Abstract
Bacteria swim by rotating long thin helical filaments, each driven at its base by a reversible rotary motor. When the motors of peritrichous cells turn counterclockwise (CCW), their filaments form bundles that drive the cells forward. We imaged fluorescently labeled cells of Escherichia coli with a high-speed charge-coupled-device camera (500 frames/s) and measured swimming speeds, rotation rates of cell bodies, and rotation rates of flagellar bundles. Using cells stuck to glass, we studied individual filaments, stopping their rotation by exposing the cells to high-intensity light. From these measurements we calculated approximate values for bundle torque and thrust and body torque and drag, and we estimated the filament stiffness. For both immobilized and swimming cells, the motor torque, as estimated using resistive force theory, was significantly lower than the motor torque reported previously. Also, a bundle of several flagella produced little more torque than a single flagellum produced. Motors driving individual filaments frequently changed directions of rotation. Usually, but not always, this led to a change in the handedness of the filament, which went through a sequence of polymorphic transformations, from normal to semicoiled to curly 1 and then, when the motor again spun CCW, back to normal. Motor reversals were necessary, although not always sufficient, to cause changes in filament chirality. Polymorphic transformations among helices having the same handedness occurred without changes in the sign of the applied torque.
The peritrichous bacterium Escherichia coli executes a random walk: an alternating sequence of runs (relatively long intervals during which the cell swims smoothly) and tumbles (relatively short intervals during which the cell changes course) (8). A cell is propelled by several helical flagellar filaments, each attached by a hook (a universal joint) to a reversible rotary motor (7). During runs, the filaments coalesce into a bundle that pushes the cell forward (24). When viewed from behind the cell, the bundle rotates counterclockwise (CCW), and, to balance the torque, the cell body rotates clockwise (CW). Tumbles are initiated by CW motor rotation (21). Based on studies of Salmonella using dark-field microscopy, it was thought that the motors change direction synchronously, causing the bundle to fly apart (24, 25). Based on studies using fluorescence microscopy, it became apparent that different filaments can change directions at different times and that a tumble can result from a change in direction of as few as one filament (30). During a tumble, the reversed filament comes out of the bundle and transforms from normal (a left-handed helix with a pitch of 2.3 μm and a diameter of 0.4 μm) to semicoiled (a right-handed helix with half the normal pitch but normal amplitude) and then to curly 1 (a right-handed helix with half the normal pitch and half the normal amplitude). The change in direction of the cell's track generated by the tumble occurs during the transformation from normal to semicoiled, so at the beginning of the subsequent run, the cell swims for a time with left-handed filaments in a bundle turning CCW and a right-handed filament outside the bundle turning CW, both pushing the cell body forward. When the reversed motor switches back to CCW rotation, the single filament regains its normal conformation and rejoins the bundle. However, more exotic things can happen; for example, several filaments can undergo polymorphic transformations, and bundles can go directly from normal to curly 1 or from normal to a mixture of normal and semicoiled or curly 1 (30). For recent reviews of bacterial motility and chemotaxis, see references 4 and 31, and for recent reviews of the flagellar rotary motor, see references 1, 6, and 11.
A limitation in our previous study of swimming behavior (30) was the fact that images were recorded at 60 Hz, a rate lower than the rate of filament rotation, so rotation frequencies could not be measured and directions of rotation were inferred from filament shape and cell motion. Here, to better understand swimming in a dilute aqueous buffer or in a buffer containing methylcellulose, we recorded the motion of fluorescently labeled cells at 500 Hz. Methylcellulose was used because it was included in early tracking experiments (8) as a viscous agent to suppress Brownian motion and make cells easier to follow; however, it did not alter the run-tumble statistics (our unpublished data). Using frame-by-frame analysis, we measured the swimming speed, the rate of rotation of the cell body, and the rate of rotation of the flagellar bundle. We also measured the rate of rotation of single filaments on cells stuck to glass and in buffer. We compared the shapes of normal filaments when they were spinning to their shapes when they were stalled. We estimated values for motor torque and for filament stiffness.
MATERIALS AND METHODS
Labeling cells.
E. coli strain AW405 (3) was grown as described previously (30). All subsequent steps were carried out at room temperature (23°C). Bacteria were washed twice by centrifugation (2,000 × g, 10 min) and gentle resuspension with motility buffer (MB) (0.01 M potassium phosphate, 0.067 M NaCl, 10−4 M EDTA; pH 7.0) and once with MB at pH 7.5. In the final preparation (0.5 ml), the bacteria were concentrated 20-fold to 0.5 ml. One package of Cy3 monofunctional succinimidyl ester (PA23001; Amersham Pharmacia Biotech, Newark, NJ) and 25 μl of 1.0 M sodium bicarbonate were added to the bacterial suspension. Labeling was performed for 90 min with stirring by gyration at 100 rpm. Excess dye was removed by washing the bacteria with MB+ (motility buffer containing 0.002% Tween 20 [Sigma-Aldrich, St. Louis, MO] and 0.5% glucose). Tween was added to prevent labeled cells from sticking to glass but was omitted in experiments in which cells were stuck to glass. In some experiments, MB+ was supplemented with 0.18% hydroxypropylmethylcellulose (3,500 to 5,600 cP; H7509 lot 90K0802; Sigma-Aldrich, St. Louis, MO). Bulk viscosities (0.93 and 3.07 cP for MB+ and MB+ with 0.18% methylcellulose, respectively) were determined at 23°C with a Cannon-Ubbeholde viscometer, as described previously (9).
Preparing slides.
The suspension of labeled bacteria was diluted between 25- and 50-fold with MB+. About 50 μl of labeled bacteria was sealed within a thin ring of Apiezon M grease (Fisher Scientific, Pittsburgh, PA) between a coverslip (22 by 44 mm) and a microscope slide. The coverslip was seated carefully to eliminate air bubbles and then squeezed to form a chamber about 50 μm thick. Samples were used immediately and for up to about 1 h. We have no evidence that the preparations became anaerobic, but glucose was added to allow the cells to swim without oxygen. In any event, the cells remained vigorously motile for an hour or more, and their swimming speeds were similar to those observed elsewhere (e.g., by tracking [23]).
Acquiring images.
Bacteria were observed at room temperature (23°C) with a Nikon Diaphot 200 epifluorescence microscope using a phase-contrast objective (Nikon PlanApo 60/1.4 oil DM) and a 4× or 5× camera relay lens. Images were acquired with a high-speed (500-Hz) black and white charge-coupled-device camera modified for low-light conditions (HSC 500x2; J C Labs, La Honda, CA). Illumination was provided by an argon ion laser (Stabilite 2017; Spectra-Physics, Mountain View, CA) run at 514 nm, using a fluorescence cube with a D514/10x excitation filter, a 527 DCLP dichroic mirror, and an E535LP emission filter (C7408; Chroma Technologies, Brattleboro, VT). The vertical sync pulse from the camera was used to synchronize rotation of a slotted wheel that generated ∼0.2-ms exposures (one exposure per frame). The microscope was configured in the standard epifluorescence mode, with the illumination restricted to a circle about 40 μm in diameter matching the camera's field of view. The laser power at the back focal plane of the objective was 100 to 300 mW. Cells were faintly illuminated in phase contrast, using a tungsten filament light source, making it possible to visualize cell bodies and to focus prior to laser illumination. Images were captured at a rate of 500 frames/s directly to a personal computer equipped with an I-60 analog video capture board and IDEA software (both obtained from Foresight Imaging, Lowell, MA). Images were acquired for 1 s. After a few initial frames of phase-contrast illumination, the laser was switched on, guaranteeing that the start of high-intensity exposure was known. This procedure was used to minimize laser damage to cells during image acquisition, since intense light, especially at short wavelengths, is known to interfere with motor function (32). In order to image stationary filaments on stuck bacteria, cells were exposed to continuous laser illumination; filaments stopped rotating within a few seconds.
Analyzing images.
Using ImageJ (http://rsb.info.nih.gov/ij/), AVI files were converted to TIF stacks, and the motion of a cell was followed over a convenient number of frames. If the cell body had a distinctive mark or pattern of flagellation, its rotation rate was determined by counting the number of video frames for one revolution of that reference point. Filament rotation rates for bundles of swimming bacteria or single filaments of stuck bacteria were determined either by counting the number of frames required for the distal tip to complete one revolution or by following an individual wavecrest until it propagated one pitch length (see Fig. 1, 4, and 5). All measurements of rotation rates were obtained within the few first video frames of laser illumination. Distances were calibrated from recorded images of an objective micrometer (Fischer Scientific, Pittsburgh, PA). Images of single flagellar filaments were fitted to helical curves using custom code written in MATLAB (The MathWorks, Natick, MA). The shape analysis involved fitting a recorded image to a helical curve defined by eight parameters, including three physical parameters (helix pitch [p], diameter [d], and contour length [L]), three rotation parameters (α, β, and γ), and two displacement parameters (Δx and Δy). We chose images containing flagella that were practically coplanar with the image plane, so the tilt out of that plane (β) could be ignored. The proximal end of the filament was often indistinguishable from the bright cell body, so we fixed the contour length by eye before fitting. Together, these factors reduced the total number of parameters from eight to six. Starting from a canonical form (a helix with a pitch of 2.3 μm and a diameter of 0.50 μm aligned with the x axis), we allowed sequential rotations α and γ around the x and z axes, followed by translation (Δx and Δy) to bring the curve into approximate register with the recorded image. Since the microscope viewed “from above” (along the z axis), we actually observed the projection of the rotated, translated helix in the xy plane. The rotation (α) changed the helix's phase, and γ rotated the helix within the plane of view. We represented the helical curve by 100 equally spaced points and performed the rotations and translation numerically. Conceptually, the best fit is the curve that passes through the most, brightest pixels of an image. We linearly interpolated between the measured pixel values to estimate the picture brightness at each of the 100 points along the curve and maximized the sum of the 100 values, which represented the total brightness “captured” by the curve. The maximization was carried out by using a MATLAB routine, starting with the initial approximate fit, sequentially freeing each parameter, and refitting. Values for L were calculated from the axial length (z) of the flagellum (measured by hand) and the fitted pitch and diameter according to the formula 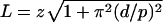 .
.
FIG. 1.
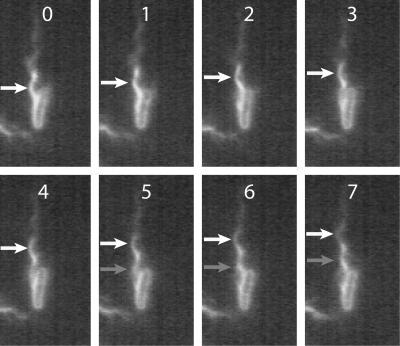
Consecutive images (500 video frames/s) of a cell swimming toward the bottom of the field, propelled by a normal flagellar bundle. The position of an individual helical wavecrest is indicated by white arrows. As the wave propagates away from the cell body, a second crest (gray arrow in frame 5) appears at the original position of the first crest, identifying a complete CCW revolution of the filament. Frame numbers can be converted to elapsed time by multiplying by 0.002 s. Details of this motion are seen more clearly in the movie file “500 Hz swimming.avi” in the supplemental material.
FIG. 4.
Consecutive images (500 video frames/s) of a stuck cell spinning a single flagellar filament. The position of an individual helical wavecrest is indicated by white arrows. As the wave propagates away from the cell body, a second crest (gray arrow in frame 3) appears at the original position of the first crest, identifying a complete CCW revolution of the filament. Frames 4 and 5 are identical; the filament has stopped rotating. The white arrows in frames 6 to 11 indicate the retrograde motion of a helical wavecrest toward the cell body. As the wave propagates toward the cell body, a second crest (gray arrow in frame 11) appears at the original position of the first crest, identifying a complete CW revolution of the filament. Details of this motion are seen more clearly in the movie file “500 Hz reversal 1.avi” in the supplemental material.
FIG. 5.
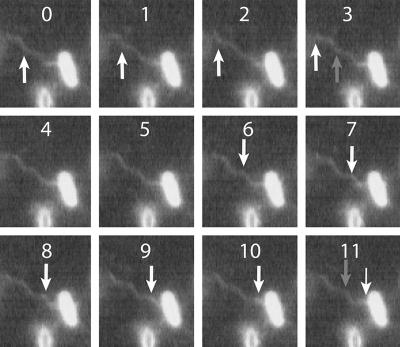
Consecutive images (500 video frames/s) of a stuck cell spinning a single flagellar filament. The position of an individual helical wavecrest is indicated by white arrows as the wave propagates away from the cell body (frames 0 to 4). In frames 5 to 9 filament rotation stops. In frames 10 to 14, the distal end of the filament remains stopped, while a short-pitch region of the transformed filament, indicated by a gray arrow, appears in frame 14. The proximal region is now inclined toward the left of the cell's longitudinal axis (compare frames 1 and 14). Details of this motion are seen more clearly in the movie file “500 Hz reversal 2.avi” in the supplemental material.
RESULTS
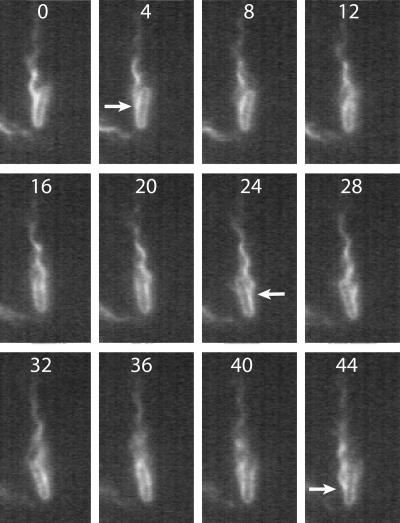
Rotation rates and swimming speeds for a sample of 50 to 100 cells, swimming in MB+ and MB+ with 0.18% methylcellulose, are shown in Table 1. Figure 1 shows a typical swimming cell to illustrate our measurement technique. CCW rotation of a normal left-handed bundle appeared as a wave propagating away from the cell body. The wave moved one wavelength between frames 0 and 5 (a time span of 0.01 s), indicating that the bundle rotation rate was ∼100 Hz. Figure 2 shows every fourth frame for the same cell; the cell body completed one revolution between frames 4 and 44 (a time span of 0.08 s), indicating that the body rotation rate was ∼12.5 Hz. In frame 4, the cell body angled toward the lower left corner of the frame and the bundle appeared to its left; in frame 24, the cell body angled toward the lower right corner of the frame and the bundle appeared to its right; in frame 44, the orientations were the same as those in frame 4. The flagellar bundle and the cell body must turn in opposite directions, since bundle and body torques balance (5), so the flagellar motors were spinning at ∼112.5 Hz, the sum of the bundle and body rates. This cell swam at a speed of ∼25 μm/s.
TABLE 1.
Data for cells with normal bundles swimming in MB+ or in MB+ with 0.18% methylcellulosea
| Medium | Body rotation rate (Hz) | Bundle rotation rate (Hz) | Motor rotation rate (Hz) | Cell speed (μm/s) |
|---|---|---|---|---|
| MB+ | 24 ± 12 (53) | 130 ± 40 (73) | 163 ± 43 (53) | 25 ± 8 (73) |
| MB+ with methylcellulose | 23 ± 11 (58) | 72 ± 28 (94) | 92 ± 31 (58) | 33 ± 11 (94) |
The values are means ± standard deviations. The numbers in parentheses are numbers of cells.
FIG. 2.
Every fourth image for the cell shown in Fig. 1. The arrow in frame 4 indicates where a filament arises from the bacterium's surface and joins the bundle. After one-half revolution of the cell body, the bundle appears on the opposite side of the cell (frame 24); after one full revolution, it reappears on the original side of the cell (frame 44). Details of this motion are seen more clearly in the movie file “500 Hz swimming.avi” in the supplemental material.
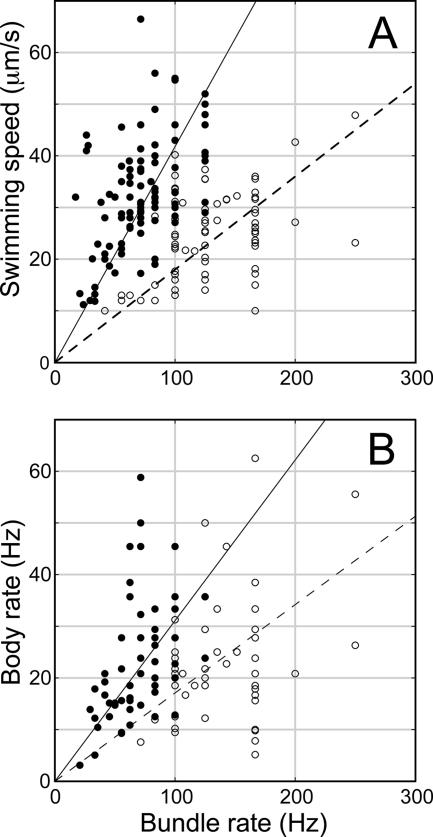
In Fig. 3A, the swimming speeds shown in Table 1 are plotted as a function of bundle rotation rates for cells in MB+ and MB+ with 0.18% methylcellulose. In both media the relationship was approximately linear, with an average speed-to-rate ratio, called the v-f ratio by Magariyama et al. (27), of 0.180 μm in MB+ and 0.418 μm in methylcellulose. This indicates that bacteria translated about 8% and 18% of the flagellar pitch, respectively, per revolution of the flagellar bundle. That is, the cells moved 8% or 18% as fast as they would have moved if the flagella had bored through the medium without slip, i.e., like a corkscrew through a cork. For some bacteria, we also determined the counterrotation rate of the cell body, which is plotted as a function of the bundle rotation rate in Fig. 3B. Again, the relationships were approximately linear; the cell bodies rotated 0.171 and 0.311 times as fast as the flagellar bundles in MB+ and in MB+ with methylcellulose, respectively.
FIG. 3.
Swimming speed (A) and body rotation rate (B) as a function of the bundle rotation rate in MB+ (○) or MB+ with 0.18% methylcellulose (•). The slopes of the linear regression lines are as follows: 0.180 μm for the dashed line and 0.418 μm for the solid line in panel A; and 0.171 for the dashed line and 0.311 for the solid line in panel B.
For a subset of the cells in Table 1, we generated a more complete data set that also included body length, bundle length, and body wobble angle (Table 2). We examined the extended data set for correlations between dynamic parameters (cell and bundle rotation rates, swimming speed, and body wobble) and also between dynamic parameters and cell geometry (bundle length, cell width, and cell length). One might expect that bundle length would correlate with either swimming speed or the bundle rotation rate, but we found no such relationship. Other than the dependence on the rotation rate (Fig. 3), the only additional important factor affecting swimming speed was the body wobble angle, which was anticorrelated with speed for cells swimming both in buffer and, less significantly, in methylcellulose. Only the bundle and motor rotation rates and, to a lesser extent, body wobble were affected by the addition of methylcellulose. The correlations between rotation rates and swimming speeds were significantly stronger for cells in methylcellulose than for cells in buffer.
TABLE 2.
Data for cells with normal bundles swimming in MB+ or in MB+ with 0.18% methylcellulosea
| Medium | Body length (μm) | Body width (μm) | Body wobble angle (°)b | Body rotation rate (Hz) | Bundle length (μm)c | Bundle rotation rate (Hz) | Motor rotation rate (Hz)d | Cell speed (μm/s) |
|---|---|---|---|---|---|---|---|---|
| MB+ | 2.5 ± 0.6 | 0.88 ± 0.09 | 46 ± 24 | 23 ± 8 | 8.3 ± 2.0 | 131 ± 31 | 154 ± 30 | 29 ± 6 |
| MB+ with methylcellulose | 2.0 ± 0.4 | 0.86 ± 0.08 | 36 ± 17 | 21 ± 11 | 10.0 ± 1.5 | 67 ± 24 | 87 ± 31 | 31 ± 10 |
The values are the means ± standard deviations for 32 cells in each medium.
The angle swept out by the axis of the cell body as it rolls about the bundle axis.
The distance between the back end of the cell body and the distal end of the bundle.
Since the cell body and bundle rotate in opposite directions, the motor rotation rate is the sum of the body and bundle rotation rates.
Figure 4 shows 12 consecutive frames from a movie of a normal filament rotating in isolation on a stuck cell. In frames 0 through 3, the filament completed one CCW revolution, indicating that the rate was ∼167 Hz. The filament stopped between frames 4 and 5 and then rotated in the opposite direction, completing one CW revolution between frames 6 and 11 (∼100 Hz). We presumed that between frames 4 and 5 the motor changed direction and the hook unwound and then rewound in the opposite sense. This is an example of a filament that remained left-handed while being spun CW. Such events occurred infrequently, about once in 100 reversals. Although we observed several instances of CW-rotating filaments, in most cases the filament moved out of the focal plane, making its rotation rate difficult to measure.
Under our buffer conditions, the normal, left-handed form is the only stable filament geometry at rest. To cause the filament to change to another form, in particular to a right-handed form, force must be applied to it. Based on consideration of the signs of the torque involved, only CW rotation of a left-handed filament would “untwist” it toward the right-handed forms. Thus, motor reversal is required (although not sufficient, as shown in Fig. 4) to cause any polymorphic transformation of the normal form. Under our conditions, the right-handed forms are not stable at rest; they can be maintained only by the application of torque from CW rotation of the motor. We have never seen a right-handed, CW-rotating filament spontaneously revert to the normal form, although we presume that this would occur, even without a motor reversal, if the applied torque dropped significantly below the normal, fully energized level. When the torque changes sign, as it does upon motor reversal, the filament always goes back to normal. Motor reversal is required (and is sufficient) to cause helicity-changing polymorphic transformation of the right-handed forms. Certain mutations in the hook-associated protein at the base of the filament can upset this balance. For instance, in sag mutants (mutants unable to swim in 0.28% agar but otherwise normal), CCW rotation can drive a normal filament to the left-handed straight form and CW rotation can drive a curly 1 filament to the right-handed straight form (18).
Every reversal observed included a pause of at least one video frame between sequences of rotation; we have never seen an entire filament rotating CCW in one frame and CW in the next frame. It is possible for the distal end of a filament to stop rotating while a polymorphic transformation occurs in its proximal end, as shown in Fig. 5. Initially, such a filament rotated CCW at about 125 Hz, completing one revolution between frames 0 and 4, as indicated by the progression of the arrow toward the distal tip of the filament. In frames 5 through 9 the rotation appears to stop, and the most proximal portion of the filament changes its inclination with respect to the cell body, moving slightly to the left. In frames 10 through 14, the distal end of the filament remains stopped, while a short-pitch region of transformed filament appears (indicated by an arrow in frame 14); compare the proximal filament positions in frames 1 and 14. All helices with shorter-than-normal pitch and a small radius are right-handed (13); therefore, the change in helicity that we observed must have been caused by a period of CW rotation of the motor. The total length of this pause (eight frames, or 0.016 s) is consistent with the winding up of the flagellar hook and the accumulation of added twist in the transformed segment. In subsequent frames the filament resumed CCW rotation (not shown).
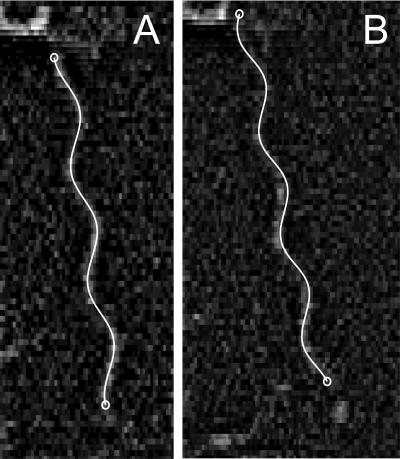
Table 3 shows the results of measurement of 24 normal filaments rotating in isolation on cells that were stuck to a glass surface. As shown by these data and the fits illustrated in Fig. 6, the shapes of spinning and stopped filaments were indistinguishable.
TABLE 3.
Helical parameters for normal filaments on stuck bacteriaa
| Movement | Pitch (μm) | Diam (μm) | Contour length (μm) | Rotation rate (Hz) |
|---|---|---|---|---|
| CCW | 2.22 ± 0.20 | 0.39 ± 0.05 | 7.1 ± 1.8 | 111 ± 20 |
| Stopped | 2.28 ± 0.15 | 0.42 ± 0.05 | 7.1 ± 1.7 | 0 |
The values are the means ± standard deviations for 24 filaments.
FIG. 6.
Typical single-frame images overlaid with a projection of the best-fit helical form. The same flagellar filament is shown in the two panels; it is stopped in panel A and moving in panel B. Since the length of the flagellum was not relevant for our purposes, we sometimes fit to slightly less than the full-length filament, as in panel A. For scale, the pitch is 2.3 μm.
DISCUSSION
Following Magariyama et al. (27), we applied resistive force theory (20) to the single-filament data in Table 3, with a swimming speed (v) of 0. We used a filament angular velocity (ω) of 2π × 111 Hz, a helix radius (r) of 0.2 μm, a helix pitch (P) of 2.22 μm, a filament radius (ρ) of 0.012 μm, and a filament contour length (L) of 7.1 μm, obtaining a filament torque of 370 ± 100 pN nm. Motors run at nearly constant torque up to frequencies of about 175 Hz (15), so it is puzzling that this value is >10-fold less than the stall torque for the flagellar motor measured with optical tweezers (10), ∼4,600 pN nm. This discrepancy led us to examine more recent estimates for motor torque obtained by spinning latex beads on flagellar stubs. Working within the low-speed, high-torque limit with spheres whose diameters ranged from 1.0 to 2.1 μm, Fahrner et al. (19) obtained rotation speeds ranging from 78 to 8.6 Hz. These measurements yielded a mean torque of 1,370 ± 50 pN nm, in agreement with the value of 1,260 pN nm obtained recently using rotating 1-μm beads (28), which we believe to be closer to the mark; however, this value is still substantially larger than 370 pN nm. Thus, either the resistive force theory predicts a torque that is too low, or a substantial burden is imposed by rotation of the filament near a glass surface. According to resistive force theory, the drag coefficient of an isolated, translating helix is inversely proportional to ln(2p/ρ) − 0.5 (27). When the helix is placed close to a surface, hydrodynamic shielding by the surface changes this expression to ln(2l/ρ), where l is the distance to the surface (22). A 4-fold or 12-fold increase in the drag coefficient, which would bring the single-filament torques into agreement with the previously described torque (1,370 pN nm or 4,600 pN nm), corresponds to a proximity of 0.02 μm or 0.01 μm. These distances are rather small (approximately 1/10 the radius of the helix), but not impossibly so.
The filament is sufficiently stiff that we were not able to detect differences in the shape of a normal filament when it was spinning or stopped, as shown in Table 3 and Fig. 6. Based on a simple elastic model of the filament (16), the axial force (F) and torque (Γ) required to deform a filament with natural, unstressed pitch (p0) and radius (r0) to a new pitch (p) and radius (r) are
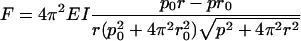 |
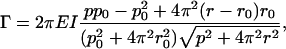 |
where EI is the flagellar stiffness. Since the forces are generated or dissipated uniformly along the length of the rotating filament, F, on average, is half of the thrust generated by the filament, and Γ, on average, is half of the torque applied by the motor. If we take the natural pitch and radius from the data for the stopped filaments (Table 3) and account for uncertainties by allowing a range of axial forces and torques (0.25 pN < F < 0.85 pN and −1,500 pN nm < Γ < −300 pN nm) and a three-standard-deviation range for the CCW form parameters (2.19 μm < p < 2.37 μm and 0.17 μm < r < 0.23 μm), a self-consistent set of numbers requires that the flagellar stiffness be greater than 5.5 pN μm2. This is reasonably consistent with the measured stiffness, 3.5 pN μm2 (16).
The hook is known to be more flexible than the filament; in fact, it changes its twist by about one full turn during a motor reversal (12). The transformation from normal to semicoiled involves supertwisting by about 3 rad/μm or about 1.25 turns per pitch (13); at a motor speed between 300 and 100 Hz, transformation of a single pitch would require between 0.008 and 0.022 s. Thus, the first few rotations of the motor can be absorbed by the hook plus a polymorphic change of the proximal end of the filament, without requiring the distal end to rotate much at all, consistent with Fig. 5. If the CW interval is short enough, when the motor again turns CCW, the polymorphed sections simply propagate back down the filament and are reabsorbed into the hook. In a swimming bacterium such a brief motor reversal would not interfere with rotation of the bundle or alter the cell's trajectory and would probably be undetectable with current microscopic techniques.
Why is the single-filament rotation rate (111 Hz) (Table 3) so similar to the bundle rotation rate (130 Hz) (Table 1)? In our previous study of fluorescent flagellar filaments (30), cells of the same strain grown in the same way produced an average of 3.4 filaments per cell. This is consistent with our observations of these swimming cells, where we could usually distinguish at least three separate filaments in a bundle. At a mean motor rate of 166 Hz (Table 1), all these flagella should be running in a constant-torque regimen (15). Consider four filaments forming a compact bundle. If interactions between these filaments can be ignored, the hydrodynamic properties of the bundle should be similar to those of a single filament with roughly twice the diameter. The viscous load depends only logarithmically on this diameter, so it should be roughly 15% larger. Additionally, the bundle speed is about 15% higher than the single-filament speed, so the total torque supplied by all four motors driving the bundle is only 30% higher than the single-motor torque; i.e., each motor operates at about 32% of the single-motor torque. For a fully assembled motor operating in a fully energized cell, one would not expect to see such a dramatic torque reduction unless the motor were operating at around 300 Hz, well above the “knee” frequency (15). We believe that the motors in a swimming cell do, in fact, deliver close to peak torque but that the effective drag of the bundle is much larger than the calculation described above suggests. Either the bundle has an effective hydrodynamic radius that is 30 times larger than the single-filament radius (much looser than has been imagined [24]), or the filaments in multiply flagellated cells generate substantial internal drag. Even if the filaments were in very close contact (average separation of one filament radius, 12 nm), they would dissipate little extra power (7), but such dissipation could be accomplished by flagella dragging over the surface of the cell. A cell with a single filament can always orient itself so that the flagellum rotates clear of the body, but any additional filaments, which usually arise from points far from the axis of rotation, generally have to cross the cell body during rotation. Unlike the drag between two thin filaments, the drag against a large surface can be substantial, so added torque contributed by additional flagella might be dissipated against the cell body.
If they do not allow the cell to swim faster, why does a cell have multiple flagella? One possible explanation is that having “extra” flagella allows cells to maintain motility while dividing quickly. There is a lag of several generations between turning on flagellar synthesis and completing the first new flagellum (2). If cells did not have a reservoir of flagella when they start a growth spurt (e.g., when they encounter a newly rich medium), cell division during this lag period would produce many unflagellated, nonmotile cells. Another possibility, assuming that a cell with a single flagellum swims poorly unless that flagellum is at a cell pole, is that inserting several flagella at random points on the cell surface is easier than building a specific motor mount at one pole. Yet another possibility is that having multiple, distributed flagella allows cells to change directions more efficiently when they tumble, i.e., to try a new direction at random (8) rather than just back up (29), which searches some but not all (17) environments more efficiently.
We believe that the last factor, namely, the connection between the presence of multiple, distributed flagella and searching efficiency, is an essential component of bacterial taxis, so we hope to understand the tumbling process in E. coli in detail. Since the flagellar bundle has the largest hydrodynamic size, its orientation determines the direction of cell motion. Any motor reversal (CCW to CW) results in deflection of the cell from this trajectory, unless the motor happens to be located in line with the bundle axis. In a previous study (30), we found that normal-to-semicoiled transformation of a filament resulted in deflection of the cell body during tumbles (4). Using the high-speed camera, we were able to confirm these events. A motor reversal (CCW to CW) causes the filament to pause and then change its direction of rotation. This deflects the cell body and unwinds the filament from the bundle. The small initial deflection of the cell body is reversed as the filament transforms to the right-handed semicoiled form, changing the thrust that the filament exerts on the cell body. The tumble usually ends with the conversion of the semicoiled form to the curly 1 form, followed later by a motor reversal (CW to CCW), causing the filament to transform back to its normal form and rejoin the bundle, as shown in Fig. 7. Although this is our best reconstruction of the canonical tumble, other endings are possible. For example, if the second motor reversal (CW to CCW) occurs while the filament is still in the semicoiled form, the filament transforms directly from semicoiled back to normal, skipping the curly form entirely.
FIG. 7.
Idealized sequence of events in a tumble caused by the reversal of a single motor. The upper timeline indicates the direction of motor rotation of the filament causing the tumble, and the lower timeline indicates the behavior as judged by motion of the cell body. From left to right: 1, a bacterium swimming along its original trajectory with all left-handed normal filaments; 2, a motor reversal (CCW to CW) causing the filament to start unbundling and the cell body to deflect slightly; 3, initiation of the transformation of the filament from the left-handed normal form to the right-handed semicoiled form and the beginning of a large deflection of the cell body opposite the previous small deflection; 4, complete transformation of the filament to the semicoiled form and reorientation of the cell along a new trajectory; 5, movement of the cell along the new trajectory, propelled by a normal bundle turning CCW and a semicoiled filament turning CW which has partially transformed to the right-handed curly 1 form; 6, complete conversion of the filament to the curly 1 form, which is flexible enough to twist loosely around the bundle; 7, the motor reversing again (CW to CCW), causing the curly 1 form to revert to normal; and 8, after the filament has rejoined the bundle.
We applied resistive force theory (20, 27) to the data obtained with free-swimming cells and found that the torque required to spin the filaments is roughly the same as the torque required to spin the cell body. Assuming the same helix radius and pitch as before (0.2 μm and 2.22 μm), but treating the bundle as a single a filament having twice the radius (0.024 μm), for the 32 cells in Table 2 we obtained a bundle torque (Γbundle) of 650 ± 220 pN nm, a bundle thrust (Fbundle) of 0.41 ± 0.23 pN, a body torque (Γbody) of 840 ± 360 pN nm, and a body drag (Fbody) of 0.32 ± 0.08 pN. Chattopadhyay et al. (14) used an optical trap to measure the propulsion matrix, which connected bundle torque and bundle thrust to swimming speed and bundle angular velocity, as Γbundle = −Bv + Dω and Fbundle = −Av + Bω. Using the values of Chattopadhyay et al. for A, B, and D with our measured swimming speed and bundle rate gives a Γbundle value of 550 pN nm and an Fbundle value of 0.28 pN, in agreement with our values for these parameters. In our calculations, the body was assumed to be a prolate ellipsoid with the length and width shown in Table 2, rotating about the bundle axis at angular velocity Ω at distance m from the body center along the cell major axis, with the axes forming an angle (θ) equal to half the wobble angle, as shown in Fig. 8. The expression for the viscous drag of the cell body averaged about the bundle axis, adapted from a solution kindly provided by Tobias Löcsei and John Rallison of Cambridge University, yields a force resisting the translation of magnitude Fbody = v(A1sin2θ + A2cos2θ) and a torque resisting the rotation of magnitude Γbody = Ω[(D1 + m2A1) sin2θ + D2cos2θ]. With viscosity η, eccentricity e [e = (a2 − b2)1/2/a], and E = ln[(1 + e)/(1 − e)], the values of the coefficients are:
FIG. 8.
Cell body in the shape of a prolate ellipsoid having length 2a and width 2b swimming at velocity v along the bundle axis, with the center of its body at distance m from, and at angle θ with respect to, the bundle axis, and rolling about that axis at angular velocity Ω. θ is half the body wobble.
A1 = 32πηae3/[(3e2 − 1)E + 2e]
A2 = 16πηae3/[(1 + e2)E − 2e]
D1 = 32πηab2e3(2 − e2)/3(1 − e2)[(1 + e2)E − 2e]
D2 = 32πηab2e3/3[2e − (1 − e2)E]
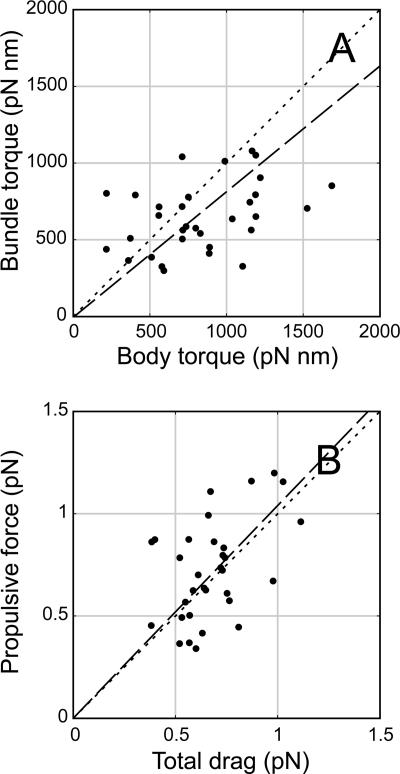
For each cell, we obtained two independent measurements of torque and force; one measurement was based on resistive force theory applied to the flagellar bundle, and the other measurement was based on the motion of the cell body. Although, as indicated above, the population averages agree quite well, there is considerable scatter on a cell-by-cell basis. Individual cells' body and bundle torques appear to be uncorrelated (Fig. 9A), perhaps due to the larger scatter in the measured values of body torque. In a typical calculation for the bundle thrust (Fbundle), the majority of the useful propulsive force produced by rotation (Fpropulsion = Bω) is immediately dissipated by dragging the large bundle behind the cell (Fself-drag = Av). Since the bundle thrust is the difference between two large numbers, it has a large experimental error, and the points in a plot of individual cells' bundle thrust versus body force (Fbundle = −Fself-drag + Fpropulsion versus Fbody), analogous to Fig. 9A, appear to be uncorrelated. Instead, we plotted the propulsive force versus the total drag (Fpropulsion versus Fbody + Fself-drag) in Fig. 9B. This figure shows that there was modest correlation and roughly equal scatter along the two axes.
FIG. 9.
(A) Plot of bundle torque (Γbundle) versus torque on the cell body (Γbody). (B) Plot of propulsive force produced by the bundle (Fpropulsion) versus total drag (Fbody + Fself-drag), calculated for 32 cells swimming in MB+. The dashed lines are least-squares linear fits; the best-fit slopes are 0.82 (A) and 1.04 (B), compared with the dotted 45° line indicating perfect agreement. One could break the bundle torque in panel A into two components and plot torques analogous to forces, as shown in panel B; however, the rotary self-drag (Bv) is so small that this would not substantially change panel A.
For cells swimming in methylcellulose, the calculated bundle and body forces do not coincide. This is not surprising, since solutions of methylcellulose are known to have a non-Newtonian viscosity (9). When a cell propelled by a constant-torque motor is subjected to a simple increase in viscosity, its rotation rate and swimming speed should decrease in proportion to η. Table 1 shows that this does not occur when viscosity is tripled by adding methylcellulose. Only the cells' bundle and motor rotation rates are substantially decreased; the body rotation rate is unaffected, and the cell speed actually increases. This qualitatively agrees with the predictions of an anisotropic viscosity model of swimming in methylcellulose (26).
In summary, assuming the validity of resistive force theory and neglecting interactions with nearby surfaces, we estimated the torque generated by an isolated filament to be ∼400 pN nm, a value substantially lower than current estimates of motor torque. Filaments are quite stiff; changes in shape between spinning filaments and stationary filaments were not detected. The torque generated by a flagellar bundle is surprisingly small, ∼700 pN nm. Evidently, a substantial fraction of the torque supplied by the several motors that drive a bundle is dissipated through internal friction within the bundle or between the bundle and the cell wall. However, the torque and thrust generated by the bundle are balanced, as they should be, by the drag computed for the cell body. Even though additional filaments in a bundle might not add much to a cell's speed, they are useful for reorientation during tumbling. CW rotation often, although not always, triggers a polymorphic transformation to a right-handed filament form. This transformation plays an important role in generating changes in the direction of swimming.
Supplementary Material
Acknowledgments
We thank William S. Ryu for computer expertise and Peter Chupity at J C Labs for modifying his camera design for low-light operation. Their support and encouragement were greatly appreciated in the initial phase of this project.
This work was supported by the Rowland Institute at Harvard and by grants AI016478 and AI065540 from the National Institutes of Health.
Footnotes
Published ahead of print on 22 December 2006.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Aizawa, S.-I. 2002. Flagella, p. 155-175. In M. Sussman (ed.), Molecular medical microbiology, vol. 1. Academic Press, San Diego, CA. [Google Scholar]
- 2.Aizawa, S. I., and T. Kubori. 1998. Bacterial flagellation and cell division. Genes Cells 3:625-634. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong, J. B., J. Adler, and M. M. Dahl. 1967. Nonchemotactic mutants of Escherichia coli. J. Bacteriol. 93:390-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg, H. C. 2004. E. coli in motion. Springer-Verlag, New York, NY.
- 5.Berg, H. C. 1993. Random walks in biology, expanded edition. Princeton University Press, Princeton, NJ.
- 6.Berg, H. C. 2003. The rotary motor of bacterial flagella. Annu. Rev. Biochem. 72:19-54. [DOI] [PubMed] [Google Scholar]
- 7.Berg, H. C., and R. A. Anderson. 1973. Bacteria swim by rotating their flagellar filaments. Nature (London) 245:380-382. [DOI] [PubMed] [Google Scholar]
- 8.Berg, H. C., and D. A. Brown. 1972. Chemotaxis in Escherichia coli analysed by three-dimensional tracking. Nature (London) 239:500-504. [DOI] [PubMed] [Google Scholar]
- 9.Berg, H. C., and L. Turner. 1979. Movement of microorganisms in viscous environments. Nature (London) 278:349-351. [DOI] [PubMed] [Google Scholar]
- 10.Berry, R. M., and H. C. Berg. 1997. Absence of a barrier to backwards rotation of the bacterial flagellar motor demonstrated with optical tweezers. Proc. Natl. Acad. Sci. USA 94:14433-14437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blair, D. F. 2003. Flagellar movement driven by proton translocation. FEBS Lett. 545:86-95. [DOI] [PubMed] [Google Scholar]
- 12.Block, S. M., D. F. Blair, and H. C. Berg. 1989. Compliance of bacterial flagella measured with optical tweezers. Nature 338:514-518. [DOI] [PubMed] [Google Scholar]
- 13.Calladine, C. R. 1975. Construction of bacterial flagella. Nature 255:121-124. [DOI] [PubMed] [Google Scholar]
- 14.Chattopadhyay, S., R. Moldovan, C. Yeung, and X. L. Wu. 2006. Swimming efficiency of bacterium Escherichia coli. Proc. Natl. Acad. Sci. USA 103:13712-13717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen, X., and H. C. Berg. 2000. Torque-speed relationship of the flagellar rotary motor of Escherichia coli. Biophys. J. 78:1036-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darnton, N. C., and H. C. Berg. Force-extension measurements on bacterial flagella: triggering polymorphic transformations. Biophys. J., in press. [DOI] [PMC free article] [PubMed]
- 17.Duffy, K. J., and R. M. Ford. 1997. Turn angle and run time distributions characterize swimming behavior for Pseudomonas putida. J. Bacteriol. 179:1428-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fahrner, K. A., S. M. Block, S. Krishnaswamy, J. S. Parkinson, and H. C. Berg. 1994. A mutant hook-associated protein (HAP3) facilitates torsionally induced transformations of the flagellar filament of Escherichia coli. J. Mol. Biol. 238:173-186. [DOI] [PubMed] [Google Scholar]
- 19.Fahrner, K. A., W. S. Ryu, and H. C. Berg. 2003. Bacterial flagellar switching under load. Nature 423:938. [DOI] [PubMed] [Google Scholar]
- 20.Holwill, M. E. J., and R. E. Burge. 1963. A hydrodynamic study of the motility of flagellated bacteria. Arch. Biochem. Biophys. 101:249-260. [DOI] [PubMed] [Google Scholar]
- 21.Larsen, S. H., R. W. Reader, E. N. Kort, W. Tso, and J. Adler. 1974. Change in direction of flagellar rotation is the basis of the chemotactic response in Escherichia coli. Nature (London) 249:74-77. [DOI] [PubMed] [Google Scholar]
- 22.Lauga, E., W. R. DiLuzio, G. M. Whitesides, and H. A. Stone. 2006. Swimming in circles: motion of bacteria near solid boundaries. Biophys. J. 90:400-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowe, G., M. Meister, and H. C. Berg. 1987. Rapid rotation of flagellar bundles in swimming bacteria. Nature 325:637-640. [Google Scholar]
- 24.Macnab, R. M. 1977. Bacterial flagella rotating in bundles: a study in helical geometry. Proc. Natl. Acad. Sci. USA 74:221-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macnab, R. M., and M. K. Ornston. 1977. Normal-to-curly flagellar transitions and their role in bacterial tumbling: stabilization of an alternative quaternary structure by mechanical force. J. Mol. Biol. 112:1-30. [DOI] [PubMed] [Google Scholar]
- 26.Magariyama, Y., and S. Kudo. 2002. A mathematical explanation of an increase in bacterial swimming speed with viscosity in linear-polymer solutions. Biophys. J. 83:733-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magariyama, Y., S. Sugiyama, K. Muramoto, I. Kawagishi, Y. Imae, and S. Kudo. 1995. Simultaneous measurement of bacterial flagellar rotation rate and swimming speed. Biophys. J. 69:2154-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reid, S. W., M. C. Leake, J. H. Chandler, C. J. Lo, J. P. Armitage, and R. M. Berry. 2006. The maximum number of torque-generating units in the flagellar motor of Escherichia coli is at least 11. Proc. Natl. Acad. Sci. USA 103:8066-8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor, B. L., and D. E. Koshland, Jr. 1974. Reversal of flagellar rotation in monotrichous and peritrichous bacteria: generation of changes in direction. J Bacteriol. 119:640-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turner, L., W. Ryu, and H. C. Berg. 2000. Real-time imaging of fluorescent flagellar filaments. J. Bacteriol. 182:2793-2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wadhams, G. H., and J. P. Armitage. 2004. Making sense of it all: bacterial chemotaxis. Nat. Rev. Mol. Cell Biol. 5:1024-1037. [DOI] [PubMed] [Google Scholar]
- 32.Wright, S., B. Walia, J. S. Parkinson, and S. Khan. 2006. Differential activation of Escherichia coli chemoreceptors by blue-light stimuli. J. Bacteriol. 188:3962-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.