Abstract
The Beijing family of Mycobacterium tuberculosis strains has been associated with epidemic spread and an increased likelihood of developing drug resistance. The characteristics that predispose this family to such clinical outcomes have not been identified, although one potential candidate, the phenolic glycolipid PGL-tb, has been shown to mediate a fulminant lethal disease in mice and rabbits due to lipid-mediated immunosuppression. However, PGL-tb is not uniformly expressed throughout the Beijing lineage and may not be the only unique virulence trait associated with this family. In an attempt to define phenotypes common to all Beijing strains, we interrogated a carefully selected set of isolates representing the five extant lineages of the Beijing family. Comparison of lipid production in this set revealed that all Beijing strains accumulated large quantities of triacylglycerides in in vitro aerobic culture. This accumulation was found to be coincident with upregulation of Rv3130c, whose product was previously characterized as a triacylglyceride synthase. Rv3130c is a member of the DosR-controlled regulon of M. tuberculosis, and further examination revealed that several members of this regulon were upregulated throughout this strain family. The upregulation of the DosR regulon may confer an adaptive advantage for growth in microaerophilic or anaerobic environments encountered by the bacillus during infection and thus may be related to the epidemiological phenomena associated with this important strain lineage.
Mycobacterium tuberculosis, the bacterial agent responsible for the disease tuberculosis (TB), causes 100 million new infections and 2 million deaths each year (49). Although early studies considered global M. tuberculosis strains to be genetically and phenotypically restricted (37), more recent efforts suggest that deletion events and single-nucleotide polymorphisms are major mechanisms of genomic plasticity with important sequelae that can impact disease (16, 42). As evolution has shaped the host response to M. tuberculosis infection, it has also shaped the bacterial adaptations to that response. Geographic restrictions in strain and human distribution have potentially created pockets of metastable host-pathogen populations, where genomic traits of both host and pathogen have reached a state of equilibrium. Global travel and immigration on a large scale may upset these equilibria by bringing into contact host and pathogen populations that have not encountered each other previously (19). There has therefore arisen an awareness of the possibility that independent clades of strains with differential virulence characteristics may be associated with unique disease manifestations (30).
One family of genotypically related M. tuberculosis strains that has attracted considerable attention over the past decade is known as “Beijing” or “W-Beijing” (3, 17). The latter term refers to the fact that the infamous multidrug-resistant W strain that spread rapidly through HIV-infected individuals in prisons and hospitals of New York City during the 1990s belongs to the much broader Beijing family (4). Members of the Beijing lineage were originally identified on the basis of their IS6110 fingerprinting and spoligotype patterns (3). More recently, however, we identified several large sequence polymorphisms (LSPs) through genomic microarray approaches that are unique to Beijing strains and enable their classification into five monophyletic subgroups (16, 41). On a global basis, Beijing strains at present account for approximately 10% of all TB cases in reported studies (14, 15). Regardless of the debate over where the lineage originated—either Central Asia or the Beijing area of China (27, 45)—it is now highly endemic throughout much of Eastern and Southeast Asia. In fact, more than 50% of TB cases across this entire region are due to infection with M. tuberculosis Beijing strains. In other parts of the world, including Russia, Cuba, Bangladesh, South Africa, and western Europe, the proportion of TB due to Beijing strains has also been found to be increasing (14). The importance of this strain family is further highlighted by the fact that the recent epidemic spread within these countries is frequently associated with drug resistance (14, 15, 17). In a large, recent Russian study, for example, multidrug-resistant TB was 2.4 times more likely to be due to infection with Beijing than non-Beijing TB isolates (13). While the propensity for epidemic spread and drug resistance are presently the most widely acknowledged epidemiological traits associated with these strains, there are a number of reports linking the Beijing family with extrapulmonary TB or with treatment failure and relapse (21, 22, 38). The reasons for the high incidence and emerging nature of these strains in multiple geographic settings are not clear, but these phenomena raise the possibility that discrete attributes of this family of strains are responsible for changes in the disease and transmission process. This was highlighted by our recent description of a phenolic glycolipid (PGL-tb) that is produced by a subset of M. tuberculosis Beijing strains that are hypervirulent within mouse survival models of pulmonary TB infection. Purified PGL-tb is able to inhibit macrophage proinflammatory cytokine release in vitro, and deletion of the pks1-15 gene region that is required for PGL-tb synthesis completely abolishes the hypervirulence phenotype in mouse and rabbit models of infection (30, 40). PGL-tb is clearly an important virulence factor for a fraction of Beijing isolates that are likely to be associated with unique disease manifestations within the human host. However, PGL-tb expression is unlikely to explain the successful epidemic spread of the entire clade of this extremely diverse collection of strains, the majority of which do not produce PGL-tb. In the present study we wanted to explore the unique properties of the M. tuberculosis Beijing lineage that may provide a molecular explanation for the important epidemiological phenomena that are currently attributed to this strain family.
The so-called “dormancy regulon” of M. tuberculosis is a transcriptional program induced by low oxygen tension or nitric oxide (NO) that is utilized by the bacterium to adapt for survival during periods of nonreplicating persistence in vitro. This regulon has been proposed to be associated with survival of the bacillus during latent TB infection, a chronic, asymptomatic state that afflicts one-third of the total global human population (34, 46). The 48-gene regulon is controlled by the transcription factor DosR, which binds DNA to induce transcription of the DosR regulon genes following phosphorylation by one of two cognate sensor kinases (31, 32). The genes contained within the DosR regulon include genes involved in anaerobic respiration and lipid metabolism. Strains with mutations in this regulon are impaired for survival under oxygen limitation (5), a condition likely to be encountered during latency in humans and possibly even during chronic phases of active disease (6, 46, 48).
MATERIALS AND METHODS
Bacterial strains and media.
M. tuberculosis strains other than CDC1551 (T. Shinnick, Centers for Disease Control and Prevention, Atlanta, GA), H37Rv (ATCC 27294), HN878 (J. Musser, Methodist Hospital Research Institute, Houston, TX), and NHN5 (J. Musser) were all collected previously as part of our comparative whole-genome analysis studies (16, 42). Unless otherwise stated, strains were cultured using 7H9 liquid medium (Difco) containing 10% albumin dextrose complex, 0.5% glycerol, and 0.1% Tween-80.
Lipid analysis.
Cultures (10 to 30 ml) were grown to an A650 of 0.2, at which point either 0.1 μCi ml−1 [1-14C] propionic acid (American Radiolabeled Chemicals), 1 μCi ml−1 [1-14C]acetic acid (GE Healthcare), or 1 μCi ml−1 [14C(U)]glycerol (American Radiolabeled Chemicals) was added, and the cultures were incubated for a further 48 h. Cell pellets were resuspended in methanol-0.3% NaCl (aqueous) (10:1), and apolar lipids were extracted twice with petroleum ether as described previously (36). Lipid extracts were spotted onto silica gel 60 thin-layer chromatography (TLC) plates (Merck), which were developed in petroleum ether-acetone (96:4). TLC plates were visualized using a Storm 860 PhosphorImager (Molecular Dynamics) or by chemical staining with 5% phosphomolybdic acid (Sigma) in ethanol. Liquid chromatography-mass spectrometry (LC/MS) was conducted on an Agilent 1100 series HPLC with attached quadrupole mass analyzer and electrospray ionization in positive ion mode. LC utilized a C18 column with isocratic elution using 70% methanol, 30% CHCl3, 0.1% ammonium hydroxide at a flow rate of 1 ml/min. Purified glycerol tridodecanoate, tripalmitate, and triarachidate were purchased from Sigma.
RNA isolation and quantitative RT-PCR.
Frozen cell pellets from cultures with an optical density at 650 nm of 0.15 were resuspended in 1 ml of TRIzol (Invitrogen) and transferred to 2-ml screw-cap tubes containing 0.2 ml of 0.1-mm-diameter glass beads (BioSpec Products). Cells were disrupted by three 30-s pulses in a FastPrep homogenizer (Thermo Electron Corporation). After centrifugation, supernatants were extracted with 300 μl of chloroform/isoamyl alcohol, and RNA was precipitated with isopropanol. RNA pellets were washed with 75% ethanol and resuspended in RNase-free water containing RNaseOUT (1 unit/μl; Invitrogen). TURBO DNase (Ambion) was used per the manufacturer's recommendations to remove contaminating DNA both before and after the RNA was purified with an RNeasy minikit (QIAGEN). SuperScript III (Invitrogen) reverse transcriptase was used to prepare randomly primed first-strand cDNA from 200 ng of each RNA sample, and cDNA prepared in this manner was diluted 1:10 prior to use in subsequent TaqMan reactions. The primers and Fam-labeled TaqMan TAMRA probes (Applied Biosystems) listed in Table 1 were used at concentrations of 300 nM and 200 nM, respectively. All TaqMan primers and probes were designed using Primer Express Software (Applied Biosystems). TaqMan reactions were carried out by using TaqMan universal PCR master mix (Applied Biosystems) and the GeneAmp 7700 sequence detection system using standard reaction conditions as recommended by the manufacturer.
TABLE 1.
Oligonucleotide primers and TaqMan probes used in qRT-PCR analysis of DosR regulon expression
| Primer or probe | Sequencea |
|---|---|
| PCR primers | |
| Rv3130c | 5′-ATCCTGACCAAACTGCACCAC-3′ |
| 5′-CCCAGCTAGCAGGTGAGTCG-3′ | |
| dosR | 5′-GATGGCAACGGCATTGAACT-3′ |
| 5′-ATCAGACAGCGCAGATCGG-3′ | |
| hspX | 5′-GAATTCGCGTACGGTTCCTTC-3′ |
| 5′-TGTCGTCCTCGTCAGCACC-3′ | |
| fdxA | 5′-GCGTGGATGTGATGGACAAG-3′ |
| 5′-ATGTAGAGCATTCGGGCGC-3′ | |
| narX | 5′-AGTCCGGAGATTGGTGGGAC-3′ |
| 5′-TTGCCGTGAGTTCGGGTAG-3′ | |
| sigA | 5′-TCGCGCCTACCTCAAAC-3′ |
| 5′-CTAGCTCGACCTCTTCCTCGG-3′ | |
| 16S rRNA | 5′-GTGGAGAAGAAGCACCGGC-3′ |
| 5′-ACGCTCGCACCCTACGTATT-3′ | |
| sigB | 5′-GGGCGATTTCATCGAGGAC-3′ |
| 5′-TCGGTGTGTAACAGTTCGGC-3′ | |
| TaqMan probes | |
| Rv3130c | 5′-CATGGCCGACGGAATCGCG-3′ |
| dosR | 5′-CCGCGATCTGTTGTCCCGCAT-3′ |
| hspX | 5′-CGCACGGTGTCGCTGCCG-3′ |
| fdxA | 5′-TGTGCAGGAGTGTCCGGTCGACTGTAT-3′ |
| narX | 5′-TGGTGTGGCAATGCGCCTCG-3′ |
| sigA | 5′-TCGGCAAGGTAGCGCTGCTCAAC-3′ |
| 16S rRNA | 5′-CTACGTGCCAGCAGCCGCGG-3′ |
| sigB | 5′-TCCGCGGAGAACGCGGTCA-3′ |
For primers, forward and reverse sequence pairs are listed.
RESULTS
PGL-tb synthesis and the M. tuberculosis Beijing lineage.
We previously demonstrated that HN878 (37) and closely related Beijing strains (W4, W10, and 210) (3) are able to synthesize PGL-tb (30). This complex glycolipid is not produced in strains CDC1551 (43) and NHN5 (37) or in the sequenced laboratory strain H37Rv (8). NHN5 lacks PGL-tb despite the fact that it possesses an intact copy of the pks1-15 gene (30). This led to the speculation that multiple, independent genetic alterations have taken place and are responsible for the loss of PGL-tb expression in diverse strain types. NHN5 was previously misclassified as a non-Beijing strain based upon available genotypic and phenotypic information (25). However, we have recently established that NHN5 is indeed a member of the Beijing family due to the presence of Beijing-specific LSPs (16, 41).
Comparative whole-genome hybridization was carried out previously in order to identify the subpopulation structure of the M. tuberculosis Beijing family of strains (16, 41). The RD105 (regions of difference) LSP appears in all Beijing strains examined to date and can be used to define the set of strains belonging to this lineage. Further subdivisions of the M. tuberculosis Beijing lineage are made on the basis of the variable appearance of the RD207, RD181, RD150, and RD142 deletions. In the present study, we refer to group 1 Beijing strains as those that contain only the RD105 LSP, while group 2 to 5 Beijing strains also contain RD207 (group 2), RD207 and RD181 (group 3), RD207, RD181, and RD150 (group 4), and RD207, RD181, and RD142 (group 5) (Fig. 1). Group 1 strains are atypical in that they do not have deletions of RD207 (the genomic deletion responsible for the characteristic spoligotype pattern of Beijing strains) and thus can be thought of as being “ancestral” to the “classical” Beijing lineage. Other strain families examined in this study include those referred to as Indo-Oceanic and Euro-American (16). Indo-Oceanic strains are defined by the deletion of RD239 (Fig. 1) and are ancestral to “modern” M. tuberculosis strains, as they lack the TbD1 deletion (7). The Euro-American lineage incorporates strains previously classified under the heading of principal genetic groups 2 and 3 (37) and includes CDC1551 and H37Rv. All strains within the Euro-American clade contain the 7-bp pks1-15 deletion, which renders them incapable of synthesizing PGL-tb (9, 30). Specific Middle Eastern and African sublineages also exist within the diverse Euro-American strain family (Fig. 1).
FIG. 1.
Global population structure of M. tuberculosis. Numbers in rectangles refer to lineage-defining LSPs. The geographic regions associated with specific lineages are indicated, as is the Beijing family subgroup structure (adapted from reference 16 with the permission of the publisher).
In order to extend our initial observations of PGL-tb expression within the Beijing lineage, an additional set of 36 Beijing isolates representing each of the five sublineages was assayed for PGL-tb synthesis as previously described (30). Briefly, in vitro cultures of each of these strains were incubated in the presence of [1-14C]propionic acid to specifically radiolabel methyl-branched lipids derived from methyl-malonyl coenzyme A. Apolar organic solvent extracts were prepared for each of the radiolabeled strains and fractionated by TLC. Each of the strains tested in this manner has its pks1-15 sequence intact (41). PGL-tb synthesis was detected in only 40% of strains tested, which further confirmed the apparent dichotomy in PGL-tb expression for the Beijing lineage. Even more striking was the finding that PGL-tb synthesis was restricted to Beijing subgroups 3, 4, and 5. Ten percent of group 3 strains that were tested produced PGL-tb, while 60% and 80% of strains within groups 4 and 5, respectively, synthesized detectable quantities of PGL-tb (Table 2). It should be noted that at present we only have a single confirmed group 2 strain in our collection. Further studies are required to definitively assess whether additional members of this subgroup are also incapable of synthesizing PGL-tb. As expected, none of the 18 non-Beijing strains tested generated PGL-tb.
TABLE 2.
Proportion of PGL-tb- and TAG-producing strains in Beijing and non-Beijing lineages of M. tuberculosis
| Lineage and group | LSP(s) | % (no. positive/no. tested) producing:
|
|
|---|---|---|---|
| PGL-tb | TAG | ||
| Beijing | |||
| 1 | RD105 | 0 (0/7) | 100 |
| 2 | RD105, RD207 | 0 (0/1) | 100 |
| 3 | RD105, RD207, RD181 | 10 (1/10) | 100 |
| 4 | RD105, RD207, RD181, RD150 | 60 (6/10) | 100 |
| 5 | RD105, RD207, RD181, RD142 | 80 (8/10) | 100 |
| Indo-Oceanic | 0 (0/5) | 0 | |
| Euro-American | 0 (0/13) | 0 | |
Triglyceride accumulation within Beijing strains.
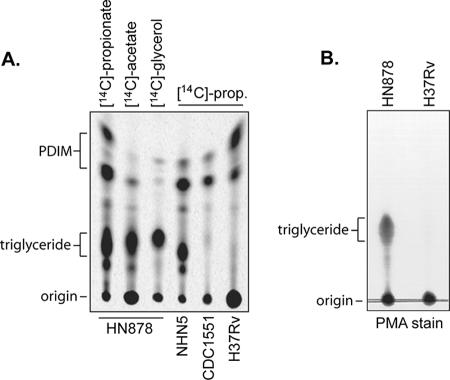
To continue our comparative studies aimed at identifying lipids that are unique to the M. tuberculosis Beijing lineage, in vitro cultures of HN878, NHN5, CDC1551, and H37Rv were incubated in the presence of [1-14C]propionic acid. Apolar organic solvent extracts were prepared for each of the radiolabeled strains and fractionated by normal-phase TLC. After a range of mobile-phase systems was explored, an abundant lipid was visualized by phosphorimaging in the HN878 and NHN5 extracts following TLC fractionation under the least polar solvent conditions tested (Fig. 2A). This lipid was absent from identical apolar lipid extracts generated from the non-Beijing strains CDC1551 and H37Rv. To confirm that the relative abundance of this lipid was not just an artifact of using [1-14C]propionic acid in the radiolabeling process, we repeated the labeling experiment with [1-14C]acetic acid (Fig. 2A) and also chemically stained TLC fractionated lipids with phosphomolybdic acid (Fig. 2B).
FIG. 2.
Triglyceride synthesis in Beijing strains. (A) Apolar lipids extracted from [1-14C]propionic acid-, [1-14C]acetic acid-, or [U-14C]glycerol-labeled cultures of the M. tuberculosis strains indicated were analyzed on silica gel TLC plates developed in petroleum ether-acetone (96:4). Radiolabeled lipids were detected by phosphorimaging. PDIM, phthiocerol dimycocerosate. (B) Apolar lipid extracts of HN878 and H37Rv cultures were fractionated as for panel A and detected by staining with 5% phosphomolybdic acid (PMA).
The relative mobility of this lipid under the solvent conditions tested suggested that it belonged to the class of lipids known as triacylglycerides (TAG) (12). This interpretation was confirmed through the ability to specifically radiolabel this material with [U-14C]glycerol (Fig. 2A). Several prior studies on the TAG of M. tuberculosis, Mycobacterium smegmatis, and Mycobacterium bovis BCG each demonstrated an enormous variety in the range of fatty acids that are able to be esterified to the three positions of the glycerol backbone, which includes several methyl-branched fatty acid species (10, 47). This observation is consistent with our ability to radiolabel the HN878 and NHN5 TAG with both [1-14C]propionate and [1-14C]acetate. Preliminary LC/MS carried out on TAG purified from HN878 lipid extracts by preparative TLC confirmed the presence of an extremely diverse mixture of TAG molecules (data not presented).
Triglyceride synthesis in genetically diverse M. tuberculosis lineages.
The set of 36 isolates representing each of the five Beijing sublineages was cultured under standard in vitro conditions in the presence of [1-14C]propionic acid. Apolar lipids were extracted and analyzed by TLC as outlined above. Each of the Beijing strains assayed in this manner was found to accumulate TAG as shown previously for strains HN878 (Beijing group 5) and NHN5 (group 3) (Fig. 3). In contrast, of the 18 non-Beijing bacterial strains representing both the Euro-American and Indo-Oceanic lineages, not a single strain was found to synthesize TAG in detectable amounts when cultured under identical conditions (Fig. 3). It should be noted that TAG storage within the Beijing strains is not simply a function of excess glycerol within the growth medium, as we also observed TAG formation when cultures were grown in the absence of exogenous sources of glycerol (data not shown).
FIG. 3.
All Beijing sublineages accumulate triglycerides. Apolar lipids extracted from [1-14C]propionic acid-labeled in vitro cultures of the Beijing and non-Beijing strains indicated were analyzed on silica gel TLC plates developed in petroleum ether-acetone (96:4). Radiolabeled lipids were subsequently visualized by phosphorimaging. The position of the triglyceride fraction specific to all Beijing strains is shown. PDIM, phthiocerol dimycocerosate.
Expression of the dormancy regulon within the Beijing lineage.
One of the genes of the dormancy (DosR) regulon, Rv3130c, has been shown to encode a protein with TAG synthase activity (10). In the same study, synthesis and accumulation of TAG were shown to occur in M. tuberculosis (H37Rv) cultures that were subjected to low-oxygen conditions for >14 days or upon short-term exposure to NO, suggesting that TAG accumulation was under DosR control. Transcription of the Rv3130c TAG synthase under these conditions is induced 20- to 40-fold (10). Disruption of Rv3130c in the H37Rv background was very recently shown to prevent TAG accumulation under these in vitro conditions (35). Because of this association, we decided to evaluate the transcriptional activity of Rv3130c along with other genes of the DosR regulon for members of the M. tuberculosis Beijing lineage.
cDNA was prepared for quantitative real-time PCR (qRT-PCR) analysis using total RNA isolated from log-phase cultures of 11 strains representing each of the five Beijing subgroups, as well as 8 non-Beijing strains representing both the Indo-Oceanic and Euro-American clades. In addition to Rv3130c, qRT-PCR was used to assay expression levels of dosR (Rv3133c) (the response regulator that mediates transcription of the dormancy regulon [29]), hspX (α-crystallin; Rv2031c), fdxA (ferredoxin; Rv2007c), and narX (nitrate reductase; Rv1736c). Aside from dosR, which is found almost adjacent to Rv3130c, these genes were selected on the basis that they are located in distinct regions of the M. tuberculosis genome. After normalizing to sigA expression levels, Rv3130c transcription was determined to be on average 10-fold greater in Beijing strains than in non-Beijing strains, consistent with the accumulation of TAG seen for the Beijing lineage (Fig. 4). The greatest difference in transcript abundance was observed for dosR, which was expressed at 50-fold-higher levels in Beijing strains than in non-Beijing strains. For the other genes of the regulon that were analyzed, the transcript levels were on average 6 times greater within the M. tuberculosis Beijing isolates (Fig. 4). In order to avoid any potential bias in these findings due to inherent differences in sigA mRNA levels between Beijing and non-Beijing strains, we reanalyzed all qRT-PCR data by normalizing to 16S rRNA. The results obtained in this manner were indistinguishable from those obtained following sigA normalization. To also confirm that the dormancy regulon was not overexpressed within the Beijing isolates as part of a more generalized “stress” response, we assayed transcription of the stress-inducible sigma factor sigB (26, 46). sigB expression did not vary significantly among the isolates tested (data not shown).
FIG. 4.
Constitutive dormancy regulon expression in the M. tuberculosis Beijing lineage. Gene expression levels within RNA samples isolated from early-log-phase in vitro cultures were analyzed by qRT-PCR. Mean transcript abundance (ng) was determined for Beijing (n = 11) and non-Beijing (n = 8) isolates (assayed in triplicate) relative to sigA transcript levels. Two independent RNA samples were analyzed for each M. tuberculosis strain, and data from a single representative experiment are presented. Error bars represent standard deviations.
DISCUSSION
The reasons for the apparent global success of Beijing strains are not yet understood but could include a variety of host-related factors, such as human population movements (27, 44), selective pressure due to increases in worldwide BCG vaccine coverage (24, 45), institutionalization (4, 39), and ineffective treatment of drug-resistant strains, leading to increased transmission periods. The possibility that features inherent in the M. tuberculosis Beijing lineage may play an important role in the success of these strains by conferring unique virulence or transmission properties has also been highlighted recently by several laboratories, including ours. A small number of Beijing strains have shown increased replication rates within in vitro-grown macrophages (2, 23, 50) as well as enhanced virulence (hypervirulence) within mouse models of pulmonary TB and rabbit models of tuberculous meningitis (24, 25, 30, 40). We have also demonstrated selective enrichment of Beijing strains over non-Beijing strains in mixed infections in mice (2).
The finding that PGL-tb expression appears to be confined to Beijing subgroups 3 to 5 (10%, 60%, and 80% of strains within groups 3, 4, and 5, respectively) supports the hypothesis that PGL-tb synthesis has been selected against on at least two independent occasions (due to the fact that all Beijing strains possess an intact pks1-15 gene, unlike the Euro-American strains). This observation also suggests that the ability to synthesize PGL-tb has been maintained (or possibly reacquired) independently in three distinct Beijing lineages. PGL-tb must confer some form of selective evolutionary advantage upon the strains that generate this complex immunosuppressive glycolipid. The selective pressure in this case may be related to a unique attribute of the host immune response within the population of patients infected with these PGL-tb-producing isolates. Obviously, the fact that PGL-tb synthesis is not uniformly distributed among the five Beijing lineages will significantly complicate any attempts to ascertain the role of this lipid in human TB disease.
The DosR regulon of M. tuberculosis comprises a set of 48 diverse genes that are coordinately regulated in response to either oxygen deprivation (hypoxia) or treatment with NO (34, 46). Both of these conditions lead to inhibition of respiration and are believed to contribute to the development of latent TB bacilli within the granulomatous environment of the human lung (28, 48). Many of the genes that comprise the dormancy regulon, including those shown to be constitutively overexpressed in M. tuberculosis Beijing strains as part of the present study (dosR, Rv3130c, hspX, fdxA, and narX), have been confirmed to be induced within the lungs of infected mice and in extracellular bacteria isolated from a mouse artificial granuloma model (20, 33). Induction of each of these genes also occurs in in vitro-cultured macrophages that have been activated via treatment with IFN-γ and is dependent upon the generation of NO within these macrophages. In this study, we have demonstrated for the first time that the M. tuberculosis Beijing lineage has a basal level of transcription for several dormancy regulon genes that is up to 50-fold higher than that in non-Beijing strains. While it still remains to be confirmed experimentally, it seems reasonable to assume that the ability to constitutively overexpress genes known to be involved in the adaptive response of M. tuberculosis to hypoxia and reactive nitrogen intermediates would confer a significant advantage upon these strains, since they would be “preadapted” to one set of environmental conditions thought to predominate in vivo during infection. Indeed, the importance of dosR for bacterial maintenance and survival under hypoxic conditions in vitro has been previously demonstrated (5, 46). The hypothesis that M. tuberculosis Beijing strains are predisposed toward metabolism and survival within microaerophilic or anaerobic environments is the subject of ongoing studies within our laboratories.
In addition to constitutive overexpression of the DosR regulon within the Beijing lineage, all Beijing strains were found to accumulate TAG. The fact that these two phenotypes were linked was highlighted recently by the discovery that Rv3130c—a member of the dormancy regulon—encodes a diacylglycerol acyltransferase (TAG synthase) whose activity is induced in response to hypoxia and NO treatment. Moreover, TAG has been reported to accumulate to detectable levels after 2 weeks within O2-depleted cultures (H37Rv) and at a considerably higher rate during NO exposure. Disruption of Rv3130c has also been demonstrated to prevent TAG accumulation under these in vitro conditions (10, 35). Upon nutrient starvation, stored TAG is able to be hydrolyzed following lipase induction, supporting a role for this lipid as an energy storage mechanism that provides a carbon and energy source to support cell growth in the face of either anoxia or an aggressive immune response (11). As such, the ability of M. tuberculosis Beijing strains to accumulate fatty acids in the form of TAG under conditions where non-Beijing strains normally do not is likely to mean that they have a readily available energy source that could afford them a clear advantage over other lineages during, for example, periods of latency and transmission. In the present study, levels of the Rv3130c transcript were 10-fold greater in Beijing strains than in non-Beijing strains under standard growth conditions, consistent with the striking accumulation of TAG in this strain family. Despite the fact that the constitutive levels of the dormancy regulon transcripts detected within the M. tuberculosis Beijing isolates appear to be somewhat less than the levels reached in other studies following NO exposure or within infected mice (20, 46), we are still able to detect significant amounts of accumulated TAG within the Beijing strains. Aside from M. tuberculosis, accumulation of TAG under low-oxygen conditions also occurs in Rhodococcus opacus (1). This led to the intriguing suggestion that TAG synthesis—by analogy to another group of storage lipids, polyhydroxyalkanoic acid—may serve as a sink for cellular reducing equivalents. Fatty acid biosynthesis requires large amounts of NADPH, such that their synthesis and storage in large quantities as TAG could avoid the accumulation of reduced cofactors during the withdrawal of O2. If this is indeed the case, then the constitutive accumulation of TAG by the M. tuberculosis Beijing lineage supports the hypothesis that these strains are more adept than strains of non-Beijing lineages at evolving their metabolism to that required for survival within host tissues.
In summary, while the role of the DosR regulon and TAG in human TB remains to be explored, this study represents the first description of a series of phenomena that occur uniquely within members of a single M. tuberculosis lineage. The ability of M. tuberculosis Beijing strains to constitutively overexpress the DosR regulon with concomitant accumulation of TAG may help to explain the epidemiological traits currently associated with this important strain family. Bearing in mind that even minor differences in inoculum can have dramatic consequences in terms of disease outcome (2), any slight advantage that Beijing strains have over other lineages—particularly early on in infection—could easily translate into a dramatic effect on a population-wide basis. In addition, if Beijing strains are predisposed to adaptive metabolic or cell wall alterations upon entering the host, this could potentially contribute to phenotypic antibiotic tolerance (18), lack of treatment effectiveness, and the development of drug resistance. It will be vital to gain insight into the range of influence that the dormancy regulon, PGL-tb, and TAG have on the metabolism and the spectrum of disease caused by Beijing strains if we are to successfully control this adaptive and widely successful emerging pathogen.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
We thank Michael Goodwin (NIAID, NIH) for his technical assistance.
Footnotes
Published ahead of print on 19 January 2007.
REFERENCES
- 1.Alvarez, H. M., and A. Steinbuchel. 2002. Triacylglycerols in prokaryotic microorganisms. Appl. Microbiol. Biotechnol. 60:367-376. [DOI] [PubMed] [Google Scholar]
- 2.Barczak, A. K., P. Domenech, H. I. Boshoff, M. B. Reed, C. Manca, G. Kaplan, and C. E. Barry III. 2005. In vivo phenotypic dominance in mouse mixed infections with Mycobacterium tuberculosis clinical isolates. J. Infect. Dis. 192:600-606. [DOI] [PubMed] [Google Scholar]
- 3.Bifani, P. J., B. Mathema, N. E. Kurepina, and B. N. Kreiswirth. 2002. Global dissemination of the Mycobacterium tuberculosis W-Beijing family strains. Trends Microbiol. 10:45-52. [DOI] [PubMed] [Google Scholar]
- 4.Bifani, P. J., B. B. Plikaytis, V. Kapur, K. Stockbauer, X. Pan, M. L. Lutfey, S. L. Moghazeh, W. Eisner, T. M. Daniel, M. H. Kaplan, J. T. Crawford, J. M. Musser, and B. N. Kreiswirth. 1996. Origin and interstate spread of a New York City multidrug-resistant Mycobacterium tuberculosis clone family. JAMA 275:452-457. [PubMed] [Google Scholar]
- 5.Boon, C., and T. Dick. 2002. Mycobacterium bovis BCG response regulator essential for hypoxic dormancy. J. Bacteriol. 184:6760-6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boshoff, H. I., and C. E. Barry III. 2005. Tuberculosis—metabolism and respiration in the absence of growth. Nat. Rev. Microbiol. 3:70-80. [DOI] [PubMed] [Google Scholar]
- 7.Brosch, R., S. V. Gordon, M. Marmiesse, P. Brodin, C. Buchrieser, K. Eiglmeier, T. Garnier, C. Gutierrez, G. Hewinson, K. Kremer, L. M. Parsons, A. S. Pym, S. Samper, D. van Soolingen, and S. T. Cole. 2002. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc. Natl. Acad. Sci. USA 99:3684-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M. A. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, R. Squares, S. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 9.Constant, P., E. Perez, W. Malaga, M. A. Laneelle, O. Saurel, M. Daffe, and C. Guilhot. 2002. Role of the pks15/1 gene in the biosynthesis of phenolglycolipids in the Mycobacterium tuberculosis complex. Evidence that all strains synthesize glycosylated p-hydroxybenzoic methyl esters and that strains devoid of phenolglycolipids harbor a frameshift mutation in the pks15/1 gene. J. Biol. Chem. 277:38148-38158. [DOI] [PubMed] [Google Scholar]
- 10.Daniel, J., C. Deb, V. S. Dubey, T. D. Sirakova, B. Abomoelak, H. R. Morbidoni, and P. E. Kolattukudy. 2004. Induction of a novel class of diacylglycerol acyltransferases and triacylglycerol accumulation in Mycobacterium tuberculosis as it goes into a dormancy-like state in culture. J. Bacteriol. 186:5017-5030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deb, C., J. Daniel, T. D. Sirakova, B. Abomoelak, V. S. Dubey, and P. E. Kolattukudy. 2006. A novel lipase belonging to the hormone-sensitive lipase family induced under starvation to utilize stored triacylglycerol in Mycobacterium tuberculosis. J. Biol. Chem. 281:3866-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dobson, G., D. E. Minnikin, S. M. Minnikin, J. H. Parlett, M. Goodfellow, M. Ridell, and M. Magnusson. 1985. Systematic analysis of complex mycobacterial lipids, p. 237-265. In M. Goodfellow and D. E. Minnikin (ed.), Chemical methods in bacterial systematics, vol. 1. Academic Press, London, United Kingdom. [Google Scholar]
- 13.Drobniewski, F., Y. Balabanova, V. Nikolayevsky, M. Ruddy, S. Kuznetzov, S. Zakharova, A. Melentyev, and I. Fedorin. 2005. Drug-resistant tuberculosis, clinical virulence, and the dominance of the Beijing strain family in Russia. JAMA 293:2726-2731. [DOI] [PubMed] [Google Scholar]
- 14.European Concerted Action on New Generation Genetic Markers and Techniques for the Epidemiology and Control of Tuberculosis. 2006. Beijing/W genotype Mycobacterium tuberculosis and drug resistance. Emerg. Infect. Dis. 12:736-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filliol, I., J. R. Driscoll, D. van Soolingen, B. N. Kreiswirth, K. Kremer, G. Valetudie, D. A. Dang, R. Barlow, D. Banerjee, P. J. Bifani, K. Brudey, A. Cataldi, R. C. Cooksey, D. V. Cousins, J. W. Dale, O. A. Dellagostin, F. Drobniewski, G. Engelmann, S. Ferdinand, D. Gascoyne-Binzi, M. Gordon, M. C. Gutierrez, W. H. Haas, H. Heersma, E. Kassa-Kelembho, M. L. Ho, A. Makristathis, C. Mammina, G. Martin, P. Mostrom, I. Mokrousov, V. Narbonne, O. Narvskaya, A. Nastasi, S. N. Niobe-Eyangoh, J. W. Pape, V. Rasolofo-Razanamparany, M. Ridell, M. L. Rossetti, F. Stauffer, P. N. Suffys, H. Takiff, J. Texier-Maugein, V. Vincent, J. H. de Waard, C. Sola, and N. Rastogi. 2003. Snapshot of moving and expanding clones of Mycobacterium tuberculosis and their global distribution assessed by spoligotyping in an international study. J. Clin. Microbiol. 41:1963-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gagneux, S., K. DeRiemer, T. Van, M. Kato-Maeda, B. C. de Jong, S. Narayanan, M. Nicol, S. Niemann, K. Kremer, M. C. Gutierrez, M. Hilty, P. C. Hopewell, and P. M. Small. 2006. Variable host-pathogen compatibility in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 103:2869-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glynn, J. R., J. Whiteley, P. J. Bifani, K. Kremer, and D. van Soolingen. 2002. Worldwide occurrence of Beijing/W strains of Mycobacterium tuberculosis: a systematic review. Emerg. Infect. Dis. 12:843-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez, J. E., and J. D. McKinney. 2004. M. tuberculosis persistence, latency, and drug tolerance. Tuberculosis (Edinburgh) 84:29-44. [DOI] [PubMed] [Google Scholar]
- 19.Hirsh, A. E., A. G. Tsolaki, K. DeRiemer, M. W. Feldman, and P. M. Small. 2004. Stable association between strains of Mycobacterium tuberculosis and their human host populations. Proc. Natl. Acad. Sci. USA 101:4871-4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karakousis, P. C., T. Yoshimatsu, G. Lamichhane, S. C. Woolwine, E. L. Nuermberger, J. Grosset, and W. R. Bishai. 2004. Dormancy phenotype displayed by extracellular Mycobacterium tuberculosis within artificial granulomas in mice. J. Exp. Med. 200:647-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong, Y., M. Cave, L. Zhang, B. Foxman, C. Marrs, J. Bates, and Z. Yang. 2006. A population-based study of deletions in five different genomic regions of Mycobacterium tuberculosis and their possible clinical relevance. J. Clin. Microbiol. 44:3940-3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lan, N. T., H. T. Lien, Le B. Tung, M. W. Borgdorff, K. Kremer, and D. van Soolingen. 2003. Mycobacterium tuberculosis Beijing genotype and risk for treatment failure and relapse, Vietnam. Emerg. Infect. Dis. 9:1633-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, Q., C. C. Whalen, J. M. Albert, R. Larkin, L. Zukowski, M. D. Cave, and R. F. Silver. 2002. Differences in rate and variability of intracellular growth of a panel of Mycobacterium tuberculosis clinical isolates within a human monocyte model. Infect. Immun. 70:6489-6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez, B., D. Aguilar, H. Orozco, M. Burger, C. Espitia, V. Ritacco, L. Barrera, K. Kremer, R. Hernandez-Pando, K. Huygen, and D. van Soolingen. 2003. A marked difference in pathogenesis and immune response induced by different Mycobacterium tuberculosis genotypes. Clin. Exp. Immunol. 133:30-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manca, C., M. B. Reed, S. Freeman, B. Mathema, B. Kreiswirth, C. E. Barry III, and G. Kaplan. 2004. Differential monocyte activation underlies strain-specific Mycobacterium tuberculosis pathogenesis. Infect. Immun. 72:5511-5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manganelli, R., E. Dubnau, S. Tyagi, F. R. Kramer, and I. Smith. 1999. Differential expression of 10 sigma factor genes in Mycobacterium tuberculosis. Mol. Microbiol. 31:715-724. [DOI] [PubMed] [Google Scholar]
- 27.Mokrousov, I., H. M. Ly, T. Otten, N. N. Lan, B. Vyshnevskyi, S. Hoffner, and O. Narvskaya. 2005. Origin and primary dispersal of the Mycobacterium tuberculosis Beijing genotype: clues from human phylogeography. Genome Res. 15:1357-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nathan, C. 2002. Inducible nitric oxide synthase in the tuberculous human lung. Am. J. Respir. Crit. Care Med. 166:130-131. [DOI] [PubMed] [Google Scholar]
- 29.Park, H. D., K. M. Guinn, M. I. Harrell, R. Liao, M. I. Voskuil, M. Tompa, G. K. Schoolnik, and D. R. Sherman. 2003. Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol. Microbiol. 48:833-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reed, M. B., P. Domenech, C. Manca, H. Su, A. K. Barczak, B. N. Kreiswirth, G. Kaplan, and C. E. Barry III. 2004. A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature 431:84-87. [DOI] [PubMed] [Google Scholar]
- 31.Roberts, D. M., R. P. Liao, G. Wisedchaisri, W. G. Hol, and D. R. Sherman. 2004. Two sensor kinases contribute to the hypoxic response of Mycobacterium tuberculosis. J. Biol. Chem. 279:23082-23087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saini, D. K., V. Malhotra, D. Dey, N. Pant, T. K. Das, and J. S. Tyagi. 2004. DevR-DevS is a bona fide two-component system of Mycobacterium tuberculosis that is hypoxia-responsive in the absence of the DNA-binding domain of DevR. Microbiology 150:865-875. [DOI] [PubMed] [Google Scholar]
- 33.Schnappinger, D., S. Ehrt, M. I. Voskuil, Y. Liu, J. A. Mangan, I. M. Monahan, G. Dolganov, B. Efron, P. D. Butcher, C. Nathan, and G. K. Schoolnik. 2003. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: insights into the phagosomal environment. J. Exp. Med. 198:693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sherman, D. R., M. Voskuil, D. Schnappinger, R. Liao, M. I. Harrell, and G. K. Schoolnik. 2001. Regulation of the Mycobacterium tuberculosis hypoxic response gene encoding alpha-crystallin. Proc. Natl. Acad. Sci. USA 98:7534-7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sirakova, T. D., V. S. Dubey, C. Deb, J. Daniel, T. A. Korotkova, B. Abomoelak, and P. E. Kolattukudy. 2006. Identification of a diacylglycerol acyltransferase gene involved in accumulation of triacylglycerol in Mycobacterium tuberculosis under stress. Microbiology 152:2717-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slayden, R. A., and C. E. Barry III. 2001. Analysis of the lipids of Mycobacterium tuberculosis, p. 229-245. In T. Parish and N. G. Stoker (ed.), Mycobacterium tuberculosis protocols. Humana Press, Totowa, NJ. [DOI] [PubMed]
- 37.Sreevatsan, S., X. Pan, K. E. Stockbauer, N. D. Connell, B. N. Kreiswirth, T. S. Whittam, and J. M. Musser. 1997. Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proc. Natl. Acad. Sci. USA 94:9869-9874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun, Y. J., A. S. Lee, S. Y. Wong, and N. I. Paton. 2006. Association of Mycobacterium tuberculosis Beijing genotype with tuberculosis relapse in Singapore. Epidemiol. Infect. 134:329-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toungoussova, O. S., A. Mariandyshev, G. Bjune, P. Sandven, and D. A. Caugant. 2003. Molecular epidemiology and drug resistance of Mycobacterium tuberculosis isolates in the Archangel prison in Russia: predominance of the W-Beijing clone family. Clin. Infect. Dis. 37:665-672. [DOI] [PubMed] [Google Scholar]
- 40.Tsenova, L., E. Ellison, R. Harbacheuski, A. L. Moreira, N. Kurepina, M. B. Reed, B. Mathema, C. E. Barry III, and G. Kaplan. 2005. Virulence of selected Mycobacterium tuberculosis clinical isolates in the rabbit model of meningitis is dependent on phenolic glycolipid produced by the bacilli. J. Infect. Dis. 192:98-106. [DOI] [PubMed] [Google Scholar]
- 41.Tsolaki, A. G., S. Gagneux, A. S. Pym, Y. O. Goguet de la Salmoniere, B. N. Kreiswirth, D. Van Soolingen, and P. M. Small. 2005. Genomic deletions classify the Beijing/W strains as a distinct genetic lineage of Mycobacterium tuberculosis. J. Clin. Microbiol. 43:3185-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsolaki, A. G., A. E. Hirsh, K. DeRiemer, J. A. Enciso, M. Z. Wong, M. Hannan, Y. O. Goguet de la Salmoniere, K. Aman, M. Kato-Maeda, and P. M. Small. 2004. Functional and evolutionary genomics of Mycobacterium tuberculosis: insights from genomic deletions in 100 strains. Proc. Natl. Acad. Sci. USA 101:4865-4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valway, S. E., M. P. Sanchez, T. F. Shinnick, I. Orme, T. Agerton, D. Hoy, J. S. Jones, H. Westmoreland, and I. M. Onorato. 1998. An outbreak involving extensive transmission of a virulent strain of Mycobacterium tuberculosis. N. Engl. J. Med. 338:633-639. [DOI] [PubMed] [Google Scholar]
- 44.van Helden, P. D., R. M. Warren, T. C. Victor, G. van der Spuy, M. Richardson, and E. Hoal-van Helden. 2002. Strain families of Mycobacterium tuberculosis. Trends Microbiol. 10:167-168. [DOI] [PubMed] [Google Scholar]
- 45.van Soolingen, D., L. Qian, P. E. de Haas, J. T. Douglas, H. Traore, F. Portaels, H. Z. Qing, D. Enkhsaikan, P. Nymadawa, and J. D. van Embden. 1995. Predominance of a single genotype of Mycobacterium tuberculosis in countries of East Asia. J. Clin. Microbiol. 33:3234-3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Voskuil, M. I., D. Schnappinger, K. C. Visconti, M. I. Harrell, G. M. Dolganov, D. R. Sherman, and G. K. Schoolnik. 2003. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J. Exp. Med. 198:705-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walker, R. W., H. Barakat, and J. G. Hung. 1970. The positional distribution of fatty acids in the phospholipids and triglycerides of Mycobacterium smegmatis and M. bovis BCG. Lipids 5:684-691. [DOI] [PubMed] [Google Scholar]
- 48.Wayne, L. G., and C. D. Sohaskey. 2001. Nonreplicating persistence of Mycobacterium tuberculosis. Annu. Rev. Microbiol. 55:139-163. [DOI] [PubMed] [Google Scholar]
- 49.World Health Organization. 2006. Global tuberculosis control—surveillance, planning, financing: WHO Report 2006 (WHO/HTM/TB/2006.362). World Health Organization, Geneva, Switzerland.
- 50.Zhang, M., J. Gong, Z. Yang, B. Samten, M. D. Cave, and P. F. Barnes. 1999. Enhanced capacity of a widespread strain of Mycobacterium tuberculosis to grow in human macrophages. J. Infect. Dis. 179:1213-1217. [DOI] [PubMed] [Google Scholar]