Abstract
Derepression of pyrG expression in Bacillus subtilis involves CTP-sensitive reiterative transcription, which introduces up to 11 extra G residues at the 5′ ends of pyrG transcripts. Insertion of three or more additional Gs at the 5′ end of the pyrG initially transcribed region abolished reiterative transcription and caused constitutive expression.
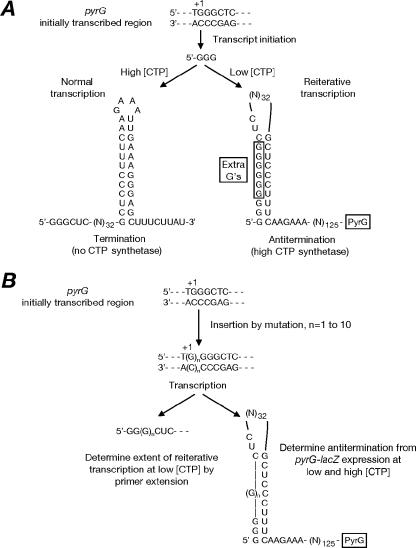
Expression of the pyrG operon, which encodes CTP synthetase, is regulated in Bacillus subtilis and several other low-GC gram-positive bacteria by the intracellular CTP level (5, 8). CTP exerts its repressive effect by a novel mechanism that involves transcription attenuation at an intrinsic terminator located within an untranslated leader region. Two short segments of conserved nucleotide sequence are required for regulation, namely, a pyrG initially transcribed region (ITR) with the sequence 5′-GGGC (nontranscribed strand) and a C-plus-U-rich sequence (GCUCCCUUUC in B. subtilis) located in the 5′ strand of the attenuator-specified terminator hairpin (Fig. 1A). Evidence that supports the mechanism for pyrG regulation illustrated in Fig. 1A has been presented (8-10). Low intracellular CTP concentrations cause transcription pausing at position +4 (normally a C) of the nascent pyrG transcript, which allows reiterative transcription (also called transcriptional slippage) to occur and introduce from 1 to 11 extra G residues before normal elongation resumes. When the intracellular CTP level is high, insertion of a C residue at position +4 proceeds readily without pausing, which precludes reiterative transcription. The extra G residues added to the 5′ ends of pyrG transcripts under CTP limitation are proposed to act as antiterminator sequences; when present, they base pair with the C-plus-U-rich 5′ strand of the terminator hairpin and preclude terminator formation (Fig. 1A). Thus, conditional reiterative transcription and the insertion of extra G residues result in CTP-sensitive antitermination and CTP-regulated transcription of the downstream pyrG gene. It is likely that this mechanism serves to regulate pyrG expression in several genera of low-GC gram-positive bacteria (10).
FIG. 1.
(A) Proposed mechanism for the regulation of B. subtilis pyrG expression by CTP-sensitive reiterative transcription and antitermination (from reference 10). The 5′ sequence of transcripts specified by the pyrG ITR depends on the intracellular CTP concentration. If the CTP level is high (left), a C residue is rapidly inserted at position +4 and the transcript encoded by the DNA is synthesized. This sequence permits formation of the leader terminator and reduced pyrG expression. If the CTP level is low (right), transcription pauses at +4, leading to reiterative transcription of a variable number of G residues. Transcripts bearing extra G residues are able to fold into an antiterminator hairpin, which suppresses termination and results in increased transcription of the downstream pyrG coding region. The nucleotides denoted by (N)32 apparently play no role in regulation, because pyrG′-lacZ fusions from which they were deleted retained normal regulation (9). (B) Rationale for experiments reported in this work. Mutational insertion of 1 to 10 additional G residues at +1 in the pyrG ITR leads to formation of transcripts with a known number of extra 5′ G residues. The effects of such mutations on reiterative transcription were determined by primer extension mapping (left). The effects of such mutations on pyrG expression were assessed with pyrG′-lacZ fusions grown under repressing and derepressing conditions (right). Note that numerous antiterminator structures can form, depending on the number of G residues inserted and the exact base pairing.
A series of pyrG transcripts with from 1 to 11 extra G residues is formed by reiterative transcription at the wild-type pyrG ITR in pyrimidine-starved cells (10). However, mutational insertion of four extra G residues at the start of the pyrG ITR (specifying the transcript sequence 5′-GGGGGGGC) was sufficient to cause constitutive pyrG expression (10). In this study we sought to answer two related questions concerning the function of the extra 5′ G residues. First, given that the addition of extra G residues permits formation of a progressively more stable antiterminator structure as more Gs are added, what number of G residues at the 5′ end of pyrG transcripts is sufficient to cause fully constitutive expression? Second, what number of G residues in the pyrG ITR is optimal for reiterative transcription in pyrimidine-starved cells? In other cases where reiterative transcription has been studied, a homopolymeric run of at least three template residues is required for transcriptional slippage (1, 3, 4, 11). Our previous studies (10) confirmed that at least three G residues in the template pyrG ITR were required for reiterative transcription. However, reiterative transcription requires melting of the DNA template and nascent transcript within the initiation complex, so it would be expected that the greater stability of longer (rG)n·(dC)n duplexes would suppress melting and reduce reiterative transcription.
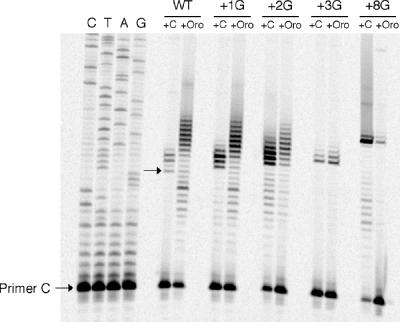
Both of these questions were addressed by construction of mutant pyrG promoter regions in which the number of G residues in the ITR was systematically increased from 3 (wild type) to 13 (Fig. 1B). Reiterative transcription in vivo with templates bearing several of the Gn insertions was characterized by primer extension mapping of the 5′ ends of pyrG transcripts to determine their lengths and relative amounts, as previously described by Meng et al. (10) (Fig. 2). Derivatives of the high-copy plasmid pEB112 (6) containing the wild-type pyrG promoter-leader region (nucleotides −49 to +81) and mutants of this region in which one, two, three, and eight G residues were inserted into the ITR were constructed as described by Meng et al. (10) and used to transform B. subtilis HC-11 (pyrB::Spcr). The pyrimidine auxotrophic pyrB host was used so that the cells could be starved for pyrimidines by growth on orotate, which is a poor pyrimidine source for B. subtilis (8, 9). Growth of the strain with cytidine, on the other hand, represses pyrG expression. High-copy plasmids containing the pyrG regions were used to produce transcripts at levels much higher than the background levels of transcripts produced from the wild-type chromosomal pyrG operon also present in strain HC-11 (which were barely detectable under the conditions used). The transformed cells were grown in minimal medium containing cytidine or orotate, i.e., repressing and derepressing conditions, respectively, and RNA was harvested from the cells as previously described (10). A single-stranded DNA (primer C) complementary to nucleotides +15 to +34 of the pyrG transcript was chemically synthesized and 5′ end labeled with [γ-33P]ATP and T4 polynucleotide kinase. The labeled DNA was used as the primer for avian myeloblastosis virus reverse transcriptase, and the primer-extended products were analyzed by electrophoresis as described previously (10). Transcripts specified by the wild-type pyrG plasmid and harvested from cells grown with excess cytidine mapped to the +1 position of the ITR, as previously reported (8, 10). In this assay transcripts that were one to four nucleotides longer were also detected in cells grown with excess cytidine (Fig. 2, WT +C). These longer transcripts may indicate that limited reiterative transcription occurred even under repressing conditions, but we believe that they are largely an artifact of using templates borne on multicopy plasmids, because they were much less prominent when transcripts from the chromosomal pyrG operon were analyzed (8, 10). Extensive reiterative transcription occurred in pyrimidine-starved cells with the wild-type template, as shown by the formation of a ladder of primer-extended products, corresponding to transcripts with extensions up to 11 nucleotides long. We have previously demonstrated that these extensions are formed by the addition of extra 5′ G residues (10). The most abundant poly(G) extensions ranged from 4 to 10 nucleotides long (Fig. 2, WT +Oro).
FIG. 2.
Primer extension mapping of pyrG transcripts extracted from the pyrimidine-requiring B. subtilis strain HC-11 bearing pEB112-derived plasmids that contained the promoter-leader region of wild-type pyrG (WT) or the corresponding region with (G)n insertion mutations (+1G, +2G, +3G, and +8G). Cells were grown on minimal medium (8) at 37°C with excess cytidine (+C) or orotate (+Oro), a poor pyrimidine source, and were harvested in mid-exponential phase as previously described (8). The sequencing ladder is labeled to correspond to the sequence of the nontranscribed strand. The arrow denotes G +1, the previously identified (10) transcription initiation site encoded by the pyrG gene. The position of 5′-end-labeled primer C (10), used both for sequencing and primer extension, is also shown.
When the transcripts specified by plasmids carrying pyrG ITRs containing one, two, three, or eight G insertions were mapped, the shortest transcripts from cells grown under repressing conditions were longer than the wild-type transcripts by one, two, three, and eight nucleotides, respectively, as expected. As with the wild-type template, some marginally extended transcripts were formed in repressed cells when the template contained insertions of one or two G residues, but these transcripts were much less abundant with the templates containing three and eight G insertions. This observation indicates that an ITR sequence of longer than five G residues suppresses reiterative transcription. This conclusion is reinforced by the mapping of transcripts from the same strains grown under derepressing conditions. A ladder of longer transcripts similar to that seen with the wild-type template was formed in starved cells containing the pyrG template with a single G insertion. When the template contained two inserted G residues, the ladder of transcripts also indicated the occurrence of reiterative transcription in pyrimidine-starved cells, but the ladder was somewhat shorter than that found with the wild-type template, and only a few of the transcripts were longer than those observed with repressed cells. When the pyrG template contained three or eight inserted G residues, little reiterative transcription occurred under any condition.
It was demonstrated previously that reiterative transcription at the pyrG ITR requires a run of three G residues at the site of transcription initiation, followed by a C or T residue (10). Here we have demonstrated that a run of four or five G residues also permits reiterative transcription, but a run of six or more G residues suppresses it. As noted above, reiterative transcription requires melting of the hybrid formed by the DNA template and the nascent transcript, so the greater stability of the (rG)n·(dC)n duplex when n is greater than 5 may preclude reiterative transcription by suppressing melting.
The effect of various G insertions on expression of corresponding pyrG′-lacZ fusions integrated into the B. subtilis chromosome was also determined. Derivatives of the pDH32 integration plasmid (2) that contained pyrG′-lacZ fusions bearing either the wild-type pyrG promoter-leader region (nucleotides −49 to +81), which specifies transcripts initiating with 5′-GGGCUC, or mutated pyrG promoter-leader regions that specified transcripts initiating with 5′-GGG(G)nCUC (where n equals 1, 2, 3, 4, 6, 8, and 10) were constructed using previously published procedures (8, 9). DNA sequencing confirmed the identities of the pyrG′ regions of the plasmids. The plasmids were integrated into the amyE locus of the chromosome of B. subtilis strain HC-11 (pyrB::Spcr) as described previously (8). (Note that two of these stains, QM401 and QM425, were prepared previously [8, 10].) The integrant strains were grown on minimal medium containing 200 μg of cytidine per ml (repressing conditions) and on the same medium containing 100 μg of orotate per ml (derepressing conditions), and β-galactosidase assays were used to assess pyrG expression under both conditions using published methods (8). As seen in previous studies (8, 9), expression of the wild-type pyrG′-lacZ fusion was derepressed 14-fold by growth under pyrimidine-limiting conditions, relative to growth on excess cytidine (Table 1). Insertion of even a single G residue in the pyrG ITR led to substantial derepression of expression. Insertion of two or more G residues resulted in essentially constitutive pyrG expression (Table 1). (The residual 1.8- to 2.5-fold-higher level of expression in pyrimidine-starved cells containing three or more inserted G residues was previously observed in a pyrG′-lacZ fusion in which the attenuator terminator was deleted [8] and results from a phenomenon unrelated to transcription attenuation.) The highest level of pyrG expression under both repressing and derepressing conditions was observed with the pyrG′-lacZ fusion containing four inserted G residues.
TABLE 1.
Effects of G insertion mutations on expression of pyrG-lacZ fusions in repressed and pyrimidine-starved B. subtilis HC-11 cells
| Strain (insertion) | β-Galactosidase activity (Miller units)a with:
|
Fold regulation | |
|---|---|---|---|
| Cytidine | Orotate | ||
| QM401 (none [wild type]) | 41 ± 4 | 574 ± 34 | 14 |
| AE101 (+1G) | 220 ± 25 | 1,240 ± 82 | 5.6 |
| AE102 (+2G) | 415 ± 30 | 900 ± 60 | 2.2 |
| AE103 (+3G) | 500 ± 29 | 910 ± 48 | 1.8 |
| QM425 (+4G) | 800 ± 27 | 1,800 ± 115 | 2.2 |
| AE104 (+6G) | 645 ± 45 | 1,610 ± 103 | 2.5 |
| AE105 (+8G) | 560 ± 63 | 1,130 ± 66 | 2 |
| AE106 (+10G) | 480 ± 54 | 870 ± 35 | 1.8 |
The data are the combined results of duplicate assays from three replicate experiments (mean ± standard deviation).
Interpretation of the effects of the G insertions on pyrG′-lacZ expression is complex, because the insertions have two consequences that are expected to have opposite effects on pyrG expression. Reiterative transcription is necessary to generate the 5′ poly(G) tails on pyrG transcripts that act as the antiterminator and suppress the downstream attenuator. From this consideration alone, one would predict that the fusions in which reiterative transcription occurred, i.e., those with one and two G insertions (Fig. 2, +1G and +2G), would behave more or less like the wild type and that those containing three or more G insertions, in which reiterative transcription was suppressed (Fig. 2, +3G and +8G), would fail to antiterminate and would be repressed under all growth conditions. In fact, all of the G insertion mutants were expressed at elevated levels; that is, they failed to terminate normally under repressing conditions. The pyrG′-lacZ fusion containing a single G insertion showed some residual regulation, which presumably resulted from its ability to carry out reiterative transcription, but was expressed at an elevated level under repressing conditions, compared to the wild-type fusion. Insertions of two or more G residues led to constitutive expression, whether reiterative transcription occurred or not. Examination of the patterns of transcript formation in these insertion strains (Fig. 2) leads us to suggest that derepression results from formation of pyrG transcripts containing six or more G residues at their 5′ ends, whether these result from reiterative transcription (wild type and one and two G insertions) or are encoded in the pyrG template (three or more G insertions). While a 5′-GGGGGG segment of the transcript is apparently sufficient to serve as an antiterminator by base pairing with the downstream GCUCCCUUUC segment of the attenuator hairpin, the highest level of expression was obtained with the four-G insertion mutant (Table 1), corresponding to a 5′ poly(G) tract of seven residues. The most abundant poly(G) tracts formed by reiterative transcription in pyrimidine-starved cells with the wild-type pyrG template contained 7 to 13 G residues (Fig. 2, WT +Oro), which are clearly sufficient for optimal antitermination.
It is evident that the pyrG DNA has been selected to contain the optimal number of rG-dC base pairs specifying the 5′ terminus of its transcripts; that number is three. A pyrG template specifying two G residues in the transcript, i.e., one in which one of the three wild-type G residues is mutated to A, fails to carry out significant reiterative transcription (10), cannot antiterminate, and is expressed at very low levels (9). A pyrG template specifying four G residues is capable of reiterative transcription but antiterminates to a significant degree even under repressing conditions, leading to suboptimal regulation. Templates specifying five or more G residues are progressively more defective in reiterative transcription and antiterminate under all conditions.
Our studies identified that a 5′ poly(G) sequence containing six G residues is highly effective in antitermination of pyrG expression, and a 5′ poly(G) sequence of seven to nine G residues is optimal for antitermination. However, the stability of the putatively optimal antiterminator stem-loop structure with (G)9C base paired to the terminator sequence GCUCCCUUUC, as calculated using Mfold (12), is approximately equal to the calculated stability of the B. subtilis pyrG leader terminator (attenuator) stem-loop, whereas antiterminator structures with fewer G residues are progressively weaker by about 0.5 kcal/mol per G residue. (Numerous possible structures are predicted by Mfold, depending on the exact sequences folded and constraints used, so such computations should be interpreted cautiously.) We suggest that the competition in vivo between antiterminator and terminator conformations of pyrG leader RNA does not occur as a simple thermodynamic equilibrium between the two folded forms. If it did, termination would be expected to equal or exceed antitermination even under derepressing conditions. Rather, we predict that the system is kinetically controlled. We propose that during pyrimidine starvation reiterative transcription leads to formation of 5′ poly(G) tracts on the transcripts that are able to form the antiterminator structure before the terminator is fully transcribed and emergent from RNA polymerase. By the time the terminator is able to form and compete with the antiterminator structure, transcription has proceeded through the terminator sequence, and RNA polymerase is committed to transcription of the entire downstream pyrG coding region. Only in the absence of the upstream 5′ poly(G) tracts and the consequent absence of interfering (albeit transient) antiterminator formation is the pyrG leader terminator able to form quickly enough to terminate within the leader region.
Reiterative transcription occurs when nucleotides are added repetitively to the 3′ end of a nascent transcript after that transcript slips toward the 5′ end relative to the DNA template strand. Slippage of the transcript generally occurs when RNA polymerase pauses because it is unable to add the next encoded nucleotide. The slippage site is a homopolymeric sequence, at least three nucleotides in length, on the template that specifies a complementary sequence in the transcript (GGG in pyrG), which undergoes elongation to longer homopolymeric segments (4). Very few systematic studies of the dependence of reiterative transcription on the length of the homopolymeric slippage site in the template are available, and most have studied reiterative transcription in vitro, not in vivo, as in this work. We have identified only one other example of reiterative transcription yielding poly(G) addition at the 5′ end of transcripts, namely, that catalyzed by T7 RNA polymerase with GTP only and a template specifying 5′-GGGN transcripts (7). In this case a template specifying two Gs or one G sharply reduced or abolished reiterative transcription, respectively. Templates specifying a sequence of four or more Gs were not studied. Reiterative transcription has been observed more frequently when the template specifies a tract of U or A residues at the 5′ end of the transcript (4). In several such cases the effects of substitution mutations in the homopolymeric segments of the template documented that a run of at least three T or A residues was required (1, 3, 11). However, only in the studies by Cheng et al. (1) of reiterative transcription and regulation of the Escherichia coli upp promoter was the effect of length of the homopolymeric segment of T residues in the template systematically examined. These workers found that a minimum of three T residues in the template was required for reiterative transcription of U residues in the upp transcript. Stepwise variation in the number of template Ts from three to eight did not alter reiterative transcription significantly in vitro but appeared to increase it in vivo, as judged from the regulation of corresponding upp-lacZ fusions. This length dependency is quite different from that observed in our studies with reiterative transcription of G residues with the pyrG template. From these differences it seems likely that the detailed dependence of reiterative transcription on template sequence will differ from system to system. It should be noted, however, that the mechanism of regulation of upp expression by reiterative transcription differs markedly from the mechanism of pyrG regulation. In the upp system reiterative transcription of U residues at high UTP levels leads to abortive initiation and reduced expression, whereas reiterative transcription at the pyrG ITR occurs at low CTP levels and leads to antitermination and elevated expression.
Acknowledgments
We gratefully acknowledge Qi Meng for providing B. subtilis strains QM401 and QM425 and developing the methods used in this work and Charles L. Turnbough, Jr., for valuable comments on the manuscript.
This research was supported by Public Health Service grant GM47112 from the National Institute of General Medical Studies.
Footnotes
Published ahead of print on 8 December 2006.
REFERENCES
- 1.Cheng, Y., S. M. Dylla, and C. L. Turnbough, Jr. 2001. A long T-A tract in the upp initially transcribed region is required for regulation of upp expression by UTP-dependent reiterative transcription in Escherichia coli. J. Bacteriol. 183:221-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grandoni, J. A., S. B. Fulmer, V. Brizzio, S. A. Zahler, and J. M. Calvo. 1993. Regions of the Bacillus subtilis ilv-leu operon involved in regulation by leucine. J. Bacteriol. 175:7581-7593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo, H. C., and J. W. Roberts. 1990. Heterogeneous initiation due to slippage at the bacteriophage 82 late promoter in vitro. Biochemistry 27:10702-10709. [DOI] [PubMed] [Google Scholar]
- 4.Jacques, J.-P., and D. Kolakofsky. 1991. Pseudo-templated transcription in prokaryotic and eukaryotic organisms. Genes Dev. 5:707-713. [DOI] [PubMed] [Google Scholar]
- 5.Jørgensen, C. M., K. Hammer, and J. Martinussen. 2003. CTP limitation increases expression of CTP synthase in Lactococcus lactis. J. Bacteriol. 185:6562-6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leonhardt, H., and J. Alonso. 1988. Construction of a shuttle vector for inducible gene expression in E. coli and B. subtilis. J. Gen. Microbiol. 134:605-609. [DOI] [PubMed] [Google Scholar]
- 7.Martin, C. T., D. K. Muller, and J. E. Coleman. 1988. Processivity in early stages of transcription by T7 RNA polymerase. Biochemistry 27:3966-3974. [DOI] [PubMed] [Google Scholar]
- 8.Meng, Q., and R. L. Switzer. 2001. Regulation of transcription of the Bacillus subtilis pyrG gene encoding cytidine triphosphate synthetase. J. Bacteriol. 183:5513-5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meng, Q., and R. L. Switzer. 2002. cis-acting sequences of Bacillus subtilis pyrG mRNA essential for regulation by antitermination. J. Bacteriol. 184:6734-6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng, Q., C. L. Turnbough, Jr., and R. L. Switzer. 2004. Attenuation control of pyrG expression in Bacillus subtilis is mediated by CTP-sensitive reiterative transcription. Proc. Natl. Acad. Sci. USA 101:10943-10948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiong, X. F., and W. S. Resnikoff. 1993. Transcriptional slippage during the transcription initiation process at a mutant lac promoter in vivo. J. Mol. Biol. 231:569-580. [DOI] [PubMed] [Google Scholar]
- 12.Zuker, M. 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31:3406-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]