Abstract
Borrelia burgdorferi undergoes an infectious cycle that requires adaptation to different hosts and marked differences in environment. B. burgdorferi copes with its different environments by regulating the expression of proteins required for survival in specific settings. The B. burgdorferi oligopeptide permease (Opp) is one of only a few transporters encoded by the B. burgdorferi genome. Opp proteins in other bacteria serve multiple environmental adaptation functions. B. burgdorferi appears to broaden the usage of this transporter by utilizing five different substrate binding proteins (OppA proteins) that interact with the integral membrane components of the transporter. Expression of the OppA proteins is individually regulated and may play different roles in adaptation to host environments. Very little is known about the mechanisms used by B. burgdorferi to regulate the expression of different OppA proteins. Here we show that the alternative sigma factors, RpoS and RpoN, regulate the expression of oppA5 but not that of other oppA genes. Using a reporter assay with Escherichia coli and gel shift binding assays, we also show that the B. burgdorferi BosR/Fur homologue interacts with the oppA4 promoter and that another candidate transcription factor, EbfC, interacts with the oppA5 promoter. Binding to the promoters was confirmed by gel shift assays. Expression of BosR/Fur in its different hosts does appear to parallel the expression of oppA4. A better understanding of the factors involved in gene regulation in B. burgdorferi will help to identify coregulated proteins that may cooperate to allow the organism to survive in a specific environment.
Borrelia burgdorferi, the causative agent of Lyme disease, is able to adapt to a diverse range of hosts that pose very different challenges to its survival. B. burgdorferi accomplishes this, in part, by tight regulation of the expression of various proteins that are utilized for critical functions in specific hosts. A well-studied example of this is the regulation of outer surface protein A (OspA) of B. burgdorferi, a protein required for binding to the tick midgut but nonessential for survival in the mammalian host (19, 28). B. burgdorferi minimizes the antigenic cost of expression of OspA by down-regulating its expression as the organism moves from its tick host to a mammalian host (21, 24).
The B. burgdorferi genome appears to be profoundly deficient in genes devoted to the biosynthesis of fatty acids, nucleic acids, and amino acids (7). As a result, the organism is highly dependent upon its external environment for acquisition of these essential nutrients. Peptides serve as a source of amino acids for many bacteria. B. burgdorferi carries a single peptide transport system that appears to be closely related to the oligopeptide permease (Opp) family of transporters. Opp transporters are ABC-type transporters with a peptide-binding protein that interacts with two inner membrane transmembrane proteins and two ATP binding proteins. The B. burgdorferi genome encodes five separate peptide-binding proteins that each appear to be capable of functioning with the integral membrane proteins of the transporter. Three of the genes (oppA1, -2, and -3) are located in the opp operon located on the chromosome, and two are on distinct plasmids (oppA4 is on cp26, and oppA5 is on lp54). It was previously shown that all five putative peptide-binding proteins are capable of facilitating the transport of small peptides and that many of the proteins have overlapping but distinct substrate preferences (13, 25).
In addition to the transport of peptides for nutritional needs, peptide transport systems have been implicated in diverse nonnutritional bacterial functions. In Bacillus subtilis, the oligopeptide permease encoded by spo0K (Opp) binds to an exported peptide, competence and sporulation stimulating factor (CSF), which acts intracellularly to signal cell density (11). CSF stimulates competence gene expression at low concentrations and inhibits competence gene expression and stimulates sporulation at high concentrations. Uptake of CSF by Opp from the extracellular environment allows B. subtilis to respond to changes in cell density. In Enterococcus faecalis, a plasmid-encoded peptide binding protein, PrgZ, with similarity to OppA, binds a peptide sex pheromone, cCF10, which signals conjugative transfer of a plasmid (12). PrgZ uses chromosomal OppBCDF to process cCF10 and reach intracellular target molecules. Escherichia coli shows chemotaxis towards a variety of dipeptides (but not tripeptides) that is mediated by dipeptide permease A (DppA), a structurally related oligopeptide transport system (1). Chemotaxis towards dipeptides appears to be mediated through Tap, which acts as a conventional signal transducer. Manson et al. have shown that Tap-mediated peptide chemotaxis requires the function of DppA but not the rest of the Dpp peptide transport system (14). More recently, for Listeria monocytogenes, OppA was shown to be critical to environmental adaptation, allowing the bacterium to grow at a low temperature and to survive intracellularly (4).
Expression of the B. burgdorferi oppA genes was previously shown to be differentially regulated under changing environmental conditions (26). In this study, we attempted to determine the mechanisms involved in the regulation of expression of B. burgdorferi oppA by examining the effects of alternative sigma factors and putative transcription factors on the activities of the oppA promoters.
MATERIALS AND METHODS
Bacterial and mouse strains.
B. burgdorferi was grown in BSK-H medium (Sigma Co., St. Louis, MO). RpoS, RpoN, and Rrp2 mutants and complemented mutants were the kind gifts of Frank Yang and Michael Norgard and have been described previously (8, 27). Low-passage, infectious B. burgdorferi strain N40 (clone D10E9) was used for all experiments not involving B. burgdorferi mutants. E. coli (strain Top 10) was used for plasmid preparation and for β-galactosidase assays. Recombinant proteins were expressed using E. coli strain BL21(DE3)/pLysS or Rosetta-gami2 (Novagen, Madison, WI). E. coli was grown in Luria-Bertani broth (for β-galactosidase assays) or 2XYT (for protein production) supplemented with the appropriate antibiotics. C3H/HeN mice were purchased from Charles River Laboratories (Wilmington, MA).
Plasmid construction.
Cloning of the oppA upstream regions has been described previously (26). Briefly, each region was individually cloned from B. burgdorferi B31 DNA through PCRs using specific primers with BamHI and BglII restriction sites. The DNA fragments obtained from PCR were ligated into a pCR2.1 vector (Invitrogen, Carlsbad, CA) for further amplification. After digestion by BamHI and BglII, the DNA fragments were recovered using a Qiaquick gel extraction kit (QIAGEN, Valencia, CA) and ligated to the promoter-probe vector pCB182 or pCB192 (20) at BamHI and BglII sites. All DNA fragments inserted into the promoter-probe vectors were checked by DNA sequencing. Plasmid DNA was purified using Qiaprep mini spin columns (QIAGEN). All restriction enzymes and primers were purchased from Invitrogen unless stated otherwise.
The pET30a vector was used for expression of the putative B. burgdorferi transcription factors. Oligonucleotides (HISHAT and HISHAB) encoding a His6 tag followed by two copies of the influenza virus hemagglutinin (HA) epitope tag and bearing flanking NdeI and KpnI restriction sites were synthesized and annealed together. The resulting His6-HA-HA tag was then cloned into the pET30a (Novagen, Madison, WI) vector by using the NdeI and KpnI sites within the multiple cloning site, thereby replacing the His6 tag and the S tag from the original vector.
The Borrelia genes hbb (BB0232), ebfC (BB0462), and bosR/fur (BB0647) were amplified by PCR from the B. burgdorferi N40 (clone D10E9) genomic DNA, using primers containing sequences specific to the individual genes plus a 5′ BamHI restriction site and a 3′ XhoI restriction site with a stop codon at the 3′ end of the reverse primer (Table 1). The amplicons were restricted with BamHI and XhoI and ligated into similarly restriction-modified pET30a. The correct insertions were confirmed by sequencing of the plasmid (Tufts University Core Sequencing Facility, Boston, MA).
TABLE 1.
Primers and strains used for this study
| Primer or strain | Sequence (5′-3′) or genotype | Usage |
|---|---|---|
| Primers | ||
| HISHAT | TAT GCA CCA CCA CCA CCA CCA CAC CGG TTA TCC TTA CGA CGT ACC TGA CTA CGC AGC AGG ATA CCC ATA CGA CGT CCC AGA CTA CGC TGG TAC | Tag generation |
| HISHAB | CAG CGT AGT CTG GGA CGT CGT ATG GGT ATC CTG CTG CGT AGT CAG GTA CGT CGT AAG GAT AAC CGG TGT GGT GGT GGT GGT GGT GCA | Tag generation |
| 0232B5 | TAG CGG ATC CAT GTC TTT TTC AAG AAG ACC AAA GG | Hbb cloning |
| 0232X3 | TAC GCT CGA GTC ATA ACC GGC ATT TAA CCT TTG ATA CC | Hbb cloning |
| 0462B5 | TAG CGG ATC CTT GGA GCA AGT GAA GTT CTG GAG G | EbfC cloning |
| 0462X3 | TAC GCT CGA GTC AAC CTC TCC TAA CTC CAT CTG G | EbfC cloning |
| 0462IF | TTG GAG CAA GTG AAG TTC TGG | EbfC qRT-PCR |
| 0462IR | AAA AGG AAG AAC TCC CAT GG | EbfC qRT-PCR |
| 0647B5 | TAG CGG ATC CGA CAA CAT AAT AGA CGT ACA TTC C | BosR/Fur cloning |
| 0647X3 | TAC GCT CGA GTC ATG TCA ATT TCT TCT ATG TTT TTA GG | BosR/Fur cloning |
| 0647QPCRF | CGA CAA CAT AAT AGA CGT ACA TTC C | BosR/Fur qRT-PCR |
| 0647QPCRR | TTT TGC CGT AAA GTA TCT TTT GGG | BosR/Fur qRT-PCR |
| recAF | GTG GAT CTA TTG TAT TAG ATG AGG CTC TCGa | RecA promoter amplification |
| recAR | GCC AAA GTT CTG CAA CAT TAA CAC CTA AAGa | RecA promoter amplification |
| oppA-4F | ATA ACC ATT GGA GAG CAA GC | OppA4 promoter amplification |
| oppA-4R | CAA GCA TCC TTA CAA GTT TT | OppA4 promoter amplification |
| oppA-5F | GCG AAT TCA TCC TTA TGA CCT TCC TTT A | OppA5 promoter amplification |
| oppA-5R | GCG CAT GCG CAG AAA TAA ATA CTT TAC | OppA5 promoter amplification |
| Strains | ||
| BL21(DE3)/pLysS | ompT hsdSB(rB− mB−) Camr | |
| Rosetta-gami2(DE3)/pLysS | trxB gor; pLysS carries rare codons; Tetr Strr Camr | |
| Top10 (F′) | F−mcrAΔ(mrr-hsdRMS-mcrBC) φ80dlacZΔM15 ΔlacX74 recA1 araD139Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG |
From reference 18.
β-Galactosidase assays.
Plasmids carrying the oppA promoter-lacZ reporter and encoding the different transcription factors were cotransformed into E. coli (BL21) by electroporation. Bacterial cultures were grown to mid-logarithmic phase under various environmental and nutritional conditions. Growth curves for all the strains were established experimentally prior to measurements of β-galactosidase activity to ensure that bacteria were collected at similar stages of growth. Bacteria were centrifuged, washed and resuspended in Z buffer (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4, 50 mM 2-mercaptoethanol, pH 7.0), and incubated on ice (17). After the absorbance was recorded at a wavelength of 600 nm, 10 μl 1% sodium dodecyl sulfate and 20 μl chloroform were added to each 0.8-ml sample. The samples were vortexed for 15 seconds and then placed at 30°C for 15 min. o-Nitrophenyl-β-d-galactopyranoside (ONPG; 160 μl of 4-mg/ml ONPG dissolved in 0.1 M sodium phosphate buffer, pH 7.0) was added and mixed vigorously for 10 seconds. The samples were incubated at 30°C for 15 to 30 min. Four hundred microliters of 1 M sodium carbonate was added to quench the reaction. The samples were clarified by centrifugation. β-Galactosidase activity was monitored by measuring the optical density at 420 nm (OD420) and the OD550. Enzyme activity was calculated based on the following equation: units of activity (U) = 1,000 × [OD420 − (1.75 × OD550)]/(time × volume × OD600). One unit of enzyme activity was defined as the amount of enzyme with the ability to hydrolyze 1 μM o-nitrophenyl-β-d-galactopyranoside per minute (17).
Recombinant protein preparation.
E. coli BL21(DE3)/pLysS containing a plasmid carrying hbb, ebfC, or bosR/fur was grown overnight at 30°C. Five milliliters of the overnight culture was added to 500 ml 2XYT containing 0.2% dextrose and 50 μg/ml kanamycin. Bacteria were grown at 30°C to an OD600 of 0.4 to 0.6. Protein production was induced by adding IPTG (isopropyl-β-d-thiogalactopyranoside) to a final concentration of 1 mM, and cells were shaken at 250 rpm for one to two additional hours. Cells were harvested, chilled on ice for 30 min, and centrifuged at 4,230 × g for 10 min at 4°C. Pellets were resuspended in 20 ml HEPES-buffered saline and centrifuged at 4,230 × g for 10 min at 4°C. The pellets were stored at −70°C until ready for lysis.
Pellets were lysed in a French pressure cell in the presence of protease inhibitors (0.1 trypsin inhibitor unit/ml aprotinin, 1 mM benzamidine, 10 μM pepstatin A, and 1 mM phenylmethylsulfonyl fluoride [Sigma-Aldrich Co., St. Louis, MO]). Lysed cells were centrifuged at 26,890 × g for 30 min at 4°C. The supernatant was decanted and centrifuged at 38,720 × g for 20 min at 4°C.
His-bind kit columns (Novagen/EMD Biosciences, Darmstadt, Germany) were used to purify the recombinant His6-tagged proteins from the supernatants. The purification was performed per the manufacturer's instructions, except that protease inhibitors (0.1 trypsin inhibitor unit/ml aprotinin, 1 mM benzamidine, and 10 μM pepstatin A) were added to the binding buffers. The expression and purity of the individual tagged proteins were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by Coomassie blue staining or by transfer to polyvinylidene difluoride membranes and immunoblotting using a mouse monoclonal anti-HA antibody (Cell Signaling, Beverly, MA).
Electrophoretic mobility shift assays (EMSAs).
DNAs for the upstream regions of oppA4 and oppA5 containing promoter activity, as described previously (26), were prepared by PCRs utilizing the primers listed in Table 1. DNAs were labeled with digoxigenin by using a gel shift assay kit (Roche Biochemicals, Indianapolis, IN). Purified EbfC or BosR/Fur was mixed with the appropriate labeled DNA at various concentrations in a buffer containing 20 mM HEPES, pH 7.6, 50 mM NaCl, 5 mM dithiothreitol, 5% glycerol, 50 μg/ml bovine serum albumin, 1 mM MgCl, and 0.1 μg poly(dI-dC) to reduce nonspecific interactions. For competition assays, unlabeled DNA with the same sequence as the labeled DNA was added in 125-fold excess to the mixture. The mixture was incubated at room temperature for 30 min before being loading into a 6% DNA retardation gel (Novex precast gel; Invitrogen, Carlsbad, CA). After electrophoresis, the gel was transferred to a nylon membrane (Bio-Rad, Hercules, CA). Blocking of the membrane, incubation with alkaline phosphatase-linked anti-digoxigenin antibody, incubation with the chemiluminescent substrate CDP-Star, and detection of alkaline phosphatase by exposure to film (Biomax; Kodak, Rochester, NY) were performed per the gel shift assay kit instructions.
Preparation of tick RNA.
Ixodes dammini ticks were obtained from a laboratory colony derived from an Ipswich, MA, population that has been determined to be free of inherited spirochetal infection. Outbred C3H mice were infected by nymphs infected with strain N40 (clone D10E9), which was maintained in alternating tick-mouse-tick passages. Larvae were allowed to feed to repletion 3 weeks after the infected nymphs engorged. Upon repletion, engorged larvae were collected and permitted to molt to the nymphal stage at 21°C and 95% relative humidity. Nymphal ticks were fed on uninfected mice for 60 h prior to removal.
Fed and unfed nymphal ticks were pooled into groups of 5 to 10 ticks, suspended in Trizol (Invitrogen), and homogenized for 30 s, using a rotor-stator homogenizer. RNAs were purified per the manufacturer's instructions.
Preparation of mouse RNA.
C3H/HeN mice (Jackson Laboratory, Bar Harbor, ME) were infected subcutaneously with 104 B. burgdorferi organisms (strain N40, clone D10E9). Mice were sacrificed at 2 weeks postinfection, and hearts were placed into RNAlater (Applied Biosystems, Foster City, CA), snap frozen in liquid nitrogen, and stored at −70°C until use. RNAs were prepared using Trizol per the manufacturer's instructions.
RT-PCR.
Total RNA from B. burgdorferi, ticks, or mouse tissue was heated to 95°C for 10 min and then chilled. Samples were then treated with RNase-free DNase I (Applied Biosystems) at 37°C for 15 min. First-strand cDNA synthesis was performed using SuperScript (Invitrogen) with random hexamer primers or gene-specific primers per the manufacturer's instructions. The generated cDNAs were used as a template for real-time PCR amplification (iCycler; Bio-Rad), using SYBR green fluorescent dye (SYBR green master mix; QIAGEN) and specific primers for each oppA gene. Cycling parameters were 50°C for 5 min and 95°C for 10 min, followed by 40 cycles of 95°C for 30 s and 55°C for 1 min. The primers used for reverse transcriptase PCR (RT-PCR) are shown in Table 1 and were described previously (26). Calculations of relative expression of the gene of interest were normalized to recA gene expression by using the ΔΔCT method, where the amount of target, normalized to an endogenous reference and relative to a calibrator, is given by the variable 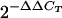 , where CT is the cycle number of the detection threshold.
, where CT is the cycle number of the detection threshold.
Statistics.
Comparisons of B. burgdorferi gene expression between control and test conditions were performed using the nonparametric Mann-Whitney U test for two-sided tails.
RESULTS
Role of alternative sigma factors in oppA expression.
The B. burgdorferi genome has been found to encode two alternative sigma factors, RpoS and RpoN. RpoN has been reported to control the expression of RpoS (8), and RpoN expression is under the control of a response regulator, Rrp2 (27). Both RpoN and RpoS appear to control the expression of a cluster of B. burgdorferi proteins that may be important in environmental adaptation. We examined whether RpoS and RpoN are involved in the regulation of expression of any of the oppA genes by utilizing deletion mutants of RpoS and RpoN (kind gifts of Frank Yang and Michael Norgard) (27). Mutant, complemented mutant, and wild-type strains of B. burgdorferi were grown at 37°C to logarithmic growth phase, and transcription of the various oppA genes was measured by quantitative RT-PCR (qRT-PCR). Deletion of RpoS, RpoN, or Rrp2 did not substantially affect transcription of oppA1, -3, or -4 (Fig. 1). Deletion of rpoS slightly decreased the expression of oppA2, by approximately 3.5-fold (P = 0.005), and the decreased expression was fully recovered in the complemented strains. Deletion of rpoN also slightly decreased the expression of oppA2, but this did not reach significance (P = 0.095). Deletion of rrp-2 did not alter oppA2 expression. Expression of oppA5 was significantly decreased in both the RpoS and RpoN mutants, by 5- to 11-fold (P ≤ 0.005). Again, expression was fully restored in the complemented mutant RpoS and RpoN strains. The deletion of rrp-2 also reduced the expression of oppA5 (eightfold), but recovery of expression was not seen in the complemented mutant, so it is unclear whether the reduction was due to a lack of Rrp2 expression or to unintended effects of the mutation.
FIG. 1.
Effects of alternative sigma factor mutations on OppA expression. Levels of oppA transcripts were measured by real-time RT-PCR in mutant B. burgdorferi strains lacking rpoN (RpoN−), rpoS (RpoS−), or rrp-2 (Rrp2−) as well as in the complemented mutant strains (RpoN−/+, RpoS−/+, and Rrp2−/+). Experiments were performed two to five times, and error bars represent standard errors of the means. The expression level for wild-type bacteria was set to 1, and all other values are expressed relative to the wild type. wt, wild-type B. burgdorferi.
Roles of identified and putative transcription factors in expression of oppA genes.
In order to better understand the mechanisms governing oppA expression, we sought to test the roles of other identified or putative transcription factors of B. burgdorferi. Hbb (BB0232), EbfC (BB0462), and BosR/Fur (BB0647) have been annotated in the B. burgdorferi genome to have similarity to DNA binding proteins or transcription factors. EbfC and BosR/Fur have been shown to affect the expression of at least one B. burgdorferi gene (2, 5, 9, 22). To test their effects on the expression of oppA genes, we utilized a transcriptional reporter fusion system in E. coli. The genes for the transcription factors were cloned into an expression plasmid, and expression of the recombinant proteins was verified by Western blotting with an antibody raised against the HA epitope tag fused to each protein. A screen for the effects of these recombinant proteins on oppA expression was performed by cotransforming E. coli expressing the recombinant protein of interest with a reporter plasmid containing the promoter region of an oppA gene fused to lacZ. The effects of the recombinant protein were then measured by performing β-galactosidase assays as previously described (26).
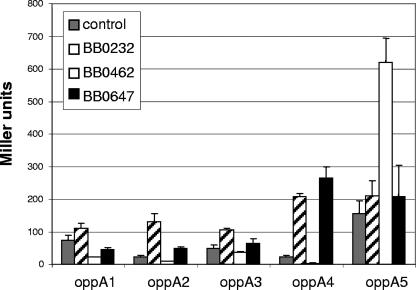
Increases in β-galactosidase activity of >3-fold were seen for Hbb with the oppA2 and oppA4 promoters, for EbfC with the oppA5 promoter, and for BosR/Fur with the oppA4 promoter (Fig. 2). A decrease in β-galactosidase activity of >3-fold was seen only for EbfC with the oppA4 promoter. As controls, multiple other B. burgdorferi proteins, including those with similarity to transcription factors of other organisms and those not predicted to have transcription factor properties, were tested, and none showed changes of >3-fold (data not shown).
FIG. 2.
Effects of putative transcription factors on activities of OppA promoters. Plasmids expressing each putative transcription factor were cotransformed into E. coli BL21(DE3) with a lacZ reporter plasmid for each oppA promoter region. Bacteria were grown to an OD600 of 0.5. Cells were harvested, and β-galactosidase activity was measured. The control is pET30a plus the specific oppA reporter plasmid. Error bars represent standard errors of the means for three experiments performed in duplicate.
Binding of BosR/Fur and EbfC to oppA4 and oppA5 promoters.
We had previously shown that the expression of oppA4 and oppA5 was highly affected by environmental conditions, whereas the expression of other oppA genes, such as oppA1, was constitutive (26). Therefore, we chose to further study the interactions of EbfC with the oppA5 promoter and of Hbb and BosR/Fur with the oppA4 promoter. Purified recombinant Hbb, EbfC, and BosR/Fur proteins were prepared for use in EMSAs (Fig. 3). The oppA4 and oppA5 promoter regions were labeled with digoxigenin and incubated in the presence or absence of recombinant protein. Unlabeled oppA4 or oppA5 promoter DNA was used as a competitor; unlabeled, irrelevant DNA of the same size was used as a control.
FIG. 3.
Purification of Hbb, EbfC, and BosR/Fur. Recombinant Hbb, EbfC, and BosR/Fur were expressed in E. coli as described in Materials and Methods. The recombinant proteins were expressed with a His6-HA-HA tag. Lysates of E. coli were clarified by centrifugation and then applied to Ni2+ columns. After being washed, recombinant proteins were eluted from the columns by using an imidazole gradient. Shown are Coomassie blue-stained gels of the purified proteins. The predicted sizes of the recombinant proteins with tags are as follows: BosR/Fur, ∼25 kDa; EbfC, ∼18 kDa; and Hbb, ∼18 kDa. Note that there is a discrepancy between the predicted size of BosR/Fur and the actual migration. Identification of the protein was performed by sequencing and Western blotting. Lane 1, BosR/Fur; lane 2, EbfC; lane 3, Hbb; lane M, markers.
Hbb bound nonspecifically to oppA4 promoter DNA as well as to multiple control regions of DNA, including the oppA1, oppA2, oppA3, and oppA5 promoters and coding regions from within the recA gene, under all tested conditions (data not shown). As a result, we did not pursue the study of Hbb further.
The addition of EbfC resulted in a shift of labeled oppA5 promoter DNA that was lost with the addition of a 125-fold excess of unlabeled oppA5 promoter DNA (Fig. 4). The addition of an equivalent amount of EbfC did not result in a shift of labeled promoter regions from other oppA genes or of the recA promoter.
FIG. 4.
Binding of BosR/Fur and EbfC to OppA promoters. Binding of putative transcription factors to oppA promoters was determined by EMSA. Purified proteins were added in increasing amounts to digoxigenin-labeled DNAs from promoter regions. Unlabeled DNA (125-fold excess) was added as a specific competitor (Comp) to wells containing the largest amount of protein. DNA from the recA coding region was used as a negative control. (A) BosR/Fur; (B) EbfC. Arrows indicate shifted DNAs.
The addition of BosR/Fur resulted in a shift of labeled oppA4 promoter that was lost with the addition of a 125-fold excess of unlabeled oppA4 promoter. The addition of an equivalent amount of BosR/Fur did not result in a shift of labeled promoter regions from other oppA genes or of the recA promoter. One previous report suggested that the presence of Zn2+ optimizes binding of BosR/Fur to its target sequences, although others have not found this to be true (5, 9). We did not see any significant changes in binding with the addition of various concentrations of Zn2+ to the binding buffer (data not shown).
These data confirm the results of the reporter screen showing that EbfC binds to the oppA5 promoter region and that BosR/Fur binds to the oppA4 promoter region. These interactions are consistent with the reporter assay data showing increases in the promoter activities of oppA4 by BosR/Fur and oppA5 by EbfC.
Comparison of expression of EbfC and BosR/Fur with oppA expression under various environmental conditions.
If EbfC and BosR/Fur regulate the expression of oppA5 and oppA4, respectively, then it is possible that their own regulation is affected by environmental conditions and parallels expression of the opp genes that they regulate. It was previously shown that oppA4 expression is greatly increased in B. burgdorferi organisms recovered from mouse tissue compared with that in organisms grown in vitro and is undetectable in organisms recovered from either unfed or feeding ticks (26). oppA5 expression was found to increase in B. burgdorferi organisms recovered from unfed ticks compared with either those from fed ticks or those from mouse tissue. We examined the expression of ebfC and bosR/fur in fed and unfed ticks and in mouse heart tissue by qRT-PCR (Fig. 5).
FIG. 5.
Relative expression of ebfC and bosR/fur under various environmental conditions. B. burgdorferi RNAs were recovered from bacteria grown in vitro in BSK-H medium at 37°C (in vitro), from unfed nymphal Ixodes ticks (unfed), from fed nymphal Ixodes ticks (fed), and from heart tissue of mice infected with B. burgdorferi for 2 weeks (heart). After the generation of cDNAs, using gene-specific primers, qRT-PCR was performed for ebfC, bosR/fur, and recA transcripts. recA transcripts were used to normalize results between samples. Transcription of the genes in organisms grown in vitro was assigned a value of 1, and expression under all other conditions was expressed relative to the in vitro expression level. All experiments were performed three to five times in duplicate. Error bars represent standard errors of the means.
The pattern of transcription of ebfC did not match the expression of oppA5 under various environmental conditions. The relative expression of ebfC decreased approximately fivefold (P = 0.014) in unfed nymphal ticks compared to that in organisms grown in vitro at 37°C. Expression in ticks that had taken their blood meal was essentially unchanged from that in organisms grown in vitro, and expression in organisms recovered from mouse heart tissue was only slightly elevated (1.5-fold). Deletion of RpoS, RpoN, or Rrp2 also did not significantly change the expression of EbfC in vitro.
The relative expression of BosR/Fur more closely followed the pattern of oppA4 gene expression. bosR/fur transcripts were undetectable in unfed ticks. Transcripts in fed ticks were decreased compared with levels in organisms grown in vitro and increased in organisms recovered from mouse heart tissue (P ≤ 0.014 for both).
DISCUSSION
In this study, we examined the roles of specific alternative sigma factors and candidate transcription factors in the expression of oppA genes of B. burgdorferi. The five oppA genes of B. burgdorferi have previously been shown to be independently regulated and to respond differentially to changing environmental conditions (26). The B. burgdorferi genome contains genes encoding only three identifiable sigma factors, namely, RpoD, RpoS, and RpoN. The alternative sigma factors, RpoS and RpoN, have been found to regulate the expression of a cluster of proteins involved in environmental adaptation and infection of mice (6, 8, 27). Expression of many of these proteins appears to be influenced by temperature. Using knockout and complemented mutant bacteria, we found that of the oppA genes, RpoS and RpoN appear to be involved in the regulation of only oppA5. This appears to be consistent with prior studies that have shown that among the B. burgdorferi oppA genes, only oppA5 expression is affected by temperature (3, 26).
Non-sigma-factor-controlled expression of oppA genes is likely mediated by additional transcriptional activators or repressors. Compared with other bacteria, B. burgdorferi has only a limited number of putative genes with similarity to known transcription factor genes. Only a few functional transcription factors have been identified in B. burgdorferi to date, and their effects on the expression of OppA proteins have not previously been studied in detail (5, 9, 10, 22). Because of the difficulty in working directly to manipulate B. burgdorferi gene expression, we employed a reporter screen using the expression of B. burgdorferi putative transcriptional factors in E. coli. While this is clearly suboptimal, use of the heterologous system allows for rapid screening of multiple protein-promoter pairs to identify promising interactions for more detailed examination. Here we show, using a reporter assay with E. coli and gel shift assays, that EbfC (BB0462) binds to the oppA5 promoter and BosR/Fur (BB0647) binds to the oppA4 promoter region. Binding of both of these proteins to the respective OppA promoters results in increased promoter activity. Although another putative transcription factor, Hbb (BB0232), showed activity in our reporter assays, we were unable to confirm this activity due to nonspecificity of binding of our recombinant protein. Previous investigators have found specific binding by Hbb (10). The difference may be due to the tags that were used in our system or to subtle differences in the host strain or in the expression and purification procedures that resulted in changes in folding and activity of the protein. Even among our constructs, we did see differences in DNA affinity between Hbb proteins expressed from different host strains of E. coli.
EbfC has only recently been identified as a functional transcription factor. Babb et al. identified EbfC by DNA affinity chromatography with the promoter region from the erpA gene of B. burgdorferi (2). Some of the Erp proteins (also known as OspE/OspF/Elp family proteins) bind to mammalian factor H proteins, which is hypothesized to protect the organism from complement-mediated killing (15, 23). Erp proteins are down-regulated in unfed ticks and up-regulated as ticks take their blood meals and in the mammalian host (16). We found that ebfC expression was greatly decreased in unfed ticks, which is consistent with its role in expression of the Erp proteins. Expression of ebfC did not match the expression of oppA5 in the tick, which increases in unfed ticks compared with fed ticks (26). This may be due in part to the effects of other mechanisms that control oppA5 expression, for example, the RpoS-RpoN system, which we found to have significant effects on oppA5 expression. It should also be noted that because of the small numbers of organisms present in tick and mouse tissues, our studies, of necessity, measured transcription, not actual protein production. It is possible that EbfC protein expression is not transcriptionally controlled and that actual protein levels differ significantly. Another possibility is that the activity of EbfC on the oppA5 promoter may not have been represented accurately by the heterologous E. coli reporter system. Babb et al. identified a putative EbfC binding motif, TGT(A/T)ACA, which was not identified in the oppA5 promoter region. It is unknown whether there are other sequences which bind EbfC or whether the requirement for the TGT(A/T)ACA sequence is stringent.
BosR/Fur (BB0647) has previously been studied by three groups of investigators (5, 9, 22). It has variously been named BosR and reported as a homolog of PerR, a stress response repressor, and also Fur, for its similarity to ferric uptake regulation protein. Recombinant BosR/Fur can bind to both Fur and Per box sequences with similar affinities (9). There are data to suggest that it may act as both a repressor and an activator in B. burgdorferi (5, 9, 22). It has been shown to bind to the promoter of napA, which is involved in the response by B. burgdorferi to oxidative stress, and to the superoxide dismutase (sodA) promoter. BosR also binds to its own promoter, indicating autoregulation, and binds to the BB0646 promoter as well, although the role for this is not yet clear (9). We found that BosR/Fur has an activator effect on the oppA4 promoter region but not on other B. burgdorferi oppA promoter regions. An analysis of the oppA4 promoter region did not reveal any segments that closely matched consensus Fur or Per binding sequences identified for E. coli or B. subtilis, although multiple partial matches were seen. The expression of bosR/fur in vivo has not previously been described. Here we show that the expression of bosR/fur is greatly increased in the heart tissue of mice compared with expression in vitro or in either fed or unfed ticks. This expression pattern parallels that of oppA4 and would be consistent with the need for increased expression of NapA under conditions of higher oxidative stress in the mammalian host.
In summary, we have now delineated some of the mechanisms by which B. burgdorferi is able to differentially regulate the expression of its oppA genes. There are undoubtedly additional mechanisms that are yet to be described that play roles in the control of these proteins. A better understanding of the mechanisms by which B. burgdorferi regulates gene expression and the proteins that are coregulated by different transcriptional factors will lead to new insights into how B. burgdorferi is able to adapt to the markedly different environments it encounters.
Acknowledgments
We thank Michael Norgard and Frank Yang for their kind gift of the RpoS, RpoN, and Rrp2 B. burgdorferi mutants. We thank Brendan O'Malley and David Lazinski for sharing unpublished information regarding the construction of the HISHAB and HISHAT oligonucleotides we used for the construction of our expression plasmids. We are indebted to Anne Kane from GRASP for her assistance with large-scale protein purification.
Work on this project was supported by grants from the National Institute of Allergy and Infectious Diseases (R01 AI44240 [L.T.H.], R01 AI50043 [L.T.H.], R01 AI51407 [J.C.], and F31 AI52495 [M.M.]). Recombinant proteins were produced with support from the Center for Gastroenterology Research on Absorptive and Secretory Processes (GRASP), with a grant from NIDDK (P30DK39428).
Footnotes
Published ahead of print on 19 January 2007.
REFERENCES
- 1.Abouhamad, W. N., M. Manson, M. M. Gibson, and C. F. Higgins. 1991. Peptide transport and chemotaxis in Escherichia coli and Salmonella typhimurium: characterization of the dipeptide permease (Dpp) and the dipeptide-binding protein. Mol. Microbiol. 5:1035-1047. [DOI] [PubMed] [Google Scholar]
- 2.Babb, K., T. Bykowski, S. P. Riley, et al. 2006. Borrelia burgdorferi EbfC, a novel, chromosomally encoded protein, binds specific DNA sequences adjacent to erp loci on the spirochete's resident cp32 prophages. J. Bacteriol. 188:4331-4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bono, J. L., K. Tilly, B. Stevenson, et al. 1998. Oligopeptide permease in Borrelia burgdorferi: putative peptide-binding components encoded by both chromosomal and plasmid loci. Microbiology 144:1033-1044. [DOI] [PubMed] [Google Scholar]
- 4.Borezee, E., E. Pellegrini, and P. Berche. 2000. OppA of Listeria monocytogenes, an oligopeptide-binding protein required for bacterial growth at low temperature and involved in intracellular survival. Infect. Immun. 68:7069-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boylan, J. A., J. E. Posey, and F. C. Gherardini. 2003. Borrelia oxidative stress response regulator, BosR: a distinctive Zn-dependent transcriptional activator. Proc. Natl. Acad. Sci. USA 100:11684-11689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher, M. A., D. Grimm, A. K. Henion, et al. 2005. Borrelia burgdorferi sigma54 is required for mammalian infection and vector transmission but not for tick colonization. Proc. Natl. Acad. Sci. USA 102:5162-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fraser, C. M., S. Casjens, W. M. Huang, et al. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 8.Hubner, A., X. Yang, D. M. Nolen, et al. 2001. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc. Natl. Acad. Sci. USA 98:12724-12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katona, L. I., R. Tokarz, C. J. Kuhlow, et al. 2004. The fur homologue in Borrelia burgdorferi. J. Bacteriol. 186:6443-6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobryn, K., D. Z. Naigamwalla, and G. Chaconas. 2000. Site-specific DNA binding and bending by the Borrelia burgdorferi Hbb protein. Mol. Microbiol. 37:145-155. [DOI] [PubMed] [Google Scholar]
- 11.Lazazzera, B. A., J. M. Solomon, and A. D. Grossman. 1997. An exported peptide functions intracellularly to contribute to cell density signaling in B. subtilis. Cell 89:917-925. [DOI] [PubMed] [Google Scholar]
- 12.Leonard, B. A. B., A. Podbielski, P. J. Hedberg, and G. M. Dunny. 1996. Enterococcus faecalis pheromone binding protein, PrgA, recruits a chromosomal oligopeptide permease system to import sex pheromone cCF10 for induction of conjugation. Proc. Natl. Acad. Sci. USA 93:260-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin, B., S. A. Short, M. Eskildsen, et al. 2001. Functional testing of putative oligopeptide permease (Opp) proteins of Borrelia burgdorferi: a complementation model in opp(−) Escherichia coli. Biochim. Biophys. Acta 1499:222-231. [DOI] [PubMed] [Google Scholar]
- 14.Manson, M. D., V. Blank, G. Brade, and C. F. Higgins. 1986. Peptide chemotaxis in E. coli involves the Tap signal transducer and the dipeptide permease. Nature 321:253-256. [DOI] [PubMed] [Google Scholar]
- 15.Metts, M. S., J. V. McDowell, M. Theisen, et al. 2003. Analysis of the OspE determinants involved in binding of factor H and OspE-targeting antibodies elicited during Borrelia burgdorferi infection in mice. Infect. Immun. 71:3587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller, J. C., and B. Stevenson. 2004. Increased expression of Borrelia burgdorferi factor H-binding surface proteins during transmission from ticks to mice. Int. J. Med. Microbiol. 37:120-125. [DOI] [PubMed] [Google Scholar]
- 17.Miller, J. H. 972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 18.Morrison, T. B., Y. Ma, J. H. Weis, and J. J. Weis. 1999. Rapid and sensitive quantification of Borrelia burgdorferi-infected mouse tissues by continuous fluorescent monitoring of PCR. J. Clin. Microbiol. 37:987-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pal, U., X. Li, T. Wang, et al. 2004. TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell 119:457-468. [DOI] [PubMed] [Google Scholar]
- 20.Schneider, K., and C. F. Beck. 1986. Promoter-probe vectors for the analysis of divergently arranged promoters. Gene 42:37-48. [DOI] [PubMed] [Google Scholar]
- 21.Schwan, T. G., and J. Piesman. 2000. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 38:382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seshu, J., J. A. Boylan, J. A. Hyde, et al. 2004. A conservative amino acid change alters the function of BosR, the redox regulator of Borrelia burgdorferi. Mol. Microbiol. 54:1352-1363. [DOI] [PubMed] [Google Scholar]
- 23.Stevenson, B., N. El-Hage, M. A. Hines, et al. 2002. Differential binding of host complement inhibitor factor H by Borrelia burgdorferi Erp surface proteins: a possible mechanism underlying the expansive host range of Lyme disease spirochetes. Infect. Immun. 70:491-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevenson, B., T. G. Schwan, and P. A. Rosa. 1995. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 63:4535-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang, X. G., J. M. Kidder, J. P. Scagliotti, et al. 2004. Analysis of differences in the functional properties of the substrate binding proteins of the Borrelia burgdorferi oligopeptide permease (opp) operon. J. Bacteriol. 186:51-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, X. G., B. Lin, J. M. Kidder, et al. 2002. Effects of environmental changes on expression of the oligopeptide permease (opp) genes of Borrelia burgdorferi. J. Bacteriol. 184:6198-6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang, X. F., S. M. Alani, and M. V. Norgard. 2003. The response regulator Rrp2 is essential for the expression of major membrane lipoproteins in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 100:11001-11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang, X. F., U. Pal, S. M. Alani, et al. 2004. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J. Exp. Med. 199:641-648. [DOI] [PMC free article] [PubMed] [Google Scholar]