 141:443-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mizrahi, V., and S. J. Andersen. 1998. DNA repair in Mycobacterium tuberculosis. What have we learnt from the genome sequence? Mol. Microbiol. 29:1331-1339. [DOI] [PubMed] [Google Scholar]
- 46.Moolenaar, G. F., C. Moorman, and N. Goosen. 2000. Role of the Escherichia coli nucleotide excision repair proteins in DNA replication. J. Bacteriol. 182:5706-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morel, P., J. A. Hejna, S. D. Ehrlich, and E. Cassuto. 1993. Antipairing and strand transferase activities of E. coli helicase II (UvrD). Nucleic Acids Res. 21:3205-3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naqvi, A., E. Tinsley, and S. A. Khan. 2003. Purification and characterization of the PcrA helicase of Bacillus anthracis. J. Bacteriol. 185:6633-6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Norby, J. G. 1988. Coupled assay of Na+,K+-ATPase activity. Methods Enzymol. 156:116-119. [DOI] [PubMed] [Google Scholar]
- 50.Ozsoy, A. Z., H. M. Ragonese, and S. W. Matson. 2003. Analysis of helicase activity and substrate specificity of Drosophila RECQ5. Nucleic Acids Res. 31:1554-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petit, M. A., E. Dervyn, M. Rose, K. D. Entian, S. McGovern, S. D. Ehrlich, and C. Bruand. 1998. PcrA is an essential DNA helicase of Bacillus subtilis fulfilling functions both in repair and rolling-circle replication. Mol. Microbiol. 29:261-273. [DOI] [PubMed] [Google Scholar]
- 52.Petit, M. A., and D. Ehrlich. 2002. Essential bacterial helicases that counteract the toxicity of recombination proteins. EMBO J. 21:3137-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petranovic, M., K. Zahradka, D. Zahradka, D. Petranovic, B. Nagy, and E. Salaj-Smic. 2001. Genetic evidence that the elevated levels of Escherichia coli helicase II antagonize recombinational DNA repair. Biochimie 83:1041-1047. [DOI] [PubMed] [Google Scholar]
- 54.Runyon, G. T., D. G. Bear, and T. M. Lohman. 1990. Escherichia coli helicase II (UvrD) protein initiates DNA unwinding at nicks and blunt ends. Proc. Natl. Acad. Sci. USA 87:6383-6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Runyon, G. T., and T. M. Lohman. 1989. Escherichia coli helicase II (UvrD) protein can completely unwind fully duplex linear and nicked circular DNA. J. Biol. Chem. 264:17502-17512. [PubMed] [Google Scholar]
- 56.Runyon, G. T., I. Wong, and T. M. Lohman. 1993. Overexpression, purification, DNA binding, and dimerization of the Escherichia coli uvrD gene product (helicase II). Biochemistry 32:602-612. [DOI] [PubMed] [Google Scholar]
- 57.SaiSree, L., M. Reddy, and J. Gowrishankar. 2000. lon incompatibility associated with mutations causing SOS induction: null uvrD alleles induce an SOS response in Escherichia coli. J. Bacteriol. 182:3151-3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sancar, A. 1996. DNA excision repair. Annu. Rev. Biochem. 65:43-81. [DOI] [PubMed] [Google Scholar]
- 59.Soultanas, P., M. S. Dillingham, S. S. Velankar, and D. B. Wigley. 1999. DNA binding mediates conformational changes and metal ion coordination in the active site of PcrA helicase. J. Mol. Biol. 290:137-148. [DOI] [PubMed] [Google Scholar]
- 60.Soultanas, P., and D. B. Wigley. 2000. DNA helicases: ‘inching forward.’ Curr. Opin. Struct. Biol. 10:124-128. [DOI] [PubMed] [Google Scholar]
- 61.Springer, B., P. Sander, L. Sedlacek, W. D. Hardt, V. Mizrahi, P. Schar, and E. C. Bottger. 2004. Lack of mismatch correction facilitates genome evolution in mycobacteria. Mol. Microbiol. 53:1601-1609. [DOI] [PubMed] [Google Scholar]
- 61a.Springer, B., L. Rand, P. Sander, E. C. Boettger, and E. O. Davis. 2004. Abstr. 104th Gen. Meet. Am. Soc. Microbiol., abstr. U-048.
- 62.Veaute, X., S. Delmas, M. Selva, J. Jeusset, E. Le Cam, I. Matic, F. Fabre, and M. A. Petit. 2005. UvrD helicase, unlike Rep helicase, dismantles RecA nucleoprotein filaments in Escherichia coli. EMBO J. 24:180-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Washburn, B. K., and S. R. Kushner. 1991. Construction and analysis of deletions in the structural gene (uvrD) for DNA helicase II of Escherichia coli. J. Bacteriol. 173:2569-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.World Health Organization. March 2004, posting date. Tuberculosis: infection and transmission in 2002. Fact sheet no. 104. World Health Organization, Geneva, Switzerland. http://www.who.int/mediacentre/factsheets/fs104/en/.
- 65.Yamaguchi, M., V. Dao, and P. Modrich. 1998. MutS and MutL activate DNA helicase II in a mismatch-dependent manner. J. Biol. Chem. 273:9197-9201. [DOI] [PubMed] [Google Scholar]