Abstract
The release of dipicolinic acid (DPA) during the germination of Bacillus subtilis spores by the cationic surfactant dodecylamine exhibited a pH optimum of ∼9 and a temperature optimum of 60°C. DPA release during dodecylamine germination of B. subtilis spores with fourfold-elevated levels of the SpoVA proteins that have been suggested to be involved in the release of DPA during nutrient germination was about fourfold faster than DPA release during dodecylamine germination of wild-type spores and was inhibited by HgCl2. Spores carrying temperature-sensitive mutants in the spoVA operon were also temperature sensitive in DPA release during dodecylamine germination as well as in lysozyme germination of decoated spores. In addition to DPA, dodecylamine triggered the release of amounts of Ca2+ almost equivalent to those of DPA, and at least one other abundant spore small molecule, glutamic acid, was released in parallel with Ca2+ and DPA. These data indicate that (i) dodecylamine triggers spore germination by opening a channel in the inner membrane for Ca2+-DPA and other small molecules, (ii) this channel is composed at least in part of proteins, and (iii) SpoVA proteins are involved in the release of Ca2+-DPA and other small molecules during spore germination, perhaps by being a part of a channel in the spore's inner membrane.
Spores of Bacillus and Clostridium species are metabolically dormant and very resistant to many stress agents including heat, radiation, and toxic chemicals (13, 24). As a consequence and because these organisms are commonly found in soils, spores of a number of these species are significant agents of food spoilage and food-borne disease (25). However, since spores are metabolically dormant, they must return to active growth to cause their deleterious effects. Spores return to active growth through the process of germination that is followed by outgrowth (16, 23). Spore germination is normally triggered by any of a variety of specific nutrients such as l-alanine and d-glucose. Metabolism of these nutrients does not trigger spore germination. Rather, the nutrients bind to specific receptors located in the spore's inner membrane, and this binding triggers the release of a variety of compounds from the spore's central region or core, including monovalent cations (H+, Na+, and K+), divalent cations (Ca2+, Mg2+, and Mn2+), and pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]). Release of the latter compound is especially important, since DPA makes up ∼10% of the total spore dry weight and ∼20% of the dry weight of the spore core where it is present in a 1:1 ratio of chelate with divalent cations, predominantly Ca2+. Indeed, during germination with nutrients, most DPA is likely released as the Ca2+ chelate (Ca-DPA). This Ca-DPA release is crucial to spore progression from germination into outgrowth, as water replaces the Ca-DPA, thus elevating the spore core water content significantly. The Ca-DPA release also triggers further events in spore germination, most importantly, the hydrolysis of the spore's peptidoglycan cortex that otherwise prevents the expansion of the spore's core. Following cortex hydrolysis, the spore core expands and takes up more water such that the core water content rises to that in the protoplast of a growing cell. This allows protein movement and enzyme action in the spore core and leads to early events in spore outgrowth including the resumption of energy metabolism and macromolecular synthesis (7, 16, 23).
Since Ca-DPA release is such a pivotal event in spore germination, knowledge of the mechanisms and control of Ca-DPA release is crucial to the complete understanding of the overall process of germination. It was suggested a number of years ago that the proteins encoded by the spoVA operon are involved in DPA uptake into the developing spore during sporulation (9). The spoVA operon encodes six proteins, the majority of which are most likely membrane proteins. Since spoVA is transcribed exclusively in the developing forespore, the SpoVA proteins are likely to reside in the spore's inner membrane, and this is the case for at least the SpoVAD protein (32). The fact that SpoVA proteins are involved in DPA uptake during sporulation has been shown using strains with either deletion or temperature-sensitive (TS) mutations in spoVA (30, 31). The spores of TS strains formed at a permissive temperature are also TS for DPA release during nutrient-induced germination (31), consistent with a role for at least some SpoVA proteins in Ca-DPA release during spore germination.
In addition to nutrients, spores can be germinated by a number of other agents including surfactants such as dodecylamine, exogenous Ca-DPA, and lysozyme treatment if spores are first decoated to allow the lysozyme to penetrate to the spore's cortex (16, 20, 23). These latter agents do not act through the spore's nutrient germinant receptors but in some cases (dodecylamine) appear to directly cause Ca-DPA release, with this release then triggering subsequent germination events.
An obvious question is whether the Ca-DPA release triggered by germination agents other than nutrients also involves SpoVA proteins, as it is possible that these other agents directly stimulate the opening of a Ca-DPA channel. In this paper, we report the results of studies that indicate that Ca-DPA release triggered by both dodecylamine and lysozyme treatment of decoated spores requires SpoVA proteins, consistent with these proteins being components of a Ca-DPA channel.
MATERIALS AND METHODS
Bacillus subtilis strains used and spore preparation.
All B. subtilis strains used are derivatives of strain PS832, a prototrophic derivative of strain 168. Strains PS3640 and PS3642 (both TS spoVA) carry TS spoVA mutations with changes in either bp 1047 or 1267, respectively, in spoVA (31). Strain PS3411 (↑SpoVA) contains the spoVA operon under the control of the strong forespore-specific promoter of the sspB gene (32). The levels of SpoVAD in the inner membrane of spores of this strain (and, by inference, levels of all SpoVA proteins) are fourfold higher than those in the inner membrane of wild-type spores (32). Strain FB113 (ΔcwlJ::tet ΔsleB::spc) (21) has antibiotic resistance genes replacing the majority of the coding sequence of the cwlJ and sleB genes that encode the two enzymes that degrade the cortex during spore germination. Either CwlJ or SleB is sufficient for cortex degradation, but cwlJ sleB spores cannot complete spore germination triggered by nutrients, although Ca-DPA release is relatively normal (21).
Spores of various strains were prepared at 37°C, except for spores of strains PS3640 and PS3642 (both TS spoVA), which were prepared at 30°C, on 2× SG medium (14) agar plates. Spores were harvested, cleaned, and stored as described previously (14, 15, 21). All spore preparations used in this work were free (>98%) of growing or sporulating cells or germinated spores as determined by examination using a phase-contrast microscope.
Spore germination.
Spores were germinated with dodecylamine without a heat shock, as this is not required for dodecylamine germination (20). Unless noted otherwise, spores were germinated at an optical density at 600 nm (OD600) of 1 in 10 mM Tris-HCl (pH 7.5). Spore germination was assessed by centrifugation of 1-ml aliquots of the germination mixture and reading the OD270 of the supernatant fluid. A sample of the starting spores was also boiled for 20 min, cooled on ice, and centrifuged, and the OD270 of the supernatant fluid was measured. Previous work showed that ∼85% of the OD270 in the supernatant fluid released from boiled wild-type spores and >95% of the OD270 in the supernatant fluid from germinating spores are due to DPA (3, 20). All experiments measuring DPA release with dodecylamine were repeated at least twice with two independent spore preparations with essentially identical results.
For germination with lysozyme, spores were first decoated and washed as described previously (1). The decoated, washed spores were suspended in hypertonic medium (17) at an OD600 of 1 and treated with lysozyme at various temperatures. Samples (1 ml) of these germination incubation mixtures were centrifuged, and the OD270 of the supernatant fluid was determined. Again, all experiments measuring DPA release upon lysozyme treatment were repeated at least twice using two independent spore preparations with essentially identical results.
Analysis of molecules released during spore germination with dodecylamine.
For analysis of the Ca2+ and DPA released during dodecylamine germination, spores at an OD600 of 2 were germinated at pH 7.5 and 37°C with 0.5 mM dodecylamine. At various times, 5-ml aliquots were centrifuged, the supernatant fluid was lyophilized, the dry residue was dissolved in 1 ml water, and DPA and Ca2+ were determined chemically as described previously (19, 33).
For analysis of other molecules released during dodecylamine germination, spores of strain FB113 (cwlJ sleB) at an OD600 of 2 were germinated at 45°C with 0.5 mM dodecylamine in 10 mM KPO4 buffer (pH 7.7). At various times, samples (10 ml) were centrifuged, the supernatant fluid was passed through a 1-ml Chelex column at 23°C to remove divalent cations, in particular Mn2+, and the run-through fraction was lyophilized (10). The pellet fraction was boiled in 1 ml water for 20 min, cooled on ice for 15 min, and centrifuged, and the supernatant fluid was treated and lyophilized as described above. For analyses of small molecules in these extracts, dry residues were dissolved in 700 μl of D2O (99.96%; Cambridge Isotope Laboratories, Andover, MA), and t-butanol (anhydrous; Aldrich, Milwaukee, WI) was added to 0.4 mM to serve as an internal standard (10). The solutions were then subjected to nuclear magnetic resonance (NMR) spectroscopy in a 500-MHz Varian Inova spectrometer. The pulse program used as well as data acquisition, processing, and display were all described previously (10). Levels of glutamic acid, a small molecule present in significant amounts in spores (12, 22), were determined by a comparison of the peak heights of signals from selected protons in this compounds to the peak heights due to known concentrations of glutamic acid added to spore extracts before Chelex chromatography. The peak heights of the selected protons of glutamic acid were linear as a function of the concentration of this amino acid over the concentration range measured in this work.
RESULTS
pH and temperature optima for dodecylamine germination.
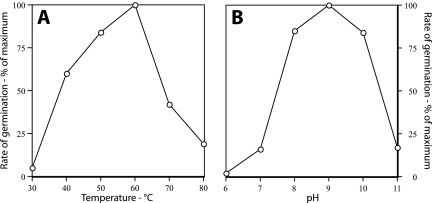
Results of previous studies on the germination of B. subtilis spores with dodecylamine have led to the suggestion of two possible mechanisms for this process (20). In one mechanism, dodecylamine interacts with the spore's inner membrane, and this interaction results in the generation of channels through which Ca-DPA can exit. Alternatively, dodecylamine may interact with a preexisting channel present in the dormant spore's inner membrane but in a closed state, with this interaction causing the channel to open. It is also possible that dodecylamine's interaction with the spore's inner membrane indirectly causes the opening of a Ca-DPA channel in this membrane. One way to distinguish between these alternative mechanisms is to examine the temperature dependence of dodecylamine germination, since the opening of a channel composed of proteins by dodecylamine would likely exhibit a distinct temperature optimum, while a purely physicochemical effect on the spore's inner membrane might not. Strikingly, the germination of wild-type B. subtilis spores with 50 μM dodecylamine exhibited a clear temperature optimum at 60°C (Fig. 1A). Germination with this agent also exhibited a pH optimum of ∼9 with 0.25 mM dodecylamine (Fig. 1B) as well as with 0.1 and 1 mM dodecylamine (data not shown).
FIG. 1.
Temperature (A) and pH (B) optima for spore germination with dodecylamine. (A) Spores of strain PS832 (wild-type) were germinated in a solution containing 10 mM Tris-HCl (pH 7.5) and 50 μM dodecylamine and at various temperatures, and the rates of spore germination were determined from the rate of DPA release as described in Materials and Methods. (B) Spores of B. subtilis strain PS832 (wild type) were germinated at 45°C, as described in Materials and Methods, with 0.25 mM dodecylamine and using 25 mM buffers of 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid adjusted to appropriate pH values with KOH (pH 6 and 7), Tris-HCl (pH 7, 8, and 9), and 2-(cyclohexylamino)ethanesulfonic acid adjusted to appropriate pH values with KOH (pH 9, 10, and 11). Rates of germination were determined by measuring DPA release as described in Materials and Methods. Values at pH 7 and 9 are the averages of values with the two different buffers that differed from each other by less than 20%.
Effect of increased levels of SpoVA proteins on dodecylamine germination.
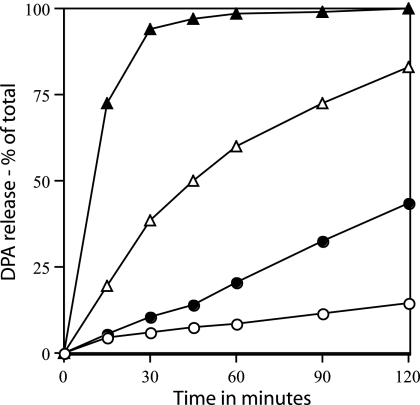
If proteins, particularly SpoVA proteins, are indeed major components of a channel for Ca-DPA in the spore's inner membrane, then increased levels of SpoVA proteins might lead to faster Ca-DPA release in dodecylamine-triggered spore germination if the action of dodecylamine is to open or create a such a channel. In order to test this possibility, we used spores of a strain in which the spoVA operon is under the control of the sspB promoter (32). This strong promoter is turned on only in the developing forespore and at the same time as the spoVA promoter (27), and this results in levels of SpoVAD in spores that are about fourfold higher than those in wild-type spores (32). Since spoVA is an operon and all genes in this operon appear to be translationally coupled (9), it seems most likely that levels of all SpoVA proteins are increased about fourfold in spores of the strain (PS3411) with spoVA under the control of the sspB promoter. Strikingly, the rates of dodecylamine germination of spores with elevated levels of SpoVA proteins were only slightly less than fourfold higher than those of wild-type spores when germination was measured at a temperature of 30, 37, or 45°C (Fig. 2 and data not shown). These results are consistent with the suggestion that SpoVA proteins are an integral part of a Ca-DPA channel, although this is by no means proven (see Discussion).
FIG. 2.
Effect of increased SpoVA levels on the rate of spore germination with dodecylamine. Spores of strain PS832 (wild type) (○ and ▵) or PS3411 (↑SpoVA) (• and ▴) were germinated in 10 mM Tris-HCl (pH 7.5) with 0.33 mM dodecylamine at either 30°C (○ and •) or 45°C (▵ and ▴), and DPA release was measured as described in Materials and Methods.
Effect of a temperature-sensitive spoVA mutation on dodecylamine germination.
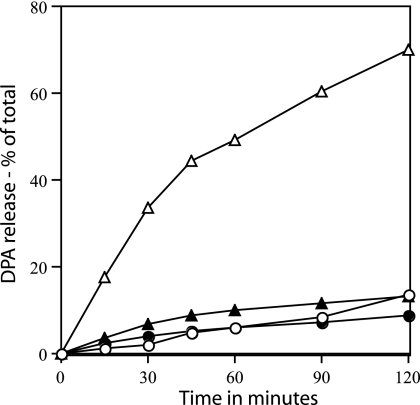
The data described above suggested not only that Ca-DPA was released during dodecylamine germination via a proteinaceous channel but also that this channel may contain SpoVA proteins. To obtain further evidence pertinent to this latter suggestion, we used spores of a strain that contained a spoVA operon with either of two TS mutations (31) and monitored dodecylamine germination at both permissive and nonpermissive temperatures. As expected (20), wild-type spores treated with dodecylamine released DPA ≥10-fold faster at 55°C than they did at 30°C (Fig. 3). However, PS3640 and PS3642 spores (both TS spoVA) germinated at similar rates at both 30°C and 55°C (Fig. 3 and data not shown).
FIG. 3.
Germination of wild-type and TS spoVA spores with dodecylamine. Spores of strains PS832 (wild-type) (○ and ▵) and PS3642 (TS spoVA) (• and ▴) were prepared at 30°C. These spores were germinated with 50 μM dodecylamine in 10 mM Tris-HCl (pH 7.5) at either 30°C (○ and •) or 55°C (▵ and ▴), and DPA release was measured as described in Materials and Methods.
Inhibition of dodecylamine germination by Hg2+.
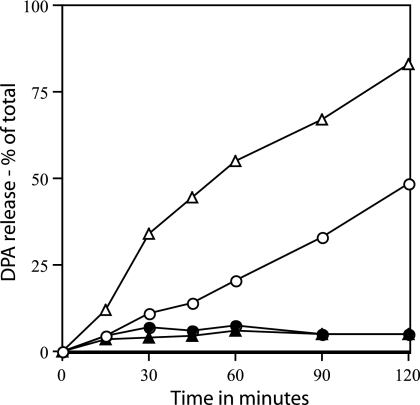
The results described above strongly suggested that SpoVA proteins are involved in DPA release during spore germination triggered by dodecylamine. This is consistent with previous work indicating that proteins are involved in DPA release during spore germination triggered by nutrients or Ca-DPA, since both of the latter processes are inhibited by HgCl2 (5). However, previous work also suggested that proteins may not be involved in this DPA release during dodecylamine germination, since this DPA release appears not to be inhibited by HgCl2 (5). However, in the latter work, dodecylamine was used at 1 mM, and HgCl2 was also used at 1 mM. In order to more rigorously test the ability of HgCl2 to inhibit the Ca-DPA release triggered by dodecylamine germination, HgCl2 was used at 3 mM with 50 μM dodecylamine (Fig. 4). Under these conditions, there was a large inhibition of Ca-DPA release from either wild-type spores or spores with elevated levels of SpoVA proteins.
FIG. 4.
Inhibition of dodecylamine germination by HgCl2. Spores of strains PS832 (wild type) (○ and •) and PS3411 (↑SpoVA) (▵ and ▴) were germinated with 50 μM dodecylamine in 10 mM Tris-HCl (pH 7.5) at 45°C without (○ and ▵) or with (• and ▴) 3 mM HgCl2, and DPA release was measured as described in Materials and Methods.
Release of other small molecules during dodecylamine germination.
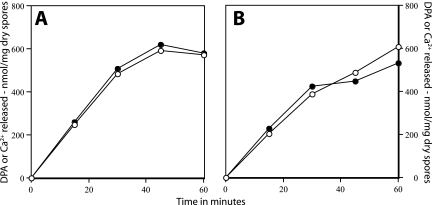
While the data mentioned above were consistent with the involvement of SpoVA proteins in DPA release during spore germination triggered by dodecylamine, an obvious question is whether this release includes both Ca2+ and DPA. Chemical analyses indicated that the amount of Ca2+ released during dodecylamine germination paralleled the amount of DPA released, as Ca2+ and DPA were present in the supernatant fluid from dodecylamine-germinated spores in almost a 1:1 ratio (Fig. 5A and B). Since this result was obtained with both wild-type and FB113 (cwlJ sleB) spores (Fig. 5A and B), this finding also indicates that cortex degradation is not needed for Ca-DPA release triggered by dodecylamine germination, since FB113 spores cannot degrade their cortex (21).
FIG. 5.
Ca2+ and DPA release during spore germination by dodecylamine. Spores of strains PS832 (wild type) (A) and FB113 (cwlJ sleB) (B) were germinated at an OD600 of 2 and at 37°C with 0.5 mM dodecylamine in 10 mM Tris-HCl (pH 7.5). At various times, 10-ml aliquots were centrifuged, the supernatant fluid was lyophilized, the dry residue was dissolved in 1 ml water, and Ca2+ and DPA were measured chemically as described in Materials and Methods. Symbols: ○, DPA; •, Ca2+.
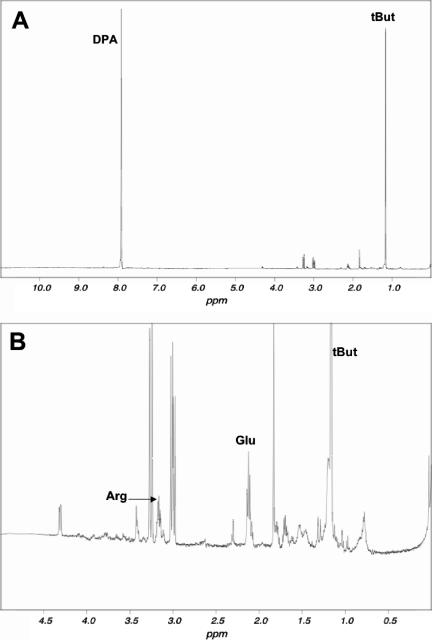
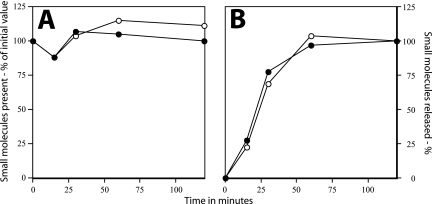
Dormant spores of Bacillus species contain high levels of a number of small molecules in addition to DPA and its associated divalent cations (22). One such molecule is glutamic acid, which is present at 20 to 100 μmol/g (dry weight) in spores of a number of Bacillus species (12, 22). Proton peaks coming from glutamic acid were readily identified in NMR spectra of small molecules extracted from dormant spores of B. subtilis strain FB113 (Fig. 6A and B), and the level of this compound was ∼25 μmol/g dry spores. The level of glutamic acid was similar in spores of B. subtilis PS832 (wild type) (data not shown). A second obvious question is whether glutamic acid is released upon spore germination with dodecylamine. Spores of strain FB113 were again used for the measurement of the release of this small molecule during dodecylamine germination, since these spores lack both cortex lytic enzymes and thus cannot degrade the peptidoglycan cortex during spore germination. This is important for measuring release of small molecules, since if dodecylamine germination proceeds through the cortex lysis that takes place with wild-type spores, molecules as large as adenine nucleotides are released (20). This adenine nucleotide release may be due to the eventual killing of fully germinated wild-type spores by dodecylamine, presumably via a disruption of plasma membrane of these spores (18). In addition, while FB113 spores initiate germination with dodecylamine or nutrient germinants and release Ca-DPA, they do not undergo events early in spore outgrowth such as the degradation of spore protein and amino acid metabolism that might alter spore levels of amino acids (21) (data not shown). Indeed, levels of glutamic acid remained relatively constant during the germination of FB113 spores with dodecylamine (Fig. 7A). The most notable observation made in this experiment was that FB113 spores incubated with dodecylamine also released ≥90% of their glutamic acid in parallel with the release of DPA (Fig. 7B). Similar analyses indicated that the great majority of the pool of free arginine of dormant spores was also released in parallel with DPA during dodecylamine germination (data not shown). In addition, while the identity of most of the compounds giving the additional large peaks flanking the arginine peaks in the NMR spectrum shown in Fig. 6B have not yet been identified, these compounds were also rapidly released during spore germination with dodecylamine (data not shown).
FIG. 6.
NMR spectrum of a dormant spore extract. Spores of strain FB113 (cwlJ sleB) were extracted with boiling water, the supernatant fluid was processed, and its NMR spectrum was determined as described in Materials and Methods and is shown at both low (A) and high (B) resolutions. Peaks labeled as due to protons of arginine, DPA, and glutamic acid as well as the t-butanol (tBut) added as an external standard were identified from control experiments with pure compounds alone or added to extracts prior to Chelex chromatography. The NMR spectrum of a wild-type spore extract (strain PS832) was essentially identical to that of the extract from FB113 spores.
FIG. 7.
Release of small molecules during spore germination by dodecylamine. Spores of strain FB113 (cwlJ sleB) were germinated at 37°C with 0.5 mM dodecylamine in 10 mM KPO4 buffer (pH 7.4), and levels of (A) total DPA and free glutamic acid in the culture and (B) glutamic acid and DPA in the supernatant fluid from spores were measured as described in Materials and Methods. Symbols: ○, glutamic acid; •, DPA.
Role of SpoVA proteins in DPA release during lysozyme germination.
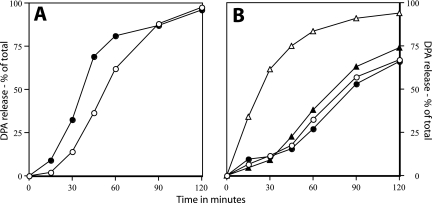
In addition to dodecylamine, spores can also be germinated by the digestion of the cortical peptidoglycan with lysozyme if spores are first decoated to allow the access of exogenous lysozyme to the cortex. The germination of decoated spores by lysozyme does not require either of the spore's cortex lytic enzymes, nor are the spore's nutrient germinant receptors required (21, 23). While DPA is released early in lysozyme germination of decoated spores, the mechanism of this DPA release is not known. We measured DPA release during lysozyme germination of decoated spores of the wild-type strain as well as of decoated spores overexpressing SpoVA proteins or with a TS mutation in spoVA. These experiments were carried out in a hypertonic medium to minimize the rupture of lysozyme-germinated spores due to internal osmotic pressure, as the use of this medium is essential to maintain spore viability during lysozyme germination (17). Decoated spores with elevated levels of SpoVA protein released DPA significantly faster than decoated wild-type spores during lysozyme germination at 30°C (Fig. 8A). In addition, while decoated wild-type spores released DPA more rapidly with lysozyme at 45°C, this was not the case for decoated spores with a TS mutation in spoVA (Fig. 8B). Note that the concentration of lysozyme used at 45°C in the latter experiment was lower than that used at 30°C in order to slow spore germination at the higher temperature to allow an accurate measurement of Ca-DPA release.
FIG. 8.
DPA release from spores of various strains germinated with lysozyme. (A) Decoated spores of strains PS832 (wild type) (○) and PS3411 (↑SpoVA) (•) were germinated with lysozyme (2.5 μg/ml) in hypertonic medium at 30°C, and DPA release was measured as described in Materials and Methods. (B) Decoated spores of strains PS832 (wild type) (○ and ▵) and PS3640 (TS spoVA) (• and ▴) were germinated with lysozyme at 2.5 μg/ml at 30°C (○ and •) or with lysozyme at 0.83 μg/ml at 45°C (▵ and ▴), and DPA release was measured as described in Materials and Methods.
DISCUSSION
A major conclusion from this work is that dodecylamine, and, by inference, all cationic surfactants, triggers spore germination by opening channels in the spore's inner membrane that allow the release of Ca-DPA as well as at least two other small molecules from the spore core. However, there must be a size limitation on the molecules that can pass through this channel, since while Ca-DPA, arginine, and glutamic acid were released upon dodecylamine treatment of spores that cannot degrade their cortex, previous work showed that AMP release is not triggered by dodecylamine treatment of these spores (20). In contrast, wild-type spores treated with dodecylamine do release AMP (20). Presumably, with wild-type spores, the triggering of Ca-DPA release by dodecylamine is followed by cortex lysis, and dodecylamine then disrupts the fully germinated spore's plasma membrane (derived from the dormant spore's inner membrane), leading to the release of molecules as large as AMP. However, with FB113 spores that cannot degrade the spore cortex, the spore's inner membrane remains in the somewhat compressed state characteristic of the dormant spore (8) due to some action of the cortex, and presumably, dodecylamine cannot fully disrupt the structure of this compressed inner membrane.
A second major conclusion from this work is that it is through a proteinaceous channel that Ca-DPA, and presumably arginine and glutamic acid, is released upon treatment with dodecylamine. This is suggested by the fact that Ca-DPA release triggered by dodecylamine germination shows a specific temperature optimum. If the effect of dodecylamine was to increase spore inner membrane permeability by a physicochemical effect, then one might have expected the rate of this process to continue to increase with increasing temperature.
The third notable conclusion from this work is that SpoVA proteins are involved in the release of Ca-DPA and presumably other small molecules during spore germination triggered by either dodecylamine or lysozyme. This has been suggested previously for Ca-DPA release in spore germination triggered by nutrients (9, 30, 31) and is further supported by this work. The correlation between rates of spore germination with either dodecylamine or lysozyme and levels of SpoVA proteins (but see below) as well as the temperature sensitivity of these germination processes with spores that have TS mutations in the spoVA operon are strong evidence that SpoVA proteins are directly involved in the release of Ca-DPA and presumably other small molecules early in spore germination triggered by these agents. The inhibition of DPA release by HgCl2 in dodecylamine germination is also evidence that this process requires proteins. However, we do not know how SpoVA proteins function to allow the release of Ca-DPA and other small molecules in spore germination and the uptake of Ca-DPA in sporulation and, most importantly, how the release of Ca-DPA is regulated in spore germination. It may be that the SpoVA proteins are integral components of the Ca-DPA channel, although it is certainly possible that these proteins act only on the Ca-DPA channel to affect its function.
While rates of DPA release during lysozyme germination were clearly affected by the level or temperature sensitivity of SpoVA proteins, the dependence of DPA release on SpoVA protein levels in particular appeared to be less than that for dodecylamine germination. This may be because there is significant DPA release from lysozyme-germinated spores through a SpoVA-independent pathway, perhaps due to rapid swelling of the spore's inner membrane upon lysozyme treatment (8), since lysozyme digests both the spore's cortex and germ cell wall. In contrast, during spore germination with nutrients or dodecylamine, peptidoglycan degradation is not essential for DPA release, and, even so, endogenous lytic enzymes degrade only the spore cortex.
At first glance, it may seem unusual that dodecylamine treatment of spores unable to degrade their cortex would trigger the release of not only Ca-DPA but also the spore's pools of glutamic acid as well as arginine. While the release of small molecules from spores by dodecylamine treatment might be due to a nonspecific effect of this surfactant on the permeability of the spore's inner membrane, there is significant evidence that the rapid release of small molecules in addition to Ca-DPA also takes place in nutrient germination. Thus, with B. megaterium spores, the rapid release of H+, K+, and Na+ ions precedes the release of Ca-DPA during nutrient germination (28), and the large amount of free amino acids generated by the degradation of dormant spore core protein is also released early in the outgrowth of spores whose germination is triggered by nutrients (26, 29). We have also found that nutrient germination of FB113 spores is accompanied not only by the release of Ca-DPA but also by the release of glutamic acid (our unpublished results). In addition, in preliminary work (our unpublished results), we have found that pressures of 150 or 500 MPa, which trigger spore germination either by activating the spore's nutrient germinant receptors or by triggering Ca-DPA release, respectively (2), also cause the release of glutamic acid in parallel with that of Ca-DPA from spores unable to degrade their cortex. Thus, it is possible that in the early minutes of spore germination, there is a large increase in the permeability of the spore's inner membrane, allowing the release of a number of spore small molecules, including Ca-DPA.
If, as seems likely, dodecylamine triggers spore germination by activating the release of small molecules, particularly Ca-DPA, from the spore core, how does dodecylamine trigger this release? There appear to be two possible mechanisms: either dodecylamine directly opens a channel in the spore's inner membrane or the interaction of dodecylamine with the inner membrane alters this membrane's properties, and this in turn indirectly opens a channel. At present, we cannot decide between these possibilities, although there is evidence that favors both. A direct interaction of a cationic surfactant with a proteinaceous membrane channel is not unprecedented, as there is at least one instance in which a proteinaceous membrane channel is opened, most likely directly, by an interaction with cationic surfactants (11). The increase in the rate of dodecylamine germination as SpoVA levels were increased, the temperature sensitivity of dodecylamine germination in spores with a TS mutant in SpoVA proteins, and the inhibition by Hg2+ of Ca-DPA release triggered by dodecylamine are consistent with dodecylamine acting to trigger the movement of Ca-DPA through a channel composed at least in part of SpoVA proteins. However, it is possible that the rate-limiting step in dodecylamine germination is the Ca-DPA movement itself and that the initial effect of dodecylamine is not on the channel itself but on the spore's inner membrane. It is also known that oxidative damage to dormant spores, most likely to the spore's inner membrane, greatly increases the rate of Ca-DPA release triggered by dodecylamine (4, 6). While the nature of the oxidative damage that leads to this effect is not known, this damage also results in a large increase in the passive permeability of the spore's inner membrane to molecules such as methylamine and decreases the ability of spores to retain Ca-DPA at normally sublethal temperatures (4, 6). This suggests that dodecylamine may well have general effects on the spore's inner membrane. Clearly, more remains to be learned about the mechanism of spore germination triggered by cationic surfactants and the mechanism of the release of Ca-DPA and other small molecules in spore germination.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (GM-19698).
We are grateful to Paul Wahome and Mark W. Maciejewski for assistance in the initial experiments measuring spore small molecules by NMR and to Donna Cortezzo for determining the pH optimum of dodecylamine germination.
Footnotes
Published ahead of print on 8 December 2006.
REFERENCES
- 1.Bagyan, I., M. Noback, S. Bron, M. Paidhungat, and P. Setlow. 1998. Characterization of yhcN, a new forespore-specific gene of Bacillus subtilis. Gene 212:179-188. [DOI] [PubMed] [Google Scholar]
- 2.Black, E. P., K. Koziol-Dube, D. Guan, J. Wei, B. Setlow, D. E. Cortezzo, D. G. Hoover, and P. Setlow. 2005. Factors influencing the germination of Bacillus subtilis spores via the activation of nutrient receptors by high pressure. Appl. Environ. Microbiol. 71:5879-5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabrera-Martinez, R.-M., F. Tovar-Rojo, V. R. Vepachedu, and P. Setlow. 2003. Effects of overexpression of nutrient receptors on germination of spores of Bacillus subtilis. J. Bacteriol. 185:2457-2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortezzo, D. E., K. Koziol-Dube, B. Setlow, and P. Setlow. 2004. Treatment with oxidizing agents damages the inner membrane of spores of Bacillus subtilis and sensitizes the spores to subsequent stress. J. Appl. Microbiol. 97:838-852. [DOI] [PubMed] [Google Scholar]
- 5.Cortezzo, D. E., B. Setlow, and P. Setlow. 2004. Analysis of the action of compounds that inhibit the germination of spores of Bacillus species. J. Appl. Microbiol. 96:725-741. [DOI] [PubMed] [Google Scholar]
- 6.Cortezzo, D. E., and P. Setlow. 2005. Analysis of factors influencing the sensitivity of spores of Bacillus subtilis to DNA damaging chemicals. J. Appl. Microbiol. 98:606-617. [DOI] [PubMed] [Google Scholar]
- 7.Cowan, A. E., D. E. Koppel, B. Setlow, and P. Setlow. 2003. A soluble protein is immobile in dormant spores of Bacillus subtilis but is mobile in germinated spores: implications for spore dormancy. Proc. Natl. Acad. Sci. USA 100:4209-4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowan, A. E., E. M. Olivastro, D. E. Koppel, C. A. Loshon, B. Setlow, and P. Setlow. 2004. 2004. Lipids in the inner membrane of dormant spores of Bacillus species are immobile. Proc. Natl. Acad. Sci. USA 101:7733-7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Errington, J. 1993. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol. Rev. 57:1-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loshon, C. A., P. G. Wahome, M. W. Maciejewski, and P. Setlow. 2006. Levels of glycine betaine in growing cells and spores of Bacillus species and lack of effect of glycine betaine on spore properties. J. Bacteriol. 188:3153-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinac, B., J. Adler, and C. Kung. 2000. Mechanosensitive ion channels of E. coli activated by amphipaths. Nature 348:261-263. [DOI] [PubMed] [Google Scholar]
- 12.Nelson, D. L., and A. Kornberg. 1970. Biochemical studies of bacterial sporulation and germination. XVIII. Free amino acids in spores. J. Biol. Chem. 245:1128-1136. [PubMed] [Google Scholar]
- 13.Nicholson, W. L., N. Munakata, G. Horneck, H. J. Melosh, and P. Setlow. 2000. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 64:548-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination and outgrowth, p. 391-450. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & Sons, Chichester, England.
- 15.Paidhungat, M., B. Setlow, A. Driks, and P. Setlow. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J. Bacteriol. 182:5505-5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paidhungat, M., and P. Setlow. 2002. Spore germination and outgrowth, p. 537-548. In J. A. Hoch, R. Losick, and A. L. Sonenshein (ed.), Bacillus subtilis and its relatives: from genes to cells. American Society for Microbiology, Washington, DC.
- 17.Popham, D. L., J. Helin, C. E. Costello, and P. Setlow. 1996. Muramic lactam in peptidoglycan of Bacillus subtilis spores is required for spore outgrowth but not for spore dehydration or heat resistance. Proc. Natl. Acad. Sci. USA 93:15405-15410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rode, L. J., and J. W. Foster. 1960. Germination of bacterial spores by long chain alkyl amines. Nature 188:1132-1134. [DOI] [PubMed] [Google Scholar]
- 19.Rotman, Y., and M. L. Fields. 1967. A modified reagent for dipicolinic acid analysis. Anal. Biochem. 22:168. [DOI] [PubMed] [Google Scholar]
- 20.Setlow, B., A. E. Cowan, and P. Setlow. 2003. Germination of spores of Bacillus subtilis with dodecylamine. J. Appl. Microbiol. 95:637-648. [DOI] [PubMed] [Google Scholar]
- 21.Setlow, B., E. Melly, and P. Setlow. 2001. Properties of spores of Bacillus subtilis blocked at an intermediate stage of spore germination. J. Bacteriol. 183:4894-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Setlow, P. 1994. Mechanisms which contribute to the long-term survival of spores of Bacillus species. J. Appl. Bacteriol. 76:49S-60S. [DOI] [PubMed] [Google Scholar]
- 23.Setlow, P. 2003. Spore germination. Curr. Opin. Microbiol. 6:550-556. [DOI] [PubMed] [Google Scholar]
- 24.Setlow, P. 2006. Spores of Bacillus subtilis: their resistance to radiation, heat and chemicals. J. Appl. Microbiol. 101:514-525. [DOI] [PubMed] [Google Scholar]
- 25.Setlow, P., and E. A. Johnson. Spores and their significance. In M. P. Doyle and L. R. Beuchat (ed.), Food microbiology: fundamentals and frontiers, 3rd ed., in press. American Society for Microbiology, Washington, DC.
- 26.Setlow, P., and G. Primus. 1975. Protein metabolism during germination of Bacillus megaterium spores. I. Protein synthesis and amino acid metabolism. J. Biol. Chem. 250:623-630. [PubMed] [Google Scholar]
- 27.Sun, D., P. Stragier, and P. Setlow. 1989. Identification of a new σ-factor involved in compartmentalized gene expression during sporulation of Bacillus subtilis. Genes Dev. 3:141-149. [DOI] [PubMed] [Google Scholar]
- 28.Swerdlow, B. M., B. Setlow, and P. Setlow. Levels of H+ and other monovalent cations in dormant and germinated spores of Bacillus megaterium. J. Bacteriol. 148:20-29. [DOI] [PMC free article] [PubMed]
- 29.Tovar-Rojo, F., R.-M. Cabrera-Martinez, B. Setlow, and P. Setlow. 2003. Studies on the mechanism of the osmoresistance of spores of Bacillus subtilis. J. Appl. Microbiol. 95:167-179. [DOI] [PubMed] [Google Scholar]
- 30.Tovar-Rojo, F., M. Chander, B. Setlow, and P. Setlow. 2002. The products of the spoVA operon are involved in dipicolinic acid uptake into developing spores of Bacillus subtilis. J. Bacteriol. 184:584-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vepachedu, V. R., and P. Setlow. 2004. Analysis of the germination of spores of Bacillus subtilis with temperature sensitive spo mutations in the spoVA operon. FEMS Microbiol. Lett. 239:71-77. [DOI] [PubMed] [Google Scholar]
- 32.Vepachedu, V. R., and P. Setlow. 2005. Localization of SpoVAD to the inner membrane of spores of Bacillus subtilis. J. Bacteriol. 187:5677-5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yingst, D. R., and J. F. Hoffman. 1983. Intracellular free Ca and Mg of human red blood cell ghosts measured with entrapped arsenazo III. Anal. Biochem. 132:431-448. [DOI] [PubMed] [Google Scholar]